Abstract
Background Bone morphogenetic proteins (BMPs) have the potential to improve clinical outcome after hip revision surgery by improving graft incorporation and implant fixation. However, impaction of cancellous bone grafts and TCP/HA bone substitute mixed with OP-1 device in a bone chamber in goats in a previous study led to reduced fibrous tissue ingrowth after 4 weeks. New bone formation was not promoted by OP-1. In the current study we examined whether this reduction represented a final loss of ingrowth or was just a delay, and whether the reduction can be overcome and ultimately results in a better late ingrowth.
Methods Bone chambers with impacted allografts and impacted TCP/HA granules mixed with 2 doses of OP-1 device were implanted in proximal medial goat tibias. Impacted allografts and TCP/HA not treated with OP-1 served as controls. After 8 weeks, the incorporation was evaluated using histology and histomorphometry.
Results Histology revealed evidence of bone graft incorporation, which proceeded in a similar way in both allografts and TCP/HA, with and without the addition of OP-1. After 8 weeks, no difference in bone ingrowth was found between the OP-1 groups and their controls. It was only in the allografts that the addition of OP-1 resulted in more fibrous tissue ingrowth.
Interpretation We conclude that the previously observed delay in fibrous tissue ingrowth can be only partially overcome.
Growth factor-enhanced allograft incorporation could improve clinical outcome after hip revision surgery by accelerating new hip formation and thereby improving implant stability (Lind et al. Citation2001). Bone morphogenetic proteins (BMPs) provide an opportunity to achieve faster and more extensive remodeling (Cook et al. Citation2000). They stimulate bone formation (Cook et al. Citation1998) and graft remodeling (Tägil et al. Citation2000). However, also BMP stimulated bone resorption has been observed both in vitro and in vivo (Kanatani et al. Citation1995, Kaneko et al. Citation2000) and in vivo (Laursen et al. Citation1999, Jensen et al. Citation2002, McGee et al. Citation2004). Accelerated resorption may have negative consequences for bone impaction grafting, since resorption before the formation of bone may compromise implant fixation. One solution would be an osteoconductive material providing initial stability after reconstruction (Oonishi Citation1991, Oonishi et al. Citation1997, Tägil et al. Citation2003). The role of BMPs in hip revision surgery might then be to serve as a promotor of bone formation in combination with a slow resorbing or unresorbable graft material (Aspenberg and Åstrand Citation2002, Åstrand and Aspenberg Citation2002).
In a previous study, we investigated the early effects of OP-1 device (OP-1 attached to a collagen carrier) on the incorporation of impacted morselized cancellous bone and tri-calcium phos-phate/hydroxy-apatite (TCP/HA) in an unloaded bone chamber in goats (Hannink et al. Citation2006). After 4 weeks, new bone formation was not promoted by the OP-1 device, and there were also no signs of accelerated resorption. However, a dose-related inhibition of vascularization and fibrous tissue ingrowth was found.
In general, in impacted bone grafts, the migration of cells within the allograft is compromized and vascularization is delayed for several weeks (Ling et al. Citation1993, Buma et al. Citation1996, Schreurs et al. Citation1996). In an unloaded bone chamber in rats, at 6 weeks the bone ingrowth into densely impacted allografts was delayed relative to bone ingrowth in allografts that had not been impacted (Tägil and Aspenberg Citation1998). An impacted graft acts as a hindrance for the ingrowing tissue or vessels, and this effect may be dependent on the degree of impaction. By adding OP-1 solution to the impacted bone grafts in the same unloaded model, bone ingrowth increased dramatically (Tägil et al. Citation2000). In contrast, in our previous study the collagen carrier might initially have further delayed bone and fibrous tissue ingrowth into the impacted graft material by filling up the space between the impacted graft material (Hannink et al. Citation2006).
Thus, although the collagen type I carrier may not be optimal for use in bone impaction grafting, in clinical cases better late ingrowth may appear despite an early delay (Wang et al. Citation2000). The present study was designed to determine whether the decrease in ingrowth represented a final loss or was just a delay. If the latter, could the OP-1 device overcome this initial delay and did this ultimately result in a better late ingrowth?
Animals and methods
Experimental design
12 mature Dutch milk goats (Capra hircus sana) (mean weight 47 (40–56) kg) were obtained from the Central Animal Laboratory, Radboud University Nijmegen. