Abstract
Background In cemented THA, aseptic loosening of the cup is more common than loosening of the stem, while periprosthetic osteolysis of the socket resulting in difficult reconstruction problems has emerged as the most significant problem with cementless cup fixation.
Patients and methods 90 patients (96 hips) scheduled for THA were stratified in three groups according to the method of fixation of the acetabular component: acrylic bone cement with fluoride (Cemex-F), porous-coated press-fit cup with ceramic coating (Trilogy, uncemented) and acrylic cement with gentamicin (Palacos). All patients received the Spectron EF stem. Acetabular bone mineral density was measured with dual-energy X-ray absorptiometry (DXA) 1 week postoperatively, and after 12 and 24 months. The periprosthetic BMD was evaluated in 5 ROIs positioned around the acetabular component.
Results In the uncemented sockets, the BMD had decreased proximally and medially to the cup after 2 years. The difference was significant in the proximal region as compared to the control group (Palacos). No difference was noted between the 2 groups with cemented components after 2 years. Stepwise linear regression analysis showed that loss of periprosthetic BMD in the proximal high-pressure region after 2 years increased with higher postoperative BMD and when the uncemented design had been used.
Interpretation Contrary to previous studies of cemented stems, the use of fluoride cement did not influence the periprosthetic BMD 2 years after the examination. Increased loss of BMD with use of uncemented press-fit cups in the region in which osteolytic lesions are commonly found suggests that stress shielding may initiate the development of this complication. Longer follow-up will, however, be necessary to substantiate this hypothesis.
Little attention has been paid to the measurement of periprosthetic changes of BMD in the pelvis after THA. Periprosthetic osteolysis with or without loosening of the socket, resulting in difficult reconstruction problems, has emerged as the most significant problem associated with cementless cup fixation. In cemented THA, aseptic loosening of the cup is more common than loosening of the stem. In a 30-year follow-up of the Charnley arthroplasty, Callaghan et al. (Citation2004) reported that revision of the cup was almost three times more frequent than revision of the stem. According to the Swedish Hip Register, and based on follow-up of 93,000 THAs, 65% of all reoperations are caused by the need to change the acetabular component (Malchau et al. Citation1993).
Computer simulations of stress-related bone remodeling around uncemented acetabular components using finite-element analysis predict bone loss of up to 50% medial to the prosthesis because of stress shielding. In uncemented press-fit cups, postoperative changes in bone mass should also be expected in the region of the acetabular rim, since this is the main site of load to the pelvis (Levenstone et al. Citation1993), as long as the acetabulum is underreamed.
Fluoride has been added to acrylic bone cement to improve the interface and reduce the risk of prosthetic loosening. Sodium fluoride influences the bone mineral structure by forming fluorapatite (Larsen and Thorsen Citation1984), which is more stable to osteoclastic resorption (Posner et al. Citation1963) and by activation of the osteoblast resulting in increased bone trabecular volume (Vigorita and Suda Citation1983, Baud et al. Citation1988). We have previously studied such a cement when used to fixate the stem (Digas et al. Citation2005). To our knowledge, there has been no clinical study comparing fluoride-containing cement with a standard acrylic cement for fixation of the acetabular component.
In a previous report our group observed differences in the early pattern of migration between cemented and uncemented press-fit cups, suggesting different load transfer and postoperative bone remodeling (Digas et al. Citation2004). The metal backing of the uncemented design will make this construct stiffer and more susceptible to localized bone resorption caused by stress-shielding. In this study, we compared the changes of BMD using DXA analysis in three types of fixation up to 2 years post-operatively. The first group consisted of cups fixed with fluoride-containing cement and the second one had uncemented porous cups with additional outer ceramic coating. Cups inserted with Palacos with gentamicin were used as controls. Our hypothesis was that the fluoride-containing cement would reduce postoperative periprosthetic loss of BMD, whereas the uncemented design would cause loss of more bone mineral, especially centrally since the acetabulum was underreamed according to the current standard technique.
