Abstract
Introduction For endoprosthetic knee surgery, intensive postoperative pain therapy is necessary. We therefore evaluated whether the combination of continuous psoas compartment and sciatic analgesia (PSC) is as effective as epidural analgesia (EPI) and whether it provides better analgesia than patient-controlled intravenous analgesia with piritramide (PCA).
Methods We studied 63 patients who underwent total knee arthroplasty (TKA). The PSC group received a combination of continuous psoas and sciatic nerve block, the EPI group an epidural analgesia, and the PCA group an intravenous patient-controlled piritramide pump. Pain scores, satisfaction, flexion and side effects were recorded.
Results Pain scores (0–10) were higher in the PCA group (on movement, day 1/day 2: 7.0/6.5) than in the EPI group (5.0/5.0) and the PSC group (4.0/3.5). Postoperative opioid consumption over 48 h was higher in the PCA group (51 mg) than in the EPI group (0 mg) and the PSC group (0 mg). There were no differences in functional recovery. Pruritus occurred more frequently in the PCA and EPI groups than in the PSC group. Patients receiving a PSC and EPI were more satisfied than those treated with PCA.
Interpretation Analgesia with PSC catheters or EPI catheter is superior to PCA regarding pain levels, analgesic requirements, and patient satisfaction. There was no difference in functional outcome between the 3 groups.
Postoperative pain is still a major concern: most patients have moderate, severe, or extreme pain (Apfelbaum et al. Citation2003). Pain after knee arthroplasty is known to be intense (Mahoney et al. Citation1990). It impais early intensive physiotherapy and rehabilitation. In clinical routine, intravenous PCA with opioids or epidural analgesia is widely used. Sensory transmission of pain after elective knee arthroplasty occurs via the lumbar (femoral and obturator nerve) and sacral plexus (sciatic nerve), and continuous peripheral nerve blocks have been reported to be effective (Singelyn et al. Citation1998, Capdevila et al. Citation1999). However, femoral nerve blocks do not reliably cover the obturator nerve (Marhofer et al. Citation2000, Bouaziz et al. Citation2002) and addition of obturator nerve blocks improves postoperative analgesia (McNamee et al. Citation2002, Macalou et al. Citation2004). The lumbar plexus block, using the posterior approach, should reliably block both the femoral and obturator nerve (Parkinson et al. Citation1989). However, Kaloul et al. (Citation2004a) found that analgesia after total knee arthroplasty with psoas block is equivalent to femoral block in terms of pain levels and opioid consumption. This unexpected result may be due to the lack of sciatic blocks, which have been reported to significantly influence early postoperative pain levels (Pham Dang et al. Citation2005, Morin et al. Citation2005).
Whether or not the theoretically optimal continuous plexus blocks (psoas plus sciatic) provide the best analgesia has not been tested yet. We therefore compared the efficacy of continuous psoas and sciatic block (PSC), epidural analgesia (EPI), and patient-controlled intravenous analgesia with opioids (PCA) after knee arthroplasty.
Patients and methods
Patient selection and study design
This prospective study was approved by the local ethical review board (Berlin), and all patients gave their written informed consent.
Patients admitted for knee arthroplasty were included in the study. Patients were excluded if one or more of the following conditions was present: infection near the possible catheter insertion sites, coagulation disorders, preexisting neurological disorders, known allergies to local anesthetics, ASA classification IV or V, age younger than 18 years, inability to understand the PCA device, pregnancy or lactation period, severe liver or kidney insufficiency, and psychiatric disorders.
Informed consent was obtained from 77 patients over a period of 13 months (2004–2005) and patients were allocated to 1 of 3 study groups (PSC, EPI, or PCA) by patient preference. Of these individuals, 10 patients were excluded because surgery was postponed, 1 patient from the PSC group refused to take part in this study just before premedication was given, 2 patients in the PSC group had to be excluded because of impossible placement of the psoas catheter. 1 patient in the EPI group had to be excluded because the epidural catheter could not be placed. Finally, 63 patients were included for analysis ().
Patients were premedicated with midazolame at 0.1 mg/kg. All nerve and plexus blocks were performed using electrical nerve stimulation with a 17-G tuohy needle (Arrow StimuCath Set) and a nerve stimulator (Stimuplex HNS 11; Braun, Helsungen, Germany). Optimal stimulation was defined as recognizable twitches at 0.3–0.5 mA with an impulse width of 0.1 ms. In the PSC group, the lumbar plexus was identified with the patient in the lateral position and the operative side up, as described by Capdevila et al. (Citation2002).
