Abstract
Background Skeletal trauma and immobilization are well-known risk factors for deep vein thrombosis (DVT) and pulmonary embolism (PE). While prophylaxis against thromboembolic complications has become routine after major orthopedic surgery, whether or not prophylaxis after minor surgery and lower limb immobilization is necessary is still under debate.
Methods In a double-blind, placebo-controlled study, 272 consecutive patients were randomized to receive either thromboprophylaxis with Dalteparin (n = 136) or placebo (n = 136) for 5 weeks after ankle fracture surgery. All patients received 1 week of initial treatment with Dalteparin before randomization. A unilateral phlebography was performed when the cast was removed.
Results The overall incidence of DVT was 21% (95% CI: 13–29%) in the Dalteparin group and 28% (CI: 19– 37%) in the placebo group (risk ratio = 0.8, CI: 0.6–1.1; p = 0.3). The incidence of proximal DVTs was 4% and 3%, respectively. No major bleeding occurred.
Interpretation We found no significant difference in the incidence of DVT between the 2 treatment groups and our results do not support prolonged thromboprophylaxis. The overall incidence of DVT was high, reflecting the potential risk of PE and post-thrombotic syndrome after ankle fracture surgery. Most of the DVTs were asymptomatic, however, and were located in distal veins.
Patients with orthopedic injuries risk developing a deep vein thrombosis (DVT) (Geerts et al. Citation1994) due to factors such as the trauma itself, the surgical procedure, and the subsequent immobilization. Ankle fracture is one of the most common lower limb fractures, having an incidence of more than 100 per 100,000 person years (Court-Brown et al. Citation1998, Jensen et al. Citation1998). The treatment options for displaced ankle fractures include immobilization in a plaster cast alone, or surgery with subsequent immobilization in a plaster cast or in an orthosis (Jensen et al. Citation1998). There is no general consensus regarding thromboprophylaxis during lower limb immobilization (Geerts et al. Citation2004). Here we present a comparative study of thromboprophylaxis in patients with surgically treated ankle fractures.
The purpose of this study was to evaluate the efficacy of a prolonged regimen of Dalteparin, and to compare its efficacy with that of placebo in patients surgically treated for ankle fracture and subsequently immobilized in plaster cast or orthosis.
Methods
Study design and patients
This was a prospective, parallel-group, singlecenter study with an open-labeled treatment with 5,000 U of subcutaneous Dalteparin once daily for 1 week, followed by a randomized double-blind and placebo-controlled treatment with 5,000 U of subcutaneous Dalteparin once daily until the plaster cast was removed after 44 days (± 2 days). The study was approved by the local research ethics committee (approval no. S.199/99) and performed according to the principles of the Declaration of Helsinki. All patients received written and verbal information about the trial, and consent was obtained from each one of them before randomization.
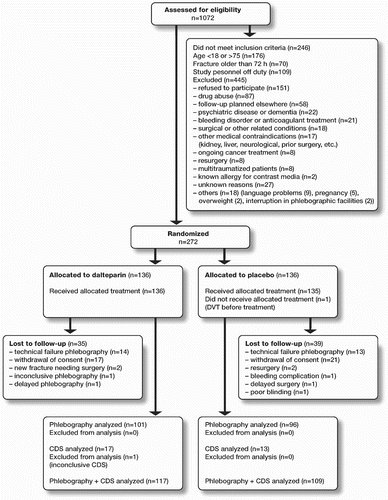
The study was conducted at Stockholm Söder Hospital between May 2000 and March 2004. All patients admitted to the hospital with an ankle fracture were recorded during this period. The inclusion criteria were defined as patients 18–75 years of age admitted to the hospital with an acute (0–72 h) ankle fracture and accepted for surgery. Exclusion criteria included inability or refusal to sign the informed consent for participation in the study, ongoing treatment with anticoagulant therapy, known allergy for contrast media, planned follow-up at another hospital, inability to comply with the study instructions (due to, for example, drug or alcohol abuse, cognitive dysfunction, etc.), known kidney disorder, nephrectomy or kidney transplantation, a recent thromboembolic event (within 3 months), recent surgery (within 1 month), known malignancy, a current bleeding disorder, pregnancy, treatment with high doses of acetylsalicylic acid (≥ 325 mg) or other platelet inhibitors, and multitrauma (injuries involving more than one organ system in addition to the musculoskeletal system, or patients with multiple fractures). 1,072 patients were admitted to the hospital with an ankle fracture during the study period. 826 patients fulfilled the inclusion criteria, 717 of whom were admitted when study personnel were on duty. Of these 717 patients, 272 were included in the study and 445 were not considered because of exclusion criteria (Figure).