Patients
90 patients (96 hips) who were on our waiting list for THR agreed to participate. Median age was 67 (31–81) years and median weight was 70 (38–107) kg (). All types of preoperative diagnoses were included. The choice of fixation was stratified and randomized based on age (≤55/<55 years), sex, diagnosis (primary osteoarthrosis, inflammatory arthritis/long-term cortisone treatment, sequel after femoral neck fracture) and bone quality according to preoperative DXA measurements (less or equal to vs. higher BMD than age- matched controls). The last variable was excluded from the stratification protocol in patients with osteosynthesis in their hip. The stratification was designed to create 3 main groups (two cemented groups and one hybrid group). In the first cemented group, fluoride-containing cement (Cemex-F (C-F); Tecres S.p.A., Italy) and in the second group Palacos cum gentamicin (C-P) (Schering Plough, Germany) was used.In both cemented groups, the same types of cement were used to fixate both components. The two types of cements have different chemical composition with a smaller proportion of monomer in the fluoride-containing cement (Nivbrant et al. Citation2001, Digas et al. Citation2005). In the group with uncemented cup (UC), a cemented stem was used. In this group the fixation of the femoral component was again randomized to either of the two cements.
Table 1. Patient data, implant data and Harris hip and pain scores. Median (range)
We used reflection all-polyethylene cups (Smith & Nephew, Memphis, TN) manufactured using the ram-extrusion technique and sterilized with ethylene oxide (EtO). All hips in the uncemented groups were operated with a porous press-fit cup with additional HA/TCP coating (Trilogy; Zimmer Inc., Warsaw, IN) (Thanner et al. Citation2000, Digas et al. Citation2003). Additional fixation with 1 screw was done in 1 hip; 28 cups had 2 screws and the remaining 8 had 3 screws inserted in the 3 screw holes available. All patients received Spectron stems (Smith & Nephew, Memphis, TN) made of cobalt chromium alloy. We used 28-mm femoral heads made of cobalt chromium in all but 3 hips, which received heads made of zirconium (1 C-F, 2 UC). None of the 6 bilaterally operated patients underwent the same method of fixation on both sides (1 patient C-F and C-P, 3 patients UC and C-P, 2 patients C-F and UC).
We used a modified Hardinge approach and third-generation cementing technique. The Palacos with gentamicin cement was prechilled to 8°C, whereas the Cemex-F cement was kept at room temperature. According to the manufacturer, Cemex-F should be mixed at atmospheric pressure. To reduce the number of confounding factors, we obtained agreement from the manufacturer that it was acceptable to mix Cemex-F under reduced atmospheric pressure. The same mixing system (Optivac hip system for mixing the cement is manufactured from Biomet AB, Sjöbo, Sweden)was used for both cements. The patients were mobilized the day after the operation and were allowed as much weight bearing as could be tolerated.
In addition to DEXA measurements of acetabular bone remodeling, these patients were also followed with radiostereometry, conventional radiography and Harris Hip Score. The results of these studies for up to 2 years have been reported (Digas et al. Citation2004). The local ethics committee approved our study.
Bone densitometry
Acetabular bone mineral density was measured using a Lunar DPX-L (n = 312 examinations) or Lunar DPX-IQ (n = 112 examinations) densitometer (Lunar Corporation, Madison, WI). Comparison of BMD obtained with this equipment and calibrated standards has shown almost identical results (r < 0.99) (Mazess et al. Citation1989).
These measurements were made preoperatively (used for stratification only), 1 week postoperatively, and after 1 and 2 years. During scanning, the patient was placed in the supine position. A foot brace and knee supports were used to obtain standardized positions. The pelvic scan was centered on the acetabular component. It was 15 cm wide, started at the lower border of the inferior pubic ramus and continued proximally to the lower limit of the ipsilateral sacroiliac joint.