Table 1. Demographic dataa
A stimulating catheter was advanced 3 cm beyond the tip of the needle by using continuous stimulation to verify correct placement of the catheter without any fluid injection. 25 mL of 0.75% ropivacaine was injected after repeated aspiration.
The sciatic catheter was inserted using the subgluteal approach, as described by di Benedetto et al. (Citation2002), with the patient remaining in the lateral position. Stimulating catheter advancement was performed as described for the lumbar plexus catheter. Sciatic block was performed with 25 mL 1% prilocaine to enable early postoperative testing of peroneal motor function (Levesque and Delbos Citation2005, Nercessian et al. Citation2005).
The EPI group received a combined spinal/epi-dural anesthesia at the L2-3 or L3-4 interspace (17G tuohy needle, Becton Dickinson, Dura safe plus Set). Spinal anesthesia was performed with 0.5% bupivacaine at 0.2 mg/kg.
Perioperative management
Induction time (placing of i.v. line until ready for surgery) was measured. After placement of the catheters and after completion of the sensory block, standardized general anesthesia was performed if necessary (PCA) or if preferred by the patient (PSC and EPI).
2–3 μg/kg of intravenous fentanyl was given for induction and intravenous propofol at 2–3 mg/kg was given until loss of consciousness. Propofol was given for maintenance, according to clinical needs. Laryngeal masks without relaxation were used in order to ensure ventilation. All patients received 2 g of metamizole intravenously before the end of surgery.
Postoperative assessment
The PSC group received a continuous infusion of 0.2% ropivacaine at a rate of 8 mL/h in each catheter after regression of sciatic motor block and checking of peroneal motor function. The EPI group received an infusion of 0.1% ropivacaine plus 0.5 μg/mL sufentanil at a rate of 8 mL/h, when a sensory block regression of 2 segments was completed. Catheter rates were adjusted twice daily by the acute pain service to maintain sensory block covering the surgical site, and to avoid motor blocks (in the range of 4–10 mL/h for each catheter in the PSC group and 5–12 mL/h in the EPI group). Intravenous PCA was provided with piritramide (1.5 mg bolus with a lockout interval of 5 min).
Local anesthetic infusions and opioid i.v. PCA were maintained for 48 h postoperatively and removed thereafter. Rescue analgesia was provided (if NAS < 3 during rest and NAS < 5 during motion) with 1 g metamizole, and if still no adequate analgesia was obtained, with 3 mg i.v. piritramide. On the evening of the second postoperative day, tramadol was given: in the case of i.v. PCA, in the evening of the first postoperative day to bridge discontinuation of catheter or PCA analgesia. A urinary catheter was given to all female patients in the EPI group, or on request (e.g. due to incontinence).
From the day after surgery until discharge, the patients performed active and passive knee and hip flexion and extension exercises against gravity twice daily. Getting up from the bed was encouraged as soon as possible, followed by ambulation with a walker. The degree of knee flexion tolerated by patients was recorded by the physiotherapist on a daily basis.
The patients were seen at least twice daily by two specially trained observers (KP and AW) who assessed analgesic consumption (piritramide, ropivacaine, metamizole), pain scores at rest and during physiotherapy using NRS scoring (NRS = 0 for no pain; NRS = 10 for worst imaginable pain), patient satisfaction (using a 6-point scale: 1 = best satisfaction, 6 = worst) and side effects such as pruritus and hypotonia (reduction of systolic blood pressure of more than 20%). The patient’s lower extremity muscle strength and sensation were assessed twice daily (using Janda scores).
Thromboembolic prophylaxis was given with low molecular weight heparin (40 mg enoxaparine) once a day, starting on the evening before surgery. Thromboembolic events were recorded if diagnosed.
After 6–9 months, a standardized telephone interview was carried out with all patients to determine their general state of health, knee pain, and consumption of pain medication. Three attempts were made to get in contact with them.
