Abstract
Background Postoperative pain after total knee arthroplasty (TKA) can be difficult to manage and may delay recovery. Recent studies have suggested that periarticular infiltration with local anesthetics may improve outcome.
Methods 80 patients undergoing TKA under spinal anesthesia were randomized to receive continuous femoral nerve block (group F) or peri- and intraarticular infiltration and injection (group I). Group I received a solution of 300 mg ropivacaine, 30 mg ketorolac, and 0.5 mg epinephrine by infiltration of the knee at the end of surgery, and 2 postoperative injections of these substances through an intraarticular catheter.
Results More patients in group I than in group F could walk < 3 m on the first postoperative day (29/39 vs. 7/37, p < 0.001). Group I also had significantly lower pain scores during activity and lower consumption of opioids on the first postoperative day. No differences between groups were seen regarding side effects or length of stay.
Interpretation Peri- and intraarticular application of analgesics by infiltration and bolus injections can improve early analgesia and mobilization for patients undergoing TKA. Further studies of optimal drugs, dosage, and duration of this treatment are warranted.
Postoperative pain management after total knee arthroplasty (TKA) is often multimodal and includes intravenous (i.v.) opioids, epidural analgesia, or peripheral nerve blocks in combination with oral analgesics and cryotherapy. These treatments are associated with side effects such as nausea, sedation, hypotension, urinary retention, and partial motor block. Alternative methods of analgesia are therefore of interest.
Recent studies on application of local anesthetics into the knee at the end of TKA have shown that this approach has several advantages compared to other regional or purely systemic approaches. In a blind study (Bianconi et al. Citation2003), 37 patients who underwent total hip or knee arthroplasty were randomized to receive either an intraoperative infiltration with ropivacaine followed by infusion for 55 hours, or saline. Intensity of postoperative pain, consumption of rescue analgesics, and length of hospital stay were significantly reduced in the group that received ropivacaine. Open studies have shown similar results (Rasmussen et al. Citation2004, Isaac et al. Citation2005).
Prompted by the positive results, we decided to carry out a randomized study to compare the relative benefits of peri- and intraarticular analgesic treatment with femoral nerve block as a reference, which is the standard treatment for postoperative pain after TKA at our institution. As intraarticular infusion implies a risk of losing substantial amounts of local analgesic to the drain (Nechleba et al. Citation2005), we decided to evaluate another approach in which extensive periarticular infiltration was performed. In order to prolong the analgesia beyond the period provided by the infiltration, subsequent bolus injections were given via an intraarticular catheter while clamping the drain.
Patients and methods
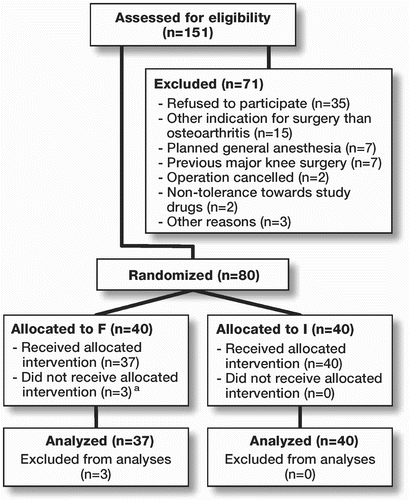
80 patients scheduled for primary TKA at the Department of Orthopedic Surgery, Aarhus Hospital, were enrolled in this prospective randomized trial after written informed consent was obtained. The enrollment period began in April 2005 and lasted until April 2006. Inclusion criteria were TKA on the basis of osteoarthritis and planned spinal anesthesia. Primary exclusion criteria were lack of mental ability to provide informed consent, neuropathic pain or sensory disorders in the leg to be operated, previous major bone surgery in the knee joint, and intolerance to the study drugs. Secondary exclusion criteria were failure of spinal anesthesia and re-operation or trauma to the knee within the study period. The trial was approved by the local ethics committee (no: 20050003, 2005) and was conducted in accordance with Helsinki Declaration II.