All patients were operatively treated according to basic principles and immobilized after surgery in a plaster cast or an orthosis, a choice made by the surgeon. Patients were included consecutively and randomized after surgery. All patients received 1,000 mL Dextran 60 on admission to the hospital. Thereafter, 5,000 U Dalteparin was given subcutaneously (s.c.) once daily for 7 days, starting on the evening after surgery or on the evening before surgery in cases of delayed surgery (more than one day after admission). The patients received the study drug during the remaining period of immobilization. The study drug was prefilled into identical syringes at a volume of 0.2 mL and contained either placebo (9% (w/v) sodium chloride) or 5,000 U Dalteparin (Fragmin; Pharmacia &Upjohn/Pfizer Inc.) in subcutaneous formulation. All patients were trained in self-injection techniques by a study nurse before leaving the hospital.
Mandatory outpatient visits took place 2 weeks and 6 weeks postoperatively. After removing the cast, both legs were examined for clinical signs of DVT and unilateral phlebography was performed (+1 day). Color duplex sonography (CDS) was used in cases where the phlebography failed, most often due to difficulties in establishing venous access. Compliance to study medication was estimated by calculating the number of syringes used at the end of the study.
Assessment of efficacy
Primary efficacy (primary endpoint) was assessed by the number of patients in each treatment group with phlebography-verified distal and/ or proximal DVT and/or pulmonary embolism (PE). The primary endpoint analysis was performed according to the “intention-to-treat” (ITT) principle and patients all receiving at least 1 dose of study medication were included if assessable phlebography was achieved at the end of study. Additional per-protocol analysis was carried out for patients with a compliance to the study medication of more than 86% (i.e. they had missed less than 6 syringes during the study period).
Secondary efficacy (secondary endpoints) included the incidence of phlebography or CDSverified distal and/or proximal DVT and/or PE, and the incidence of DVT when using different types of immobilization (i.e. plaster cast or orthosis).
Phlebography
A mandatory unilateral ascending phlebography was performed on the final day of administration of the study drug (or at the latest on the day after), or earlier if thrombosis was suspected, using a modified form of the Rabinov and Paulin technique (Citation1972). The only criterion for a fresh thrombosis was a constant intraluminal filling defect of the same shape in at least 2 images. Proximal DVT was defined as thrombosis involving the popliteal vein, or more proximal veins, with or without involvement of the calf veins. The phlebograms were preliminarily evaluated for the presence or absence of thrombosis by the radiologist on duty. An independent radiologist who was blinded to the randomization and previous imaging findings subsequently carried out a standardized, final evaluation. In the event of discrepancy between the primary and secondary evaluation, consensus was reached with a third, blinded, independent reader.
Spiral CT and/or scintigraphy
If PE was suspected, spiral CT or ventilation/perfusion scintigraphy was performed. The diagnosis of PE was based on the criteria of Biello et al. (Citation1979) for high probability of PE.
Color duplex sonography
All examinations were carried out using a Siemens Acuson Sequoia duplex imager (C512; Siemens, Mountain View, CA) with a 6L3 linear-array transducer with pulsed and color-flow Doppler recording. The examination was carried out by 1 of 4 vascular technologists, all of whom had more than 5 years of experience in diagnosing DVT using the CDS technique. A standard procedure was used that included the evaluation of all deep proximal and distal veins, including muscle veins. Proximal DVT was defined as a thrombosis that involved the popliteal vein or any more proximal veins, with or without involvement of the calf veins. The diagnostic procedure and the criteria for diagnosis of DVT have been described previously (Lapidus et al. Citation2006). Briefly, the DVT diagnosis was based on a compression test of the affected blood vessel, which was visualized by means of a color Doppler flow. All positive examinations and those considered technically difficult were reviewed by a trained vascular physician, who established the diagnosis and classified the examination as negative, positive, or inconclusive.
