Abstract
Background Increasing resistance rates towards conventional antibiotics necessitate investigations of the efficacy of newly developed antibiotics. Thus, in a rat study, we compared the efficacy of moxifloxacin and vancomycin in the treatment of a local Staphylococcus aureus bone infection.
Method The femoral medullary cavities of 36 Wistar rats were contaminated with 100 μL of an oxacillin-sensitive Staphylococcus aureus strain (ATCC 29213) at 108cfu/mL. On the seventh day, antibiotic treatment with moxifloxacin (10 mg/kg twice daily i.p.) or vancomycin (15 mg/kg twice daily i.p.) was commenced in 12 animals each. 12 control animals were left untreated. After 21 days, the infected femurs were explanted and the bacterial counts (cfu/g) were determined.
Results In the control group, a median of 3.42 × 106cfu/g (LQ/UQ 1.09 × 106/ 1.55 × 107) was cultured, with a median of 2.53 × 106cfu/g (LQ/UQ 1.95 × 106/ 4.25 × 106) in the vancomycin group and a median of 2.49 × 105cfu/g (LQ/UQ 2.84 × 104/ 3.75 × 105) in the moxifloxacin group.The bacterial count was reduced by treatment with moxifloxacin both in comparison with the control group (p < 0.001), and in comparison with treatment with vancomycin (p < 0.001). There was no statistically significant difference between the vancomycin group and the control group (p = 0.53).
Interpretation In contrast to vancomycin, moxifloxacin proved to be an effective antibiotic for the treatment of bone infections due to Staphylococcus aureus in our animal model.
Treatment of bacterial osteomyelitis in adults is based on surgical debridement and long-term antibiotic treatment. As many of the antibiotics that penetrate the bone can only be given intravenously, the treatment is usually associated with hospital admission lasting for weeks. The socioeconomic burden is therefore high.
The patient would experience more comfortable treatment if antibiotic agents could be administered orally, but the latter must reach sufficiently high tissue concentrations and demonstrate adequate antibiotic efficacy against the typical pathogens of osteomyelitis such as coagulase-positive and coagulase-negative Staphylococci. In this respect, the development of modern quinolones appears promising. Moxifloxacin is a group 4 fluoroquinolone with high activity against a broad spectrum of gram-positive, gram-negative and anaerobic bacterial pathogens. At present, moxifloxacin is used mostly for the treatment of respiratory infections. To date, there have been no adequate experimental or clinical data published in the international literature regarding treatment of bone infections with moxifloxacin.
In microbiological and pharmacological studies, an improved activity against gram-positive pathogens—often the cause of bacterial osteomyelitis—has been confirmed for moxifloxacin compared with older quinolones (Dalhoff Citation1999, Jones et al. Citation1999, Hoogkamp-Korstanje et al. Citation2000, Kaatz et al. Citation2002, Ince et al. Citation2003).
We assessed the efficacy of moxifloxacin in bone infections in a controlled animal study and compared it with vancomycin, a bactericidal substance that is broadly effective against gram-positive bacteria but which has poor tissue penetration.
Animals and methods
Animals
We used 36 male Wistar rats (Charles River, Sulzfeld, Germany) aged 12–14 weeks at the start of the study and with an average body weight of 453 g (SD 29). The animal study was conducted after approval by the local and state animal protection committee (approval application no. 621-2531.1-12/03).
Bacterial strain
We used an oxacillin-sensitive Staphylococcus aureus strain (ATCC 29213) for bacterial contamination.
Experimental osteomyelitis
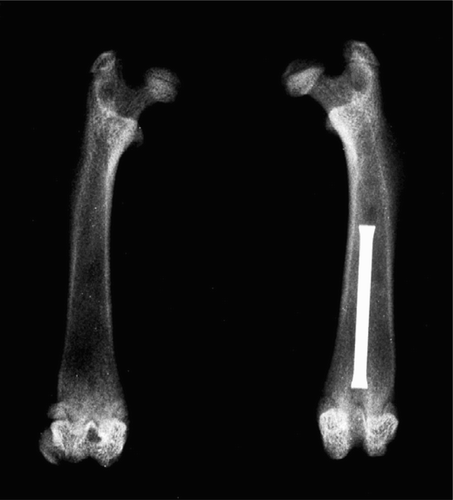
The animals were anesthetized with 90–120 mg/kg BW ketamine 10% (WDT, Garbsen, Germany) and 6–8 mg/kg BW xylazine (Bayer Vital, Leverkusen, Germany), and the knee of the left hind leg was opened through a parapatellar incision. After patellar dislocation and exposure of the femoral condyles, the femoral medullary cavity was opened and widened gradually with Kirschner wires up to a diameter of 1.6 mm retrogradely, as far as the proximal metaphysis. 100 μL of the bacterial suspension containing 108cfu/mL Staphylococcus aureus was then administered into the medullary cavity and a sterile hollow needle (16 G, 1.5 mm in length) was inserted retrogradely (). The intercondylar opening into the medullary cavity was closed with sterile bone wax, the joint was irrigated with 5 mL physiological saline and the wound was closed.