Patients were randomized immediately prior to the operation (by the use of sequentially numbered, opaque, sealed envelopes) into 2 treatment groups. Group F received a femoral nerve block followed by infusion of local anesthetic, and group I received intraoperative infiltration of local anesthetic followed by bolus injections. All operations were performed under spinal anesthesia (at level L2–3/L3–4 with 3 mL hyperbaric bupivacaine, 5 mg/mL) by 1 of 3 surgeons using tourniquet control, a medial parapatellar approach, and patellar resurfacing. Patients received 2 g dicloxacillin i.v. before surgery, and 10 mg/kg tranexamic acid at release of the tourniquet and 3 hours later. Antithrombotic therapy (fondaparinux) was started after surgery and given for 5 days. Patients were not given urinary catheter a demeure, but were offered intermittent catheterization as needed. Drains were removed on the day after surgery. Continuous passive motion (CPM) was started in the recovery room and continued until 8 a.m. on the second postoperative day, as an adjunct to conventional physiotherapy.
Regional techniques
Group F (femoral nerve catheter) received a femoral nerve block prior to spinal anesthesia. Following well-known landmarks (Winnie et al. Citation1973), an insulated Tuohy 18-gauge needle attached to a peripheral nerve stimulator was inserted in proximity to the femoral nerve at the level of the inguinal ligament. While advancing/re-directioning the needle, an initial current of 2 mA was gradually reduced to achieve twitches of the quadriceps muscle at 0.4 mA. At this point, a bolus of 20 mL ropivacaine (10 mg/mL) was injected through the needle (after negative aspiration test for blood) and subsequently a 20-gauge catheter was introduced 4–8 cm into the femoral sheath. In the recovery room, the femoral nerve catheter was connected to an infusion pump, and 10 mL/h ropivacaine (2 mg/mL) was infused for 48 h. If needed, a bolus of 20 mL could be given through the pump once every 8 h. 4 mg morphine (0.4 mg/mL) and 50 mg bupivacaine (5 mg/mL) was given intraarticularly through the drain after skin closure.
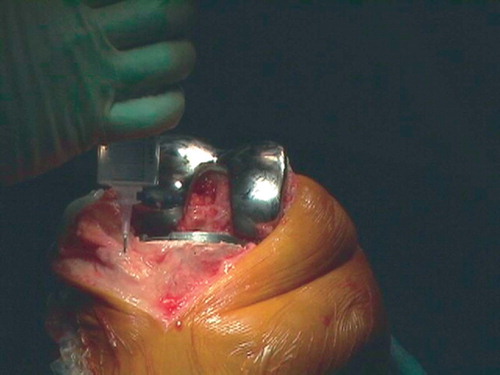
Group I (intraarticular catheter) received extensive infiltration of the surgical site with a solution of 150 mL ropivacaine (2 mg/mL), 1 mL ketorolac (30 mg/mL), and 1 mL epinephrine (0.5 mg/mL). The solution was given in 3 × 50 mL syringes. The first 100 mL was given after cementing of the modular prosthesis, before installing the polyethylene part: 50 mL into the posterior part of the capsule and in the intercondylar area and 50 mL into the anterior part of the capsule, the collateral ligaments, and along the femur and tibia (). A drain was positioned laterally. An intraarticular 20-gauge (epidural) catheter was introduced separately from the drain, proximal-lateral to the incision under the lateral collateral ligament and towards the posterior part of the capsule. After closure of the capsule, the remaining 50 mL was infiltrated into the subcutaneous tissue. At 10 p.m. on the day of surgery, a solution of 20 mL ropivacaine (10 mg/mL), 1 mL ketorolac (30 mg/mL), and 1 mL epinephrine (0.5 mg/mL) was injected into the intraarticular catheter. A second dose was given the following morning, 30 min before physiotherapy or at 10 a.m. at the latest. The catheter was removed immediately after the second bolus.