Assessment of safety
Adverse events were recorded from patient history at 2- and 6-week follow-up. The patients were also encouraged to report all kinds of unexpected events (including bleeding events) during and after the study. All adverse events, as judged by the investigator, were documented. In the event of major bleeding—defined as bleeding requiring blood transfusion/resurgery, or bleeding at a critical site (intraocular, intracranial, intraspinal, or retroperito-neal)—the study was discontinued and the patient was treated according to standard protocols at our department. All other local bleeding was defined as minor and the investigator decided (together with each patient) whether their participation in the study should continue. Concurrent antidiabetic treatment with metformin was temporarily discontinued at least 48 h before phlebography.
Blood samples
Serum creatinine, hemoglobin, platelet count, activated partial thromboplastin time (APTT), and either prothrombin ratio (PR) or international normalized ratio (INR), were measured as baseline variables before randomization.
Statistical analysis and data management
We carried out a power analysis based on the fracture subgroup analysis from the work of Kujath et al. (Citation1993), where the incidence of DVT was 10% in the treatment group and 29% in the control group. We estimated that 188 patients (94 in each randomized group) would be required to detect a risk reduction of 20% with a power of 80% at the 5% significance level. The drop-out rate was estimated to be 20%, and thus the study would require 235 patients. All primary analyses were performed according to the “intention-to-treat” (ITT) principle, and patients receiving at least one dose of study medication were included in the results if assessable phlebography was obtained at the end of study. CDS was used as the endpoint in an additional secondary analysis for patients with an inconclusive or absent phlebography (ITT sample). A per-protocol analysis was carried out for patients who had compliance to the study medication of more than 86% and had an assessable phlebography at the end of the study. Type of immobilization (orthosis or plaster cast) and a subsequent DVTs was analyzed according to (ITT) principle.
The differences between groups were assessed using Student's t-test for continuous variables and Fisher's exact test test for categorical variables. An α-level of less than 0.05 was considered significant in all tests. The significance levels given refer to two-tailed tests. The results are expressed as mean and standard deviation (SD). The data were analyzed using SPSS statistical software. Study design, data entry, editing, and analyses were all carried out by the investigators.
Results
Patient characteristics
136 patients were randomized to the group for prolonged treatment with Dalteparin, and 136 were randomized to the placebo group. Of the 272 randomized patients, 197 patients (72%) were included in the primary analysis of efficacy, 101 patients in the Dalteparin group and 96 patients in the placebo group. 75 patients, 35 in the Dalteparin group and 40 in the placebo group, were excluded from the analysis ().
Table 1. Breakdown of patient numbers.
A minor bleeding complication occurred in 2 patients (1 from each group) and in both cases the study treatment was stopped by patient request on the grounds of discomfort. A symptomatic DVT occurred subsequently in one of these patients, and this patient was therefore included in the analysis of efficacy. The other adverse events that were reported were of a technical nature unrelated to the study medication (3 patients, for example, required resurgery with an additional syndesmotic screw). The failures in carrying out successful phlebography were due to difficulties in establishing venous access (), except for one case where the patient was too heavy for the X-ray table.
The mean age of the study population was 48 (18–76) years, and 54% of the patients were female (). Baseline demographic data, type of fracture, operation time, tourniquet time, and other clinical characteristics including the type of immobilization used after surgery were all similar in the 2 treatment groups. A plaster cast was used during the complete period of postoperative immobilization for 222 patients (108 in the placebo group and 114 in the Dalteparin group). 50 patients (28 in the placebo group and 22 in the Dalteparin group (p = 0.4) were immobilized in an orthosis). 3 patients were immobilized in an orthosis from day 1; all others received an orthosis after initial immobilization in a plaster cast for an average of 17 days. An orthosis was used more frequently for males (32/124) than for females (18/148) (26% vs. 12% (p = 0.005)), and more frequently for younger patients: the mean age of those with an orthosis was 45 years, compared to 49 years for those with a plaster cast (p = 0.03).
Table 2. Demographic and clinical data for all patients included in the study (n = 136 for both groups)
In the 175 patients for whom phlebography was assessed, the compliance to treatment was calculated when the cast was removed. The mean number of days on which the study medication was administered was 35 (2–40, SD 5) days. The mean duration of immobilization was 44 (SD 2) days in both treatment groups.