Statistics
Available data suggested that a detectable difference in pain levels of 2 (on a pain scale ranging from 0–10) with reported standard deviation of 2 and a power of 0.9 would require 22 patients per group, accepting a significance level of less than
0.05 (Singelyn et al. Citation1998, Capdevila et al. Citation1999). Allowing for dropouts, we decided to include 25 patients per group. For statistical analysis we used SPSS for Windows version 11.5. Data are expressed as median and interquartile range (IQR), if not stated otherwise. Demographic data, opioid requirements, and duration until ready for surgery were compared with the Mann-Whitney U test. Chi-squared test was used for achievement of functional goals and side effects. Significance was assumed at the p < 0.05 level. A nonparametric analysis of variance including multiple comparison adaptation (Brunner analysis) was performed to compare differences between groups during the whole course of treatment, regarding pain, knee flexion, and satisfaction.
Results
Mean duration until ready for surgery was lowest with general anesthesia, 15 min (IQR 25–75%: 6–19), from placing the i.v. line until ready for surgery, followed by combined spinal-epidural anesthesia, which required 30 min (IQR%:15– 35). Double catheter placement required 40 min (IQR%: 33–48).
2 sciatic, 2 psoas, and 1 epidural catheter were removed prematurely because of catheter dislocation or catheter occlusion. No signs of nerve irritation, infection, or neurological complication were observed. There were no bleeding complications either.
Pain
Pain scores at rest and during movement were higher in the PCA group than in the EPI group (p < 0.001 at rest and p < 0.001 during movement, respectively). Pain levels in group PCA were also higher than in the PSC group (p < 0.001 at rest and p = 0.001 during movement). There were no significant differences between the PSC and EPI groups (p = 0.6 at rest and p = 0.2 during movement) ().
Figure 1. Knee pain at rest (left panel) and during movement (right panel). NRS scores for pain in the total knee joint at rest and during motion during the 5 observational days are shown. Median/IQR (25th–75th percentile).
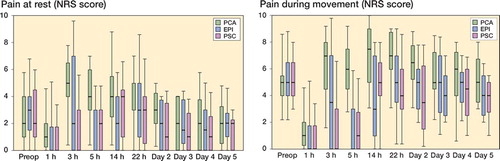
The PCA group had the highest opioid requirements of all 3 groups during the whole time course (p < 0.001). No significant difference was found between the EPI and PSC groups ().
Function
Knee flexion showed marked intragroup improvement over 3 postoperative observation days. The EPI group had better active flexion than the PCA group during the interventional days (p = 0.007). Between EPI and PSC, and also between PCA and PSC, there was no significant difference in active knee flexion (p = 0.2 and p = 0.2). Passive flexion (tolerated passive knee flexion, assisted by physiotherapists) was higher in the EPI group than in the PCA group during the first 5 postoperative days (p = 0.03). No significant differences regarding passive knee flexion were seen between the other groups.
Table 2. Postoperative opioid requirements (piritramide; mg) a
During the observational period (5 days), the EPI group had higher motor power (Janda scores) than the PCA and PSC groups (p < 0.001 and p = 0.04). There was no difference between PCA and PSC in this respect (p = 0.2).
On the first postoperative day, no significant difference was seen regarding the percentage of patients achieving functional goals. On day 1, 20 of 21 patients in the PCA group, 20 of 21 in the EPI group, and 20 of 21 in the PSC group elevated the leg in bed. On day two, 15 of 21 patients in each of the 3 groups were able to stand in front of the bed, with no differences between groups. On day 3, 16 patients in the PCA group, 19 in the EPI group, and 19 in the PSC group were able to achieve their first steps with assistance (p = 0.3).
On day 4, almost all patients were walking at least Patient satisfaction (NRS score)
a few steps with assistance from physiotherapists. On day 5, all patients in all groups walked a few steps with assistance.
Side effects
Pruritus was noted more often in the PCA group (5/21) and the EPI group (8/21) than in the PSC group (0/21) (p = 0.008, p = 0.03 and p = 0.047; postoperatively, on day 1 and on day 2, respectively). Nausea was observed (until 24 h postoperatively) most often in the PCA group (p = 0.001).
The incidence of hypotension during the postoperative phase (until day 2) was higher in the EPI group (16/21) than in the PCA group (11/21) and the PSC group (10/21) (p = 0.05). There was a gradual decrease until day 5.
Median number of days of urinary catheter use was 0, 2, and 0 in the PCA, EPI, and PSC groups, respectively (p = 0.1). 2 women in the EPI group refused the standard procedure, and had no urinary catheter placed. 1, 2, and 0 urinary catheters were inserted postoperatively in the PCA, EPI and PSC groups, respectively. No urinary infections were recorded.