On the first 3 postoperative days, the animals each received 15 mg/kg ibuprofen p.o. The drinking and feeding behavior was monitored regularly and the animals were weighed once a week.
Antibiotic treatment
7 days after experimental contamination of the medullary cavity, 12 animals were randomized by lot to each of the two treatment groups (moxifloxacin and vancomycin) or to the untreated control group. The animals in the treatment groups received intraperitoneal injections of moxifloxacin (10 mg/kg BW) or vancomycin (15 mg/kg BW) twice daily at 12-h intervals from day 7 to day 21 postoperatively. The selected dosage was based on controlled investigations in animal models with measurement of the level of antibiotics after intraperitoneal administration (Blaser et al. Citation1995).
Microbiological analysis
On day 21, the animals were killed with an overdose of pentobarbital 2 h after the last antibiotic dose. The contaminated femurs were explanted under sterile conditions and underwent microbiological examination immediately.
The preparations were each numbered to allow blinded evaluation, frozen in liquid nitrogen, homogenized in a dismembranator (Braun, Melsungen, Germany), weighed on a precision balance (Sartorius Genius Series; Sartorius AG, Göttingen, Germany) and vortexed at 250 vibrations per min in 4 mL sterile 0.9% saline solution for 1 min (Vortex Genie 2; Bender & Hobein, Zürich, Switzerland). 50 μL of each suspension was plated on tryptic soy agar plates in dilution series using a semiautomatic spiral plater (Whitley Automatic Spiral Plater; DWS, Shipley, England). After 1 day of incubation at 36°C, we quantified the bacteria by colony counting with blinding as to treatment.
Statistics
The size of the study groups was determined on the basis of preliminary studies. A standard deviation of log 0.70 was assumed and a relevant difference between treatment groups was defined to be at least log 1.0. According to the pre-hoc power analysis (SigmaStat 2.03, SYSTAT Software Inc., Point Richmond, CA), 9 animals would have been enough for each group in order to achieve a statistical power of 0.8 (type-II error ß = 0.2) and a type-I error of α= 0.05. However, to allow for possible complications, 12 animals were chosen for each of the groups, including the third (control) group.
We used t-test for statistical analysis (SPSS 11.5, SPSS Inc., Chicago IL, USA). A p-value of less than 0.05 was considered to be statistically significant.
Results
No animal died during the period of the study; thus, 36 animals could be included in the analysis. No signs of systemic septic reaction were observed in any animal postoperatively. All animals lost weight during the first 8 postoperative days: mean 28 g (SD 14). All animals subsequently gained weight again and reached or exceeded the baseline weight. The weight pattern showed no significant difference between the animals in the control group and those in the treatment groups.
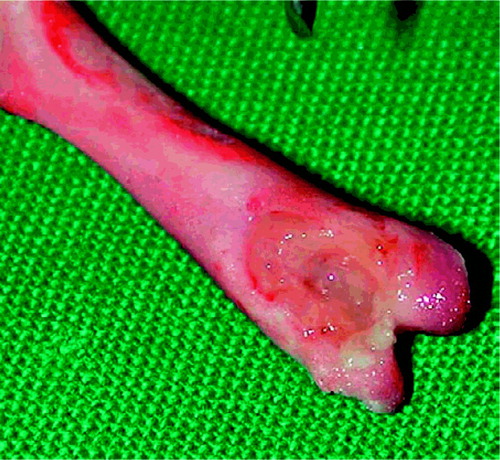
Bacteria could be cultured from all the bone preparations from the control group. Macroscopically, the bone destruction appeared to be much less marked in the animals of the moxifloxacin group than in the animals of the control and vancomycin groups (). On quantitative culture, a median of 3.42 ×106cfu/g Staphylococcus aureus (lower quartile (LQ)/upper quartile (UQ) 1.09 ×106/1.55 × 107) was cultured from the control animals, a median of 2.53 ×106cfu/g (LQ/UQ 1.95 × 106/4.2 × 106) was cultured from the animals of the vancomycin group and a median of 2.49 × 105cfu/g (LQ/UQ 2.84 × 104/3.75 × 105) was cultured from the animals of the moxifloxacin group.
The bacterial count in the bone was reduced by treatment with moxifloxacin, both compared to the control group (p < 0.001; 95% confidence interval for differences of means: log –2.33 to –0.66 cfu/g) and compared to the group treated with vancomycin (p < 0.001; 95% confidence interval: log –1.94 to –0.64 cfu/g). There was no statistically significant difference between the vancomycin group and the untreated control group (p = 0.53; 95% confidence interval: log –0.86 to +0.45 cfu/g).