Efficacy of prevention of venous thromboembolic events
The overall incidence of phlebography-verified venous thromboembolic events was 21/101 (21%;CI: 13–29%) in the Dalteparin group and 27/96 (28%; CI: 19–37%) in the placebo group (p = 0.25), with an odds ratio of 0.7 (0.4–1.3) (). 4/101 patients (4%; CI: 0–8%) in the Dalteparin group and 3/96 patients (3%; CI: 0–6%) in the placebo group had a proximal DVT. No patient presented clinical signs of PE.
Table 3. DVT in the two treatment groups, intention-to-treat analysis (ITT), and per-protocol analysis (PP)
Results of the CDS examinations that were carried out in cases with inconclusive phlebography were added to the ITT sample, but did not alter the results significantly. The incidence of DVT in this analysis was 24/117 (21%; CI: 13–29%) in the Dalteparin group and 34/109 (31%; CI: 22–40%) in the placebo group (p = 0.07).
The incidence of phlebography-verified DVT in the per-protocol analysis was 13/75 (17%; CI: 10–25%) in the Dalteparin group and 17/65 (26%;CI: 18–35%) in the placebo group. This difference was not statistically significant (p = 0.2).
Subgroup analysis of the group of patients with plaster cast immobilization (all patients with orthosis excluded) showed that the incidence of phle-bography-verified DVT was 18/86 (21%; CI: 13– 29%) in the Dalteparin group and 27/75 (36%; CI: 27–45%) in the placebo group ().
4 patients in the orthosis group sustained a DVT (3 of these were diagnosed by phlebography and 1 by CDS), but 2 of these DVT cases had been diagnosed before the orthosis was applied (during the plaster cast immobilization). The incidence of phlebography-verified DVT in the orthosis group was 3/36 (8%; CI: 3–14%) and 45/161 (28%; CI: 19–37%) in the plaster cast group. 18 patients had clinically suspected DVT during the study period and for this reason underwent an acute phlebography or CDS. 5 of these acute phlebographies and 2 of the acute CDSs were positive for DVT (1 proximal DVT and 6 distal DVTs), and 1 CDS was positive for muscle vein thrombosis (3% of the study population). 2 of these patients were in the Dalteparin group and 6 were in the placebo group. None of these patients were immobilized with an orthosis.
Discussion
This randomized placebo-controlled trial compared 1 week and 6 weeks of Dalteparin DVT prophylaxis in patients immobilized with a plaster cast or an orthosis after ankle fracture surgery. We found no significant difference between the 2 treatment groups in the incidence of DVT detected by unilateral phlebography at the end of immobilization. The incidence of DVT was 21% in the Dalteparin group and 28% in the placebo group. Most DVTs were asymptomatic and located in distal veins. No clinically significant PE or major bleeding occurred in either of the groups.
The overall incidence of DVT was higher in our study than in other studies dealing with thromboprophylaxis during plaster cast immobilization after lower limb injuries. Comparison with others is, however, difficult since previous randomized studies (Kujath et al. Citation1993, Kock et al. Citation1995, Jorgensen et al. Citation2002, Lassen et al. Citation2002) have included a heterogeneous mix of patients with different lower limb injuries, treated with or without surgery, factors that may influence the effect of the thromboprophylaxis given and the risk of DVT (Geerts et al. Citation2004). Only 1 of the previous studies (Lassen et al. Citation2002) was placebo-controlled and phlebography was mandatory in only 2 of these studies (Jorgensen et al. Citation2002, Lassen et al. Citation2002). Kujath et al. (Citation1993) and Kock et al. (Citation1995) used unblinded treatment, and ultrasound to screen for DVT. This method was poorly validated for DVT screening at that time (Wells et al. Citation1995).