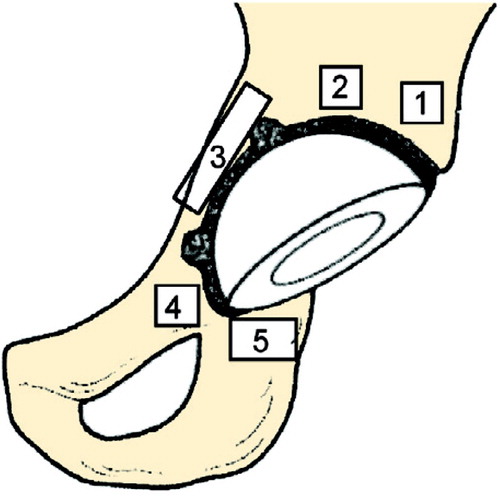
We used the paint facility of the LUNAR Orthopedic Software version 4.6 (GE Medical Systems, Madison, WI) to evaluate the BMD in 5 regions of interest (ROIs) around the acetabular component. We tried to exclude the cement mantle in the 2 cemented groups. 2 ROIs were placed proximal to the cup, the third medially, the fourth covering the superior pubic ramus, and the fifth was placed distal to the cup over the ischial tuberosity. The 2 superior ROIs and the one covering the superior ramus were of equal size (1.44 cm2). ROIs 3 and 5 were modified depending on the anatomy, but were adjusted to a constant size in one and the same patient between the examinations (Figure). In the uncemented cups, the ROIs I and II were placed so that the screws were excluded. The examiner evaluated all consecutive examinations (up to 2 years) at the same time for each patient in order to obtain as equal a position of the ROIs as possible between examinations.
15 patients with hip arthroplasty were scanned once with each type of densitometer to establish a cross calibration between the instruments. In each ROI, we found a linear correlation (mean r = 0.96, range 0.90–0.98). The mean difference ± 1 SD between the 2 densitometers was 0.9 ± 6.4%. The mean difference was used to adjust all values from the DPX-IQ device.
To evaluate the precision of the measurements, 20 patients with cemented sockets and 20 with uncemented sockets were examined twice using the DPX-IQ with a median interval of 6 (1–19) days between the examinations. In 20 further examinations, the evaluation of one examination was done twice—either by 1 or by 2 different observers—to calculate inter and intraobserver error.
The precision was expressed as the coefficient of variation (CV) according to the formula: CV% = 100 × [(δ/√2)/μ] for each ROI, where δrepresents the standard deviation of the difference between the paired BMD measurements, and μis the overall mean of all the BMD measurements for each individual ROI. The precision varied between 5% and 11% for the cemented acetabular components and between 4% and 9% for the uncemented components. The intraobserver error was less than the interobserver error ().
Table 2. Precision, intraobserver and interobserver error expressed as coefficient of variation (CV)
There were 12 missing observations (4 C-F, 4 UC, 4 C-P) in the first 2 years. The postoperative examinations were missing for 8 patients (2 C-F, 3 UC, 3 C-P). 1 patient did not attend the 2-year follow-up because of severe disease unrelated to his operated hip (C-P). 3 patients had died (3 hips: 2 C-F, 1 UC).
Statistics
We used non-parametric tests (Wilcoxon signed rank test, Mann-Whitney test, Kruskal-Wallis test, Friedman’s test) and stepwise linear regression analysis.
Results
Changes of BMD within the groups
Between the postoperative follow-up and the 2-year follow-up, the bone close to the fluoride cement showed no significant changes at all (p < 0.1, Wilcoxon signed rank test). The uncemented sockets showed reduction in bone mineral density in regions 1–3 (−3 to −17%, p = 0.001–0.04). This decrease occurred during the postoperative year (p = 0.001–0.01), without any certain further changes during the following year (p < 0.2). In regions 4 and 5, there were no statistically significant changes during the follow-up period (p < 0.6). Cups cemented with Palacos showed an increase in BMD of 14% in region 5 (p = 0.02, Wilcoxon signed rank test), whereas there was no statistically significant change in other regions (p = 0.07–0.9).