Discussion
Bone infections due to Staphylococcus aureus are a considerable problem. The increasingly critical bacterial resistance situation demands development of new antibiotic substances and their experimental investigation in suitable models.
Numerous animal osteomyelitis models have been described for pharmacological, physiological and therapeutic investigations. The most important requirements of a reliable animal model are a local infection rate of 100% and low mortality. In order to ensure the development of local bone infection, a few authors have recommended in vitro contamination of the implants before the actual operation on the experimental animal (Garcia et al. Citation1998, Monzon et al. Citation2002), or iatrogenic deterioration of the bone prior to contamination and foreign body implantation (Mayberry-Carson et al. Citation1984, Kaarsemaker et al. Citation1997). However, such additional experimental steps diminish the applicability of an osteomyelitis model to the natural development of osteomyelitis.
In the experimental model employed here, both preoperative in vitro contamination of the foreign bodies and toxic injury of the bone were omitted. Thus, the in vivo formation of the biofilm and local tissue perfusion were not influenced in the experimental model. In order to ensure the development of local osteomyelitis, we increased the quantity of Staphylococcus aureus used for contamination (to 108cfu/mL) as compared to other experimental models (Melcher et al. Citation1994, Blaser et al. Citation1995, Arens et al. Citation1996, Monzon et al. Citation2002). Even so, none of the animals died of sepsis and the local infection rate in the operated femur was 100%. In view of the median bacterial count in the femoral bone (log 6.51 cfu/g) in control animals that did not receive antibiotic treatment, one can assume that this is a reproducible and reliable infection model that permits a meaningful investigation of the efficacy of antibiotic treatment in experimental animal osteomyelitis.
Moxifloxacin has an antibacterial spectrum of activity suitable for the treatment of bone infections. In numerous in-vitro and in-vivo studies, moxifloxacin was one of the most effective substances against gram-positive bacteria and in particular, it has been shown to be many times more effective against Staphylococci than older fluoroquinolones such as ciprofloxacin (Dalhoff Citation1999, Jones et al. Citation1999, Hoogkamp-Korstanje et al. Citation2000, Kaatz et al. Citation2002, Ince et al. Citation2003). On the basis of numerous studies, the risk of development of resistance during treatment with moxifloxacin appears to be much lower than during treatment with older quinolones (Pong et al. Citation1999, Lister Citation2001, Firsov et al. Citation2003).
The pharmacokinetics of moxifloxacin enables the antibiotic to be given once daily (400 mg): oral bioavailability of 91%, a terminal half-life of 12 h and post-antibiotic effect (Müller et al. Citation1999, Rodvold and Neuhauser Citation2001, Stass et al. Citation2001, Joukhadar et al. Citation2003). The favorable bioavailability, with similar tissue concentrations of moxifloxacin after intravenous or oral administration, allows the possibility of antibiotic treatment initially started intravenously being switched to oral administration. This would help to shorten hospitalization, reduce treatment costs and avoid secondary complications such as catheter infections (Cunha Citation2001).
In our rat model, no significant reduction of bacterial counts was achieved with vancomycin as monotherapy when compared with the control group. The selected dosage of vancomycin of 15 mg/kg BW twice daily was based on controlled investigations using the animal model, with measurement of the vancomycin level after intraperitoneal administration (Blaser et al. Citation1995). The poor results for vancomycin are all the more surprising since vancomycin is one of the few alternatives in bone infections due to oxacillin-resistant Staphylococcus aureus strains in routine clinical practice. However, the results of our study are supported by observations of other researchers. Saleh-Mghir et al. (Citation2002) found no significant reduction of bacterial counts in bone after vancomycin monotherapy (60 mg/kg BW i.m. once daily) in their study of rabbits with a contaminated Silastic prosthesis in the knee (Saleh-Mghir et al. Citation2002). Zimmerli et al. (Citation1998) described similar results with glycopeptide monotherapy after investigation of a tissue chamber contaminated with Staphylococcus.
Even though a significant reduction in bacterial counts could be achieved with moxifloxacin, our study shows that antibiotic monotherapy with moxifloxacin does not appear to be sufficient to eradicate the pathogens in local osteomyelitis with Staphylococcus aureus when foreign material is left in situ.
This study was funded by an unrestricted research grant from Bayer Vital GmbH. The experiments were done, and the manuscript written, without any influence of Bayer Vital GmbH.
Author contributions
TK, JB, KL, NL, JG designed the study. TK, JB, HS, NL gathered the data. TK, JB, NL, KL analyzed the data. TK, NL, KL wrote the manuscript. MH, NL did the statistical analysis. TK, JB, HS, MH, JG, NL, KL ensured the accuracy of the data and analyses.