No thromboembolic events were recorded.
Patient satisfaction
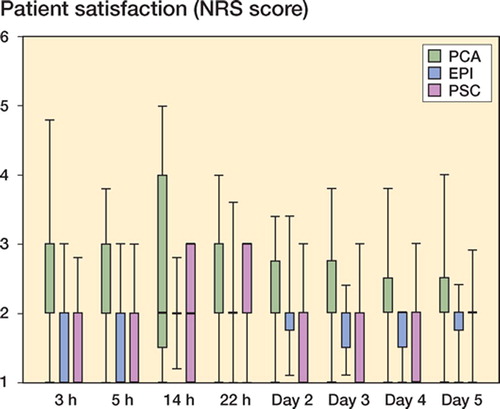
Patient satisfaction with the overall pain management was high at all times during the observation period. The PSC and EPI groups showed higher patient satisfaction until 24 h postoperatively than the PCA group (p = 0.0 and p = 0.031). No significant difference was observed between the PSC and EPI groups (p = 0.99) ().
Figure 2. Patient satisfaction. Patient satisfaction during the 5 days of observation are given (1 = very satisfied, 6 = unsatisfied; median and IQR (25th–75th percentile)). The PSC and EPI groups showed higher patient satisfaction than the PCA group (p < 0.05 and p < 0.05, respectively). No significant difference was seen between the PSC and EPI groups.
Hospital stay
There was a statistically significant difference in length of hospital stay between the three groups. The PSC group had a shorter mean length of stay (11 days; range 10–11) than the PCA group (13 days; range 11–17) and the EPI group (12 days; range 11–15) (p = 0.001 and p = 0.005, respectively).
Telephone interview
The telephone interview 6–9 months after surgery could be performed in 50/63 of the patients (17 in the PCA group, 16 in the EA group, and 17 in the PSC group). 1 patient in the PCA group died after 30 postoperative days, of unknown causes. No group-specific differences were observed for any of the questions. No patients had residual numbness, dysaesthesia, or weakness associated with the EPI or combined psoas and sciatic blockade. The majority was very satisfied with the analgesic method used, and stated that they would chose it again next time.
Discussion
Combined continuous lumbar plexus and sciatic nerve blocks for knee replacement surgery have not been tested in clinical trials yet. We found that pain therapy after total knee arthroplasty either by epidural or continuous psoas and sciatic blocks was better than by intravenous opioid PCA. This result is in line with earlier reports showing that adequate analgesia after TKA cannot be achieved with intravenous PCA alone (Singelyn et al. Citation1998, Capdevila et al. Citation1999, Chelly et al. Citation2001).
The use of continuous psoas blocks for analgesia after knee arthroplasty has been advocated because femoral analgesia blocks the obturator nerve to an insufficient degree (Bouaziz et al. Citation2002, Ganidagli et al. Citation2005). However, even though psoas blocks cover the obturator nerve better, both Kaloul et al. (Citation2004a) and Morin et al. (Citation2005) found no significant difference in pain scores and opioid consumption between continuous psoas and femoral blocks. Interestingly, both studies also found no difference in pain scores during physiotherapy. In our study, dynamic pain levels were significantly lower in both regional analgesia groups. Several reasons may account for this discrepancy. In the study reported by Kaloul, the sciatic nerve was not blocked. Because pain arising from the sciatic nerve may be relevant after knee arthroplasty (Pham Dang et al. Citation2005), this combination may be necessary. In the study by Morin et al. (Citation2005), the combined femoral and sciatic catheter group had fixed infusion rates of 14 mg/h. Accordingly, opioid consumption over 48 h was much higher in the study by Morin than in our almost opioid-free patients in both regional analgesia groups.
Significant differences in flexion were only seen between the EPI and PCA groups during the first three postoperative days, when group EPI had better active flexion than PCA. The relatively low degrees of early flexion compared to other studies (Morin et al. Citation2005) are most likely due to our physiotherapy regimen, without continuous motorized motion machine. Long-term studies have shown that the early range of flexion does not reliably indicate long-term functional outcome (Singelyn et al. Citation1998, Capdevila et al. Citation1999).