To supplement both analgesic regimens, all patients received paracetamol (1 g × 4), ibuprofen (400 mg × 3), and controlled-release oxycodone (20 mg × 2) daily. Ibuprofen was not given to patients aged over 75 years, or to patients who had a history of gastric ulcer or dyspepsia, heart-, liver- or kidney insufficiency, or allergy to non-steroid anti-inflammatory drugs (NSAIDs). Immediaterelease oxycodone (5–10 mg) was used for pain treatment if pain at rest exceeded 3 on a numeric rating scale (NRS; 0–10 scale where 0 = no pain and 10 = worst possible pain). Intravenous morphine (0.05–0.1 mg/kg) was used for the treatment of severe pain (< 7 on the NRS). Patients who were on a stable dose of another opioid prior to the operation (slow-release morphine, transdermal buprenorphine, or weak opioids) were allowed to continue the treatment postoperatively. In these cases, and in cases where patients switched drug during follow-up, consumption of opioids was converted to oxycodone equivalents. Ondansetron (the first choice) and metoclopramide were used for the treatment of nausea and vomiting. All patients received laxatives.
Outcome measures and pain assessment
Both techniques of pain control were active from the conclusion of surgery and for most of the first postoperative day. Based on this, we chose the primary outcome measures to be quality of analgesia and mobilization in the postoperative period until the end of the first postoperative day (POD 1). Quality of analgesia was assessed by consumption of opioid and with NRS for pain. Mobilization was assessed from walking distance, quadriceps function, and range of motion. The secondary outcome measure was the number of days until discharge. Adverse effects and complications were recorded.
Pain was assessed 4 times by the staff of the recovery room, using NRS (at arrival, after 1 and 2 h, and at discharge). Patients themselves recorded pain scores at rest or during CPM at 3 p.m. and 10
p.m. on the day of surgery, and at 8 a.m., 3 p.m. and 10 p.m. on PODs 1 to 3. Patients also recorded nausea, vomiting, dizziness, pruritus (on a daily basis) and constipation (on the evening of day 3). On POD 1 and POD 2, physiotherapists recorded distance walked with aid, ability to hold quadriceps tension or to lift the leg, range of motion, and highest pain score during physiotherapy. The patients' medical files and nurse observational charts were studied for possible complications until discharge.
Statistics
Calculation of sample size (n) was based on an expected difference of 10 mg oral rescue opioid, assuming a standard deviation of 15 mg in each group, an α risk of 0.05, and a β risk of 0.2. This indicated that a minimum of 36 patients should be included in each study group. To allow for incomplete data collection, 80 patients were included in the study.
Dosages of opioids were converted to oxycodone equivalents (Twycross Citation1999) and dosages of NSAIDs were converted to ibuprofen equivalents by defined daily dose (WHO International Working Group for Drug Statistics Methodology 2006). Pain was considered substantial if the pain score was ≥ 3 on the NRS; thus, the number of reported pain scores of ≥ 3 was used as a basis for comparison of analgesic effect.
Data were analyzed using the Wilcoxon/Mann-Whitney rank sum test for unpaired data (presented as medians with interquartile ranges) or Student's t-test (presented as means with standard deviations) when appropriate. Tests were performed on the original answer categories, not on the compressed data presented in the tables. A p-value of < 0.05 was considered statistically significant. Stata for Windows version 8.0 (StataCorp LP, College Station, TX) was used for statistical analysis.
Results
Patients
3 patients were excluded after randomization to the F group, due to conversion of failed spinal anesthesia to general anesthesia. 1 patient in group F discontinued the intervention that was allocated due to a malfunctioning femoral catheter. 3 patients in group I discontinued the allocated intervention: the first patient had a leakage from the drain site, and thus did not receive the full bolus injection on the evening of operation. The catheter of the second patient had accidentally slipped into the subcutis and the bolus injection on the morning after operation was not given. The third patient had the catheter accidentally cut by a nursing student during changing of the dressings and did not receive the bolus injection on the morning after operation (). The above 4 patients who discontinued their allocated interventions were included in the data analysis. The patients were similar in regard to characteristics except for NSAID consumption, as presented in .
Table 1. Patient characteristics
Pain levels
On POD 1, the patients in group I reported significantly lower pain scores during physiotherapy than patients in group F, but this difference was not apparent on POD 2. On the day of surgery and POD 1, when both techniques were in use, there was no significant difference in reported pain scores at rest ().
Table 2. Pain levels, consumption of opioids, and mobilization
Consumption of opioids
17 patients received opioids other than oxycodone and morphine (group I: n = 9; group F: n = 8). All opioids were converted to the equivalent dose of oxycodone. Patients in group I had a significantly lower consumption of opioid from the time of surgery to the end of POD 1. For the whole observation period of 4 days, however, no difference was found.
Mobilization and length of stay
Walking distance and quadriceps function were significantly improved in group I on POD 1 and 2. Similarly, an extended range of motion was noted in group I on POD 1. Length of stay in the recovery room was similar in the 2 groups (group F: 3.5 h; group I: 3.0 h; p = 0.05), although a tendency for a shorter stay was seen with patients in group I. The median length of stay in the hospital was 6 days for group F and 5 days for group I (p = 0.3).
Adverse effects
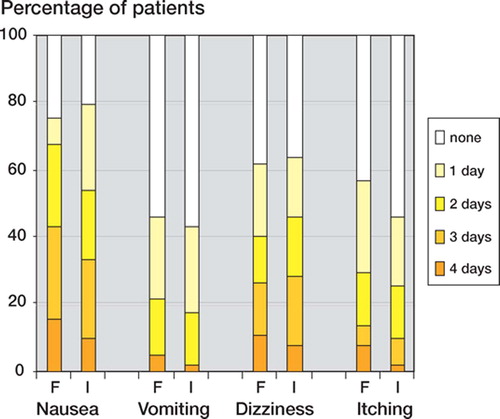
There was no significant difference in the number of days the patients in each group experienced nausea, vomiting, dizziness, or itching (p < 0.20 for each adverse effect) (). 26 patients in group F and 24 patients in group I experienced constipation (p = 0.2). Consumption of ondansetron in the study period was similar in the 2 groups (median 9 mg in both groups; p = 0.3).
Adverse events
During the study period 1 patient in group I developed an early deep infection of the knee and was treated with debridement, lavage, change of polyethylene component, and antibiotics. 1 patient in group I experienced chest pain 1 h after the first bolus. Nitroglycerin had no effect, and there were no biochemical signs of myocardial infarction. The next bolus the following morning was given uneventfully. Another patient in group I experienced a brief period of unconsciousness and tachycardia. ECG revealed bundle branch block and (previously known) atrial flutter. Coronary angiography showed normal coronary arteries and there were no biochemical signs of myocardial infarction. This patient was found to have a very low hemoglobin level. The incident occurred more than 24 h after the last bolus. 2 patients in group I developed bullae around their wounds; 1 of these patients developed necrosis of the wound, and a gastrocnemius flap and skin graft operation had to be performed. 2 cases of pneumonia (1 in each treatment group), 2 cases of gastric ulcer (both in group I), and 2 urinary tract infections (both in group I) were also observed.
Discussion
Our findings are consistent with the results of 2 recent studies in which patients were randomized to receive either peri- and intraarticular treatment and patient-controlled analgesia (PCA) with morphine, or PCA alone (Busch et al. Citation2006, Vendittoli et al. Citation2006). Both studies assessed infiltration with ropivacaine, ketorolac, and epinephrine, though Busch et al. added epimorphine to the solution and Vendittoli et al. added a postoperative bolus through an intraarticular catheter. The study by Busch et al. included 64 patients who were blinded (as was the postoperative team), and the study by Vendittoli et al. included 42 patients.