- Arens S., Schlegel U., Printzen G., Ziegler W. J., Perren S. M., Hansis M. Influence of materials for fixation implants on local infection. J Bone Joint Surg (Br) 1996; 78: 647–51
- Blaser J., Vergères P., Widmer A. F., Zimmerli W. In vivo verification of in vitro model of antibiotic treatment of devicerelated infection. Antimicrob Agents Chemother 1995; 39(5)1134–9
- Cunha B. A. Intravenous to oral antibiotic switch therapy. Drugs Today (Barc.) 2001; 37(5)311–9
- Dalhoff A. Pharmacodynamics of fluoroquinolones. J Antimicrob Chemother 1999; 43: 51–9, (Suppl B)
- Firsov A. A., Vostrov S. N., Lubenko I. Y., Drlica K., Portnoy Y. A., Zinner S. H. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47(5)1604–13
- Garcia F., Lacleriga A., Monzon M., Leiva J., Oteiza C., Amorena B. Application of a rat osteomyelitis model to compare in vivo and in vitro antibiotic efficacy against bacteria with high capacity to form biofilms. J Surg Res 1998; 79: 146–53
- Hoogkamp-Korstanje J. A., Roelofs-Willemse J. Comparative in vitro activity of moxifloxacin against Gram-positive clinical isolates. J Antimicrob Chemother 2000; 45(1)31–9
- Ince D., Zhang X., Hooper D. C. Activity of and resistance to moxifloxacin in Staphylococcus aureus. Antimicrob Agents Chemother 2003; 47(4)1410–5
- Jones M. E., Visser M. R., Klootwijk M., Heisig P., Verhoef J., Schmitz F. J. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob Agents Chemother 1999; 43(2)421–3
- Joukhadar C., Stass H., Muller-Zellenberg U., Lackner E., Kovar F., Minar E., Muller M. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob Agents Chemother 2003; 47(10)3099–103
- Kaarsemaker S., Walenkamp G., Vanderbogaard A. E. J. New model for chronic osteomyelitis with staphylococcus aureus in sheep. Clin Orthop 1997, 339: 246–52
- Kaatz G. W., Moudgal V. V., Seo S. M. Identification and characterization of a novel efflux-related multidrug resistance phenotype in Staphylococcus aureus. J Antimicrob Chemother 2002; 50(6)833–8
- Lister P. D. Pharmacodynamics of moxifloxacin and levofloxacin against Staphylococcus aureus and Staphylococcus epidermidis in an in vitro pharmacodynamic model. Clin Infect Dis 2001; 32: S33–S38, (Suppl 1)
- Mayberry-Carson K. J., Tober-Meyer B., Smith J. K., Lambe D. W., Costerton J. W. Bacterial adherence and glycocalix formation in osteomyelitis experimentally induced with Staphylococcus aureus. Infect Immun 1984; 43: 825–33
- Melcher G. A., Claudi B., Schlegel U., Perren S. M., Printzen G., Munzinger J. Influence of type of medullary nail on the development of local infection. J Bone Joint Surg (Br) 1994; 76: 955–9
- Monzon M., Garcia-Alvarez F., Lacleriga A., Amorena B. Evaluation of four experimental osteomyelitis infection models by using precolonized implants and bacterial suspensions. Acta Orthop Scand 2002; 73(1)11–9
- Müller M., Stass H., Brunner M., Moller J. G., Lackner E., Eichler H. G. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother 1999; 43(10)2345–9
- Pong A., Thomson K. S., Moland E. S., Chartrand S. A., Sanders C. C. Activity of moxifloxacin against pathogens with decreased susceptibility to ciprofloxacin. J Antimicrob Chemother 1999; 44(5)621–7
- Rodvold K. A., Neuhauser M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy 2001; 21: 233S–52S
- Saleh-Mghir A., Ameur N., Muller-Serieys, Ismael F., Lemaitre F., Massias L., Feger C., Bléton R., Crémieux A. C. Combination of Quinupristin-Dalfopristin (Synercid) and Rifampin is highly synergistic in experimental Staphylococcus aureus joint prosthesis infection. Antimicrob Agents Chemother 2002; 46(4)1122–4
- Stass H., Kubitza D., Schuhly U. Pharmacokinetics, safety and tolerability of moxifloxacin, a novel 8-methoxy-fluoroquinolone, after repeated oral administration. Clin Pharmacokinet 2001; 40: 1–9, (Suppl 1)
- Zimmerli W., Widmer A. F., Blatter M., Frei R., Ochsner P. E. Role of rifampin for treatment of orthopaedic implantrelated Staphylococcus aureus: a randomized controlled trial. JAMA 1998; 279: 1537–41