We have compared short-term and long-term thromboprophylaxis, and we have used phlebography in the primary analysis of efficacy, which is a recommended method to screen for DVT (Geerts et al. Citation2004). In the only placebo-controlled phlebographic study published, Lassen et al. (Citation2002) showed that the risk of DVT decreased significantly, from 19% to 9%, when using Reviparin during plaster cast immobilization. All patients in our study received at least one week of thromboprophylaxis, but still the incidence of DVT was higher than it was in Lassen's study, even though we used the same diagnostic criteria for DVT in the assessment of phlebograms (Zachrisson and Jansen Citation1973). This difference may relate to a different patient population; all our patients were treated surgically, as compared to 56% receiving operative treatment in the study of Lassen et al.
An inferior compliance to study medication in our study could also explain a higher incidence of DVT, although the compliance was considered acceptable when calculating the number of syringes used for each patient. The lack of significant efficacy for prolonged thromboprophylaxis with Dalteparin in our study may have been a result of incorrect sample size. Our power analysis indicated that our patient population was large enough to reveal an effect of prophylaxis, if such existed. We decided, however, to carry out a second analysis in which we added CDS as a complementary endpoint in cases where phlebography had failed. This would increase the sample size and the statistical reliability of the results. This decision was based on our previous results showing that CDS has a high sensitivity and specificity for the diagnosis of DVT after ankle fracture surgery (Lapiduset al. 2006). However, the increased sample size did not alter the results significantly. We also performed a per-protocol analysis to measure the true risk reduction for Dalteparin, but the results were unchanged. It is possible that the 7 days of treatment with Dalteparin that were given to all patients prior to the study medication equalized the effects in the 2 treatment groups. This shortcoming of the study design was necessary, however, since we considered it unethical to change (for study reasons) the DVT prophylaxis protocol used at our department.
The risk of selection bias is another limitation of our study, as in many other studies. Patients with other conditions that could increase the risk of DVT or bleeding were excluded from the study. Furthermore, only 33% (272/826) of the patients who fulfilled the inclusion criteria for the study were included, although less than 20% were excluded because they did not consent to participate. However, the incidence of symptomatic DVT in the group of patients not included in this study (2.4%) was similar to that in the study population (3%), indicating that the excluded patients had risk factors for DVT that were similar to the risk factors of those who were included. Another limitation of our study was the lack of stratification or randomization regarding the different types of immobilization (orthosis or plaster cast). Patients immobilized with plaster cast had a higher incidence of DVT than patients immobilized in an orthosis (28% vs. 8%). The risk of DVT was also significantly reduced by Dalteparin in the plaster-cast group (21% vs. 36%). However, patients in the plaster cast group differed significantly from those in the orthosis group both with regard to gender and age. The present study was not designed to compare the incidence of DVT nor the efficacy of prophylaxis in relation to different types of immobilization; thus, no conclusions can be drawn from these results.
Most postoperative DVTs are asymptomatic (Ascani et al. Citation1996) and are located in distal veins, but proximal extension of distal DVTs can be as high as 28% (Lohr et al. Citation1995). In our study 18 patients (6.6%) had symptoms indicating a DVT, and a DVT was diagnosed in 8 of these cases (3%). This incidence of DVT is similar to the incidence found at our department in the 4-year period before the study period (2% during 1997–2000) (Lapidus et al., unpublished observations) and it is similar to the incidence among the patients who were eligible but not included in this study (2.4%). In this patient population, however, it is difficult to assess the number of symptomatic DVTs objectively since most of the patients (not surprisingly) experience some discomfort or pain in the calf and ankle during the postoperative immobilization. Thus, most of the DVTs diagnosed in our study were asymptomatic and located in distal veins. The clinical significance of these often small DVTs is controversial (Wille-Jorgensen et al. Citation2005) and further studies on the long-term effects of thromboprophylaxis and the risk of post-thrombotic syndrome are required.
In conclusion, our study has shown that distal DVT is common during immobilization after ankle fracture surgery. A few patients also had a proximal DVT, reflecting the potential risk of PE, and effective thromboprophylaxis is therefore desirable. However, our results do not support prolonged thromboprophylaxis with Dalteparin.
We are grateful to Arvid Nordenhem, Pfizer Inc., for valuable assistance; Anita Söderqvist, KI SÖS, for skillful [Amer.] contributions throughout the study; Simon Printz, KI SÖS, for screening and inclusion assistance; Uffe Hylin, KI SÖS, for helpful randomization; Niklas Nyman, KI SÖS, for radiographic evaluations; and Hans Pettersson, Department of Statistics, KI SÖS, for valuable assistance.