Pruritus occurred more frequently in the PCA and EPI groups than in the PSC group. This effect may be related to application of opioids via PCA or the epidural catheter in addition to the local anesthetic (Block et al. Citation2003). Nausea up to 24 h postoperatively was commonest in the PCA group. Zaric et al. (Citation2006) investigated side effects of epidural and peripheral nerve blocks and found no relevant differences regarding pruritus and nausea, probably due to the use of equal amounts of supplementary opioids (33 mg morphine as opposed to 31 mg on day 1 postoperatively). Hypotonia occurred in all our groups, the highest incidence being in the EPI group, which confirms the results of others (Capdevila et al. Citation1999).
There was no statistically significant difference in the use of urinary catheters between groups. However, we believe that this was due to the small sample size and we would expect an elevated incidence of use of urinary catheters in group EPI. No urinary infections were recorded. Zaric et al. (Citation2006) reported an elevated incidence of urinary retention in patients receiving epidural analgesia.
All groups showed a high level of satisfaction. Lower pain scores were associated with higher patient satisfaction during the intervention period, when patients with regional anesthesia were more satisfied than those with general anesthesia ().
The length of hospital stay was different between groups. Even though the PSC group showed the shortest time in hospital, we believe that this result may relate to discharge policy rather than the method of analgesia used.
We used varying catheter rates, with median rates of 15 mg/h for epidural catheters, and of 14 mg/h for psoas catheters and 12 mg/h for sciatic catheters, i.e. 26 mg/h for both catheters. Even though Kaloul et al. (Citation2004b) found potentially toxic levels when 24 mg/h was infused, no signs of toxicity were observed in our study. Even 28 mg/h did not cause symptoms in a study by Morin et al. (Citation2005).
Routine use of a long-lasting SCI nerve block might obscure diagnosis of early compartment syndrome or perioperative nerve injury (Levesque et al. Citation2005, Nercessian et al. Citation2005). We therefore used prilocaine for sciatic single shot, and peroneal function was assessed immediately after surgery. No cases of delayed neurological diagnosis of peroneal nerve injury occurred during our study, and no other neurological complication occurred.
Our study has certain limitations. For obvious ethical reasons, blinding was not possible. As many patients are currently prejudiced toward or against either planned catheter-based procedures or general anesthesia, randomization would have biased patients’ judgement and pain levels during this pilot trial. Catheter dislocation occurred in 5 cases. However, analysis of all data was performed according to the intention to treat principle. Exclusion of prematurely removed catheters for analysis of pain values and satisfaction in the PSC and EPI groups would have given better results (data not shown).
All patients in the PCA group received standardized general anesthesia. Our design was based on the view that pain levels in the early period after surgery should be able to be differentiated between groups. If, within the PCA group, an uncertain number of patients had had spinals for surgery, early differentiation of the effects compared to other regimes would not have been possible. Clearly, the use of PCA after a spinal analgesia is a clinically relevant option.
Catheter placement takes time. We analyzed induction time from placing the i.v. line until the patient was ready for surgery and found that double catheter placement required 10 min longer than for the EPI group. This has to be taken into account for the operating room management, but may not justify case losses on a daily basis.
In summary, epidural analgesia or the combination of continuous psoas and sciatic block give better pain reduction than patient-controlled analgesia with piritramide. Psoas and sciatic block appears to have the lowest incidence of side effects.
The authors wish to thank Tanja Schink for help with the statistical analysis and the physiotherapists for their excellent efforts to contribute to this study.
Contributions of authors
CR: participated in the development of the concept of the study, collected data, and wrote the manuscript. GM and CP were involved in the concept of the study, performed the knee arthroplasties, supervised physiotherapists, and revised the manuscript. JB: perfomed the regional anesthesias, co-developed the concept of the study, and revised the manuscript. KP and AAW: participated in the developement of the study, examined patients, collected data, and revised the manuscript. LMD and AM: asked for patient consent, collected data, and revised the manuscript. TV: designed the study, analyzed the data, and wrote the manuscript.