Comparison between the groups
In the total material (all groups), the postoperative BMD was higher in the 2 proximal regions than in the medial and distal ones (Friedman’s test: p < 0.001). The postoperative BMD values varied between 0.50 and 1.38 g/cm2, 0.74 and 1.28 g/cm2and 0.76 and 1.59 g/cm2in the fluoride cemented, uncemented and Palacos cemented groups, respectively (p = 0.005–0.14, Kruskal-Wallis test) (). Overall, the postoperative BMD was higher in the Palacos group in regions 1, 2, 3 and 4. In a separate comparison between the uncemented and Palacos groups, a significant difference remained in regions 1, 2 and 3 (p < 0.03, Mann-Whitney test).
Table 3. BMD (g/cm2) postoperatively and after 1 and 2 years (mean (SD)
In region I, the BMD had increased by 12% at 2 years in the fluoride cement group, whereas a minimum decrease was noted using the 2 other types of fixation (p = 0.04, Kruskal-Wallis test). Further comparison between any of the 2 study groups and the controls indicated insignificant changes (p < 0.3) (while comparison between the two study groups showed a statistically significant difference: p = 0.02). Comparison between the 2 cemented groups in regions 2–5 revealed no further differences (p < 0.3, Mann-Whitney test).
In the uncemented group, there was a loss of BMD in region II (0–2 years: −6%), whereas the cemented Palacos group showed an average increase (+7%, p = 0.02). In region III, there was a numerical difference of 16% (−17, −1) between the two groups (UC, C-P) but this was not statistically significant (p = 0.07). In the remaining regions (I, IV, and V), the change in BMD between these two groups was more equal (p ≥ 0.20) ().
Table 4. Changes in periprosthetic BMD, 1 and 2 years postoperatively (%, mean (SD)
Regression analysis
The stepwise linear regression analysis showed that the loss of BMD in region II at 2 years increased with higher postoperative BMD (p < 0.001, r2= 0.11) and when uncemented cups were used (p = 0.001, increase of r2to 0.24). The other variables entered (age, diagnosis, weight, sex, use of Palacos or fluoride cement, migration and rotation of the cup in different directions, cup size and the proximal penetration of the femoral head to the cup) had no effect on the changes in BMD (RSA data have been presented previously in Digas et al. Citation2004). In a separate analysis only including the uncemented cups, the loss of BMD in region II (postoperatively to 2 years) increased with higher postoperative BMD (p < 0.001, r2= 0.27), radiostereometric recording of rotation around the sagittal axis 0–2 years (decreasing inclination) (p = 0.001, increase of r2to 0.45), less proximal migration of the cup (p = 0.002, increase of r2to 0.57), and smaller cup size (p = 0.04, increase of r2 to 0.64).
Discussion
Wear and focal osteolysis are probably the most important factors which limit the survival of uncemented total hip arthroplasty. Focal osteolysis occurs as a macrophage-mediated host response to implant-derived particulate debris and probably to other stimuli, resulting in local osteoclastic bone resorption (Archibeck et al. Citation2001). Adaptive remodeling may also change the periprosthetic bone mass following both cemented and uncemented total hip arthroplasty (Marchetti et al. Citation1996, Bugbee et al. Citation1997). Changes in load distribution of the acetabulum after prosthetic implantation may result in localized stress-shielding or increased load (Huiskes Citation1987, Wright et al. Citation2001). The bone tissue will react to these changes with remodeling according to Wolf’s law.