The positive results of these 2 studies are not surprising, as the analgesic treatment in the control groups mainly consisted of parenteral morphine. We chose to compare the peri- and intraarticular treatment with another regional technique. Purely systemic opioid has been replaced with epidural analgesia, which in turn has been replaced with femoral nerve block as the standard treatment after TKA at our institution. Epidural analgesia is of proven benefit, but is associated with potential problems such as motor block, urinary retention and epidural bleeding (with anticoagulation therapy), and necessitates intensive observation of patients. However, femoral nerve block also has disadvantages: the posterior part of the knee is innervated by the sciatic nerve and is therefore not anesthetized by a femoral block. This regularly causes popliteal pain and a need for supplementary treatment with systemic opioids. Furthermore, a partial motor block of the quadriceps femoris muscle is common and precludes early mobilization. If a sciatic nerve block is added to treat the popliteal pain, the partial motor block may inhibit mobilization even further (Morin et al. Citation2005).
Our study has some limitations. Firstly, blinding of patients and caregivers was not attempted. It was considered impossible to do properly, as the partial motor block present in patients in group F would be obvious to both patients and staff. The presence of 2 invasive catheters instead of 1 could also increase discomfort and risk of infection, although the risk of infection was estimated to be very small. The fact that the study was not done blind may especially have affected the consumption of supplementary opioid, as the opioid was supplied by nurses when asked for or when deemed necessary, thus allowing bias from the nursing staff. Data collection and analysis was carried out by KT, who was not blinded since catheter placement was obvious. Many other studies of analgesic treatments are not done blind due to the nature of the treatment. Secondly, it may be argued that the use of controlled-release oxycodone in both treatment groups may have reduced pain too much and “washed out” any differences between the 2 treatments. This was not the case, however, as pain scores often exceeded 3 on the NRS and supplementary opioids were needed. Thirdly, despite randomization, 18 of the 28 patients who did not receive oral NSAID due to contraindications were in group I. If anything, a more even distribution of the consumption of NSAIDs between the 2 groups would probably have accentuated the positive results found in group I. Finally, 8 patients (2 in group F and 6 in group I) received NSAIDs other than ibuprofen, the dosages of which were converted to ibuprofen equivalents. This calculation may be associated with some error.
The 3 active substances of the infiltration mixture were ropivacaine, ketorolac, and epinephrine. Ropivacaine is pharmacologically similar to bupivacaine but is associated with less cardiac and central nervous system toxicity, which allows patients to tolerate a larger dose (Mather et al. Citation2005). The addition of epinephrine helps to reduce the toxicity of the local anesthetic by reducing the rate the drug is released into the circulation. The maximum tolerated doses of local anesthetics with epinephrine administered intraarticularly or by infiltration are not properly established (Rosenberg et al. Citation2004). Our patients received an initial dose of 300 mg ropivacaine followed by 2 bolus injections of 200 mg each. No patients reported tinnitus, tingling, perioral numbness, or other toxic symptoms of local anesthetics. No toxic blood levels of ropivacaine were measured in the study by Busch et al. (using 400 mg ropivacaine for infiltration), or in the study by Vendittoli et al. (using 400 mg ropivacaine for infiltration and 150 mg for injection on the following day). Several studies have shown that NSAIDs have a clinically relevant peripheral action, and that infiltration or injection provides better pain relief than i.v. administration (Romsing et al. Citation2000). We therefore added ketorolac to the peri- and intraarticular solution. It is also established that peripheral opioid receptors are induced by inflammation (Stein et al. Citation2003), and there may be some advantage to adding morphine to the peri- and intraarticular solution as practiced by Busch et al. Although helpful in delaying release of ropivacaine to the circulation, it is possible that epinephrine caused the wound necrosis of our patient who needed a skin graft operation. Due to this concern, the subcutaneous infiltration could be performed without epinephrine. Further studies could uncover the optimal dosage and composition of the peri- and intraarticular solution.