This study was supported by a grant from Pharmacia-Upjohn, Pfizer Inc. and Karolinska Institutet. Pharmacia Upjohn/Pfizer Inc. also provided the Dalteparin that was tested in the study. The company neither planned the study nor took part in the data collection, analysis, interpretation of the data, or writing of the manuscript.
Contributions of authors
All authors participated in the design of the study and interpretation of the results. AE: analyzed the phlebographies. CL: collected the data. The article was mainly written by LL with contributions from all authors.
- Ascani A., Radicchia S., Parise P., Nenci G. G., Agnelli G. Distribution and occlusiveness of thrombi in patients with surveillance detected deep vein thrombosis after hip surgery. Thromb Haemost 1996; 75(2)239–41
- Biello D. R., Mattar A. G., McKnight R. C., Siegel B. A. Ventila-tion-perfusion studies in suspected pulmonary embolism. AJR Am J Roentgenol 1979; 133(6)1033–7
- Court-Brown C. M., McBirnie J., Wilson G. Adult ankle fractures--an increasing problem?. Acta Orthop Scand 1998; 69(1)43–7
- Geerts W. H., Code K. I., Jay R. M., Chen E., Szalai J. P. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994; 331(24)1601–6
- Geerts W. H., Pineo G. F., Heit J. A., Bergqvist D., Lassen M. R., Colwell C. W., Ray J. G.. Ches. Prevention of venous thromboembolism: the Seventh ACCP conference on Antithrombotic and Thrombolytic Therapy. 2004; 126 (3): 338S–400S
- Jensen S. L., Andresen B. K., Mencke S., Nielsen P. T. Epidemiology of ankle fractures. A prospective population-based study of 212 cases in Aalborg, Denmark. Acta Orthop Scand 1998; 69(1)48–50
- Jorgensen P. S., Warming T., Hansen K., Paltved C., Vibeke Berg H., Jensen R., Kirchhoff-Jensen R., Kjaer L., Kerbouche N., Leth-Espensen P., Narvestad E., Rasmussen S. W., Sloth C., Torholm C., Wille-Jorgensen P. Low molecular weight heparin (Innohep) as thromboprophylaxis in outpatients with a plaster cast: a venografic controlled study. Thromb Res 2002; 105(6)477–80
- Kock H. J., Schmit-Neuerburg K. P., Hanke J., Rudofsky G., Hirche H. Thromboprophylaxis with low-molecular-weight heparin in outpatients with plaster-cast immobilisation of the leg. Lancet 1995; 346(8973)459–61
- Kujath P., Spannagel U., Habscheid W. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis 1993; 23: 20–6, (Suppl 1)
- Lapidus L., de Bri E., Ponzer S., Elvin A., Norén A., Rosfors S. High sensitivity with color duplex sonography in thrombosis screening after ankle fracture surgery. J Thromb Haemost 2006; 4: 807–12
- Lassen M. R., Borris L. C., Nakov R. L. Use of the low-molecu-lar-weight heparin reviparin to prevent deep-vein thrombosis after leg injury requiring immobilization. N Engl J Med 2002; 347(10)726–30
- Lohr J. M., James K. V., Deshmukh R. M., Hasselfeld K. A. Calf vein thrombi are not a benign finding. Am J Surg 1995; 170(2)86–90
- Rabinov K., Paulin S. Roentgen diagnosis of venous thrombosis in the leg. Arch Surg 1972; 104(2)134–44
- Wells P. S., Lensing A. W., Davidson B. L., Prins M. H., Hirsh J. Accuracy of ultrasound for the diagnosis of deep venous thrombosis in asymptomatic patients after orthopedic surgery. A meta-analysis. Ann Intern Med 1995; 122(1)47–53
- Wille-Jorgensen P., Jorgensen L. N., Crawford M. Asymptomatic postoperative deep vein thrombosis and the development of postthrombotic syndrome. A systematic review and meta-analysis. Thromb Haemost 2005; 93(2)236–41
- Zachrisson B. E., Jansen H. Phlebographic signs in fresh post operative venous thrombosis of the lower extremity. Acta Radiol Diagn (Stockh) 1973; 14(1)82–96