Changes of bone mineral density (BMD) are most rapid during the first 6 months after surgery (Kröger et al. Citation1997, Rosenthall et al. Citation1999, Venesmaa et al. Citation2003), but continue for longer periods (Okano et al. Citation2002, Venesmaa et al. Citation2003, Brodner et al. Citation2004). In our study, most changes occurred during the postoperative year. Probably the major changes took place during a shorter period, but this could not be evaluated by us. The changes during the second year were small and did not reach statistical significance.
Modern uncemented implants aim at rigid fixation to the bone (Albrektsson et al. Citation1981). The bony tissue will adapt to the forces transmitted from the implant, finally resulting in equilibrium between bone apposition and resorption (Schenk Citation1995). When press-fit cups are loaded, they close and are compressed together from the side, which will transmit forces sideways to the periphery (Morscher et al. Citation1997, Wright et al. Citation2001). Cemented cups, being more elastic, may distribute the load to a wider area. As the regional BMD adjusts to its new mechanical environment, incremental attenuation of BMD in areas with no or minimal load could eventually reach a level at which the bone-implant interface becomes critically compromised.
Finite element analyses of press-fit acetabular components have also predicted stress concentrations at the rim of the implant, with associated transfer of load to the pelvic cortex and reduced stress within the trabecular bone proximal to the implant (Huiskes Citation1987, Morscher et al. Citation2002). We could not verify such a peripheral condensation of bone corresponding to ROI I around our uncemented sockets. This ROI did not, however, cover the most peripheral part of the acetabulum, and in our analysis changes of BMD anterior and posterior to the implant could not be studied.
The uncemented sockets lost bone in the high-pressure region of the acetabulum corresponding to ROI II, while in the cemented cups increase in BMD was noted in the same region at two years. Also, medially to the cup (ROI III), the uncemented socket group showed a decrease in BMD which was not large enough to result in a significant difference compared to the Palacos group at 2 years. If this trend reflects a true variation, it could be due to the comparatively thick metal backing being stiffer than whole polyethylene cups and cement.
Different degrees of periprosthetic load transmission in metal-backed and cemented cups could probably explain these variations in bone remodeling. Differences in the topography of the interface and in the location of the most rigid fixation are also important and will probably differ depending on the design. In cemented cups radiolucent lines start to develop peripherally (Hultmark et al. Citation2003), whereas uncemented porous cups inserted in an underreamed acetabulum could be expected to achieve ingrowth peripherally—which may initiate the bone resorption more centrally.
We found less loss of bone mass in the high-pressure region when the uncemented cups migrated proximally, probably because of increased stresses on the bone in this region. This observation might suggest that a small amount of proximal migration would be beneficial. If, on the other hand, the migration continues and there is inducible displacement, the load pattern will become more complicated and other biological reactions caused by wear, synovitis and pressure variations of the joint fluid during activity, may result in more complex bone remodeling with the risk of clinical loosening.
Li and Nilsson (Citation2000) evaluated bone mineral density at the proximal tibia for 2 years after total knee arthroplasty. In accordance with our findings in the acetabular region, they found that knees with high postoperative BMD displayed a postoperative decrease in BMD, whereas those with low BMD showed an increase. They also observed that knees with varus malalignment had higher levels of BMD than did those with valgus malalignment. Similar observations were made by Soininvaara et al. (Citation2004), who noted postoperative reduction in BMD in the medial metaphyseal ROI after total knee replacement in knees with varus deformity. These observations in the knee region and ours regarding the acetabulum may reflect that the abnormal load on the subchondral trabecular bone caused by the osteoarthritis has disappeared. Instead, the bone tissue adapts to the new situation dictated by the stiffness and quality of the fixation of the artificial joint.
In our two cemented groups, the cement mantle was excluded from the analysis by manual positioning of each ROI. Difficulties in consistently removing the true area with cement when repeating the analysis might be one explanation for the poorer precision mirrored by higher inter and intraobserver error for the cemented groups. The line of demarcation between cement and bone is indistinct, making attempts to completely exclude cement manually unreliable. The manual positioning of each ROI may also explain the somewhat poorer precision in the uncemented group compared with previous studies (Wilkinson et al. Citation2001).