The size of our study does not allow conclusions to be drawn regarding the risk of deep knee infection. In another study, the intraarticular catheter was in place for 72 h, and no increased risk of infection could be demonstrated (Rasmussen et al. Citation2004). To our knowledge, our study is the first to examine the combination of periarticular infiltration and 2 postoperative intraarticular bolus injections. Originally, our plan was to administer a single subsequent bolus on the morning following the operation, but an earlier bolus was introduced because a preliminary pilot patient experienced a need for supplementary analgesics at around 3–4
a.m. Patients may benefit from additional bolus injections administered later than the morning of the first postoperative day, which could be examined in further studies.
In conclusion, infiltration with multimodal drugs followed by postoperative intraarticular bolus injections seems to provide a good quality of analgesia after TKA, without increased risks. Further studies are needed to clarify the composition and dose of the analgesic solution. Also, it is not known whether patients might benefit from subsequent bolus injections beyond the morning of the first postoperative day.
No conflicts of interests declared.
Contributions of authors
All authors made significant contributions to the planning or data collection/interpretation, writing or revision of the manuscript, and approval of the final manuscript. Original idea: ET, KS. Planning and adjustments: KT, LN, FM, VH. Data collection and interpretation: KT, LN, KS. Writing of the manuscript: KT, LN, VH. Revision of manuscript: KT, LN, VH, FM, ET, KS. Approval of final manuscript: KT, LN, VH, FM, ET, KS.
The authors wish to thank Anders Odgaard for his constructive comments on the manuscript. We also wish to thank the nursing staff and physiotherapists involved for the care of study patients and data collection. The study was funded by the Danish Medical Research Council. Equipment and drugs were provided by Aarhus University Hospital.
- Bianconi M, Ferraro L, Traina G C, Zanoli G, Antonelli T, Guberti A, Ricci R, Massari L. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 2003; 91(6)830–5
- Busch C A, Shore B J, Bhandari R, Ganapathy S, MacDonald S J, Bourne R B, Rorabeck C H, McCalden R W. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg (Am) 2006; 88(5)959–63
- Isaac D, Falode T, Liu P, I'Anson H, Dillow K, Gill P. Accelerated rehabilitation after total knee replacement. Knee 2005; 12(5)346–50
- Mather L E, Copeland S E, Ladd L A. Acute toxicity of local anesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med 2005; 30(6)553–66
- Morin A M, Kratz C D, Eberhart L H, Dinges G, Heider E, Schwarz N, Eisenhardt G, Geldner G, Wulf H. Postoperative analgesia and functional recovery after totalknee replacement: comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Reg Anesth Pain Med 2005; 30(5)434–45
- Nechleba J, Rogers V, Cortina G, Cooney T. Continuous intra-articular infusion of bupivacaine for postoperative pain following total knee arthroplasty. J Knee Surg 2005; 18(3)197–202
- Rasmussen S, Kramhoft M U, Sperling K P, Pedersen J H. Increased flexion and reduced hospital stay with continuous intraarticular morphine and ropivacaine after primary total knee replacement: open intervention study of efficacy and safety in 154 patients. Acta Orthop Scand 2004; 75(5)606–9
- Romsing J, Moiniche S, Ostergaard D, Dahl J B. Local infiltration with NSAIDs for postoperative analgesia: evidence for a peripheral analgesic action. Acta Anaesthesiol Scand 2000; 44(6)672–83
- Rosenberg P H, Veering B T, Urmey W F. Maximum recommended doses of local anesthetics: a multifactorial concept. Reg Anesth Pain Med 2004; 29(6)564–75
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med 2003; 9(8)1003–8
- Twycross R G. In: Wall and Melzack's textbook of pa. 4th ed, B McMahon Stephen, Koltzenburg Martin. Churchill Livingstone, Philadelphia 1999; 1198–208
- Vendittoli P A, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M C, Varin F. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg (Am) 2006; 88(2)282–9
- WHO International Working Group for Drug Statistics Methodology WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health 2006 March 13 Available from: URL: http://www.whocc.no/atcddd/
- Winnie A P, Ramamurthy S, Durrani Z. The inguinal paravascular technic of lumbar plexus anesthesia: the ”3-in-1 block”. Anesth Analg 1973; 52(6)989–96