- Apfelbaum J L, Chen C, Mehta S S, Gan T J. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 2003; 97(2)534–40
- Block B M, Liu S S, Rowlingson A J, Cowan A R, Cowan J A, Jr, Wu C L. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 2003; 290(18)2455–63
- Bouaziz H, Vial F, Jochum D Macalou D, Heck M, Meuret P, Braun M, Laxenaire M C. An evaluation of the cutaneous distribution after obturator nerve block. Anesth Analg 2002; 94(2)445–9
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999; 91(1)8–15
- Capdevila X, Macaire P, Dadure C, Choquet O, Biboulet P, Ryckwaert Y, d'Athis F. Continuous psoas compartment block for postoperative analgesia after total hip arthroplasty: new landmarks, technical guidelines, and clinical evaluation. Anesth Analg 2002; 94(6)1606–13
- Chelly J E, Greger J, Gebhard R, Coupe K, Clyburn T A, Buckle R, Criswell A. Continuous femoral blocks improve recovery and outcome ofpatients undergoing total knee arthroplasty. J Arthroplasty 2001; 16(4)436–45
- Di Benedetto P, Casati A, Bertini L, Fanelli G. Posterior subgluteal approach to block the sciatic nerve: description of the technique and initial clinical experiences. Eur J Anaesthesiol 2002; 19(9)682–6
- Ganidagli S, Cengiz M, Baysal Z, Baktiroglu L, Sarban S. The comparison of two lower extremity block techniques combined with sciatic block: 3 in-1 femoral block vs. psoas compartment block. Int J Clin Pract 2005; 59(7)771–6
- Kaloul I, Guay J, Cote C, Fallaha M. The posterior lumbar plexus (psoas compartment) block and the three-in-one femoral nerve block provide similar postoperative analgesia after total knee replacement. Can J Anaesth 2004a; 51(1)45–51
- Kaloul I, Guay J, Cote C, Halwagi A, Varin F. Ropivacaine plasma concentrations are similar during continuous lumbar plexus block using the anterior three-in-one and the posterior psoas compartment techniques. Can J Anaesth 2004b; 51(1)52–6
- Levesque S, Delbos A. Sciatic nerve block for total-knee replacement: Is it really necessary in all patients?. Reg Anesth Pain Med 2005; 30(4)410–1
- Macalou D, Trueck S, Meuret P, Heck M, Vial F, Ouologuem S, Capdevila X, Virion J-M, Bouaziz H. Postoperative analgesia after total knee replacement: The effect of an obturator nerve block added to the femoral 3-in-1 nerve block. Anesth Analg 2004; 99(1)251–4
- Mahoney O M, Noble P C, Davidson J, Tullos H S. The effect of continuous epidural analgesia on postoperativepain, rehabilitation, and duration of hospitalization in total knee arthroplasty. Clin Orthop 1990, 260: 30–7
- Marhofer P, Nasel C, Sitzwohl C, Kapral S. Magnetic resonance imaging of the distribution of local anesthetic during the three-in-one block. Anesth Analg 2000; 90(1)119–24
- McNamee D A, Parks L, Milligan K R. Post-operative analgesia following total knee replacement: an evaluation of the addition of an obturator nerve block to combined femoral and sciatic nerve block. Acta Anaesthesiol Scand 2002; 46(1)95–9
- Morin A, Kratz C, Eberhart L, Dinges G, Heider E, Schwarz N, Eisenhardt G, Geldner G, Wulf H. Postoperative analgesia and functional recovery after Total-Knee Replacement: Comparison of a continuous posterior lumbar plexus (psoas compartment) block, a continuous femoral nerve block, and the combination of a continuous femoral and sciatic nerve block. Reg Anesth Pain Med 2005; 30(5)434–45
- Nercessian O A, Ugwonali O F, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty 2005; 20(8)1068–73
- Parkinson S K, Mueller J B, Little W L, Bailey S L. Extent of block with various approaches to the lumbar plexus. Anesth Analg 1989; 68(3)243–8
- Pham Dang C, Gautheron E, Guilley J, Fernandez M, Waast D, Volteau C, Nguyen J M, Pinaud M. The value of adding sciatic block to continuous femoral block for analgesiaafter total knee replacement. Reg Anesth Pain Med 2005; 30(2)128–33
- Singelyn F J, Deyaert M, Joris D, Pendeville E S, Gouverneur J M. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg 1998; 87(1)88–92
- Zaric D, Boysen K, Christiansen C, Christiansen J, Stephensen S, Christensen B. A comparison of epidural analgesia with combined continuous femoral-sciatic nerve blocks after total knee replacement. Anesth Analg 2006; 102(4)1240–6