We hypothesized that addition of sodium fluoride to bone cement would increase early bone formation at the interface, and thereby also the fixation of the cup. We have, however, previously reported that the use of fluoride cement does not improve the bone/cement interface. We unexpectedly observed that when fluoride-containing cement was used to fixate the stem, the degree of periprosthetic bone loss turned out to be higher than in stems cemented with Palacos with gentamicin (Digas et al. Citation2005). In the acetabulum, the use of Cemex with fluoride resulted in approximately the same amount of change as was found with use of a standard cement. The divergent findings in these 2 studies may reflect that the bone turnover, load and biological environment in the femur (with mainly cortical bone) and the acetabulum (with mainly trabecular bone) is different.
We found that compared to cemented wholepolyethylene sockets, uncemented press-fit cups lost more bone mineral in an area proximal to the socket, which is a common location for osteolytic lesion. It is probable that the early reduction of BMD proximal to our press-fit cups also occurs to the same extent with other press-fit designs, but this issue requires further investigation. If early loss of bone density is a general finding with these designs, it seems probable that stress shielding is one of several concurrent factors causing localized osteolysis around stiff metal-backed cups.
No competing interests declared.
Author contributions
GD did the measurements, statistical evaluation, and wrote the manuscript. JK designed the study, helped with the statistical evaluation and to write the article. JT designed the study with JK and operated many of the patients.
- Albrektsson T, Brånemark P I, Hansson H A, Lindström J. Osseointegrated titanium implants. Acta Orthop Scand 1981; 52: 155–70
- Archibeck M J, Jacobs J J, Roebuck K A, Glant T T. The basic science of periprosthetic osteolysis. Instr Course Lect 2001; 50: 185–95
- Baud C A, Very J M, Courvoisier B. Biophysical study of bone mineral in biopsies of osteoporotic patients before and after long-term treatment with fluoride. Bone 1988; 9: 361
- Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, Kainberger F, Gottsauner-Wolf F. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg (Br) 2004; 86: 20–6
- Bugbee W D, Culpepper W J, 2nd, Engh C A, Jr., Engh C A, Sr. Long-term clinical consequences of stress-shielding after total hip arthroplasty without cement. J Bone Joint Surg (Am) 1997; 79: 1007–12
- Callaghan J J, Templeton J E, Liu S S, Pedersen D R, Goetz D D, Sullivan P M, Johnston R C. Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg (Am) 2004; 86: 690–5
- Digas G, Thanner J, Nivbrant B, Rohrl S, Strom H, Kärrholm J. Increase in early polyethylene wear after sterilization with ethylene oxide: radiostereometric analyses of 201 total hips. Acta Orthop Scand 2003; 74: 531–41
- Digas G, Thanner J, Anderberg C, Kärrholm J. Bioactive cement or ceramic/porous coating vs. conventioanal cement to obtain early stability of the acetabular cup. Randomised study of 96 hips followed with radiostereometry. J Orthop Res 2004; 22: 1035–43
- Digas G, Thanner J, Anderberg C, Kärrholm J. Fluoride-containing acrylic bone cement in total hip arthroplasty. Randomized evaluation of 97 stems using radiostereometry and dual-energy X-ray absorptiometry. J Arthroplasty 2005; 20(6)784–92
- Huiskes R. Finite element analysis of acetabular reconstruction. Noncemented threaded cups. Acta Orthop Scand 1987; 58: 620–5
- Hultmark P, Höstner J, Herberts P, Kärrholm J. Radiographic evaluation of Charnley cups used in first-time revision: repeated observations for 7–15 years. J Arthroplasty 2003; 18: 1005–15
- Kröger H, Vanninen E, Overmyer M, Miettinen H, Rushton N, Suomalainen O. Periprosthetic bone loss and regional bone turnover in uncemented total hip arthroplasty: a prospective study using high resolution single photon emission tomography and dual-energy X-ray absorptiometry. J Bone Miner Res 1997; 12: 487–92
- Larsen M J, Thorsen A. A comparison of some effects of fluoride on apatite formation In vitro and in vivo. Calcif Tissue Int 1984; 36: 690–6
- Levenston M E, Beaupre G S, Schurman D J, Carter D R. Computer simulations of stress-related bone remodeling around noncemented acetabular components. J Arthroplasty 1993; 8: 595–605
- Li M G, Nilsson K G. Changes in bone mineral density at the proximal tibia after total knee arthroplasty: a 2-year follow-up of 28 knees using dual energy X-ray absorptiometry. J Orthop Res 2000; 18: 40–7
- Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978–1990. Acta Orthop Scand 1993; 64: 497–506
- Marchetti M E, Steinberg G G, Greene J M, Jenis L G, Baran D T. A prospective study of proximal femur bone mass following cemented and uncemented hip arthroplasty. J Bone Miner Res 1996; 11: 1033–9
- Mazess R, Collick B, Trempe J, Barden H, Hanson J. Performance evaluation of a dual-energy x-ray bone densitometer. Calcif Tissue Int 1989; 44: 228–32
- Morscher E, Berli B, Jockers W, Schenk R. Rationale of a flexible press fit cup in total hip replacement. 5-year followup in 280 procedures. Clin Orthop 1997, 341: 42–50
- Morscher E W, Widmer K H, Bereiter H, Elke R, Schenk R. Cementless socket fixation based on the “press-fit” concept in total hip joint arthroplasty. Acta Chir Orthop Traumatol Cech 2002; 69: 8–15
- Nivbrant B, Kärrholm J, Röhrl S, Hassander H, Wesslen B. Bone cement with reduced propotion of monomer in total hip arthroplasty. Acta Orthop Scand 2001; 72: 572–84
- Okano T, Hagino H, Otsuka T, Teshima R, Yamamoto K, Hirano Y, Nakamura K. Measurement of periprosthetic bone mineral density by dual-energy x-ray absorptiometry is useful for estimating fixation between the bone and the prosthesis in an early stage. J Arthroplasty 2002; 17: 49–55
- Posner A S, Eanes E D, Harper R A, Zipkin I. X-ray diffraction analysis of the effect of fluoride on human bone apatite. Arch Oral Biol 1963; 168: 549–70
- Rosenthall L, Bobyn J D, Brooks C E. Temporal changes of periprosthetic bone density in patients with a modular noncemented femoral prosthesis. J Arthroplasty 1999; 14: 71–6
- Schenk R K. Osseointegration of SULMESH-Coatings. Endoprosthetics, E Morscher. Springer, Heidelberg 1995; 60–71
- Soininvaara T A, Miettinen H J, Jurvelin J S, Suomalainen O T, Alhava E M, Kroger H P. Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta Orthop Scand 2004; 75: 600–5
- Thanner J, Kärrholm J, Herberts P, Malchau H. Hydroxyapatite and tricalcium phosphate-coated cups with and without screw fixation: a randomized study of 64 hips. J Arthroplasty 2000; 15: 405–12
- Venesmaa P K, Kröger H P, Jurvelin J S, Miettinen H J, Suomalainen O T, Alhava E M. Periprosthetic bone loss after cemented total hip arthroplasty: a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand 2003; 74: 31–6
- Vigorita V J, Suda M K. The microscopic morphology of fluoride-induced bone. Clin Orthop 1983, 177: 274–82
- Wilkinson J M, Peel N F, Elson R A, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 2001; 83: 283–8
- Wright J M, Pellicci P M, Salvati E A, Ghelman B, Roberts M M, Koh J L. Bone density adjacent to press-fit acetabular components. A prospective analysis with quantitative computed tomography. J Bone Joint Surg (Am) 2001; 83: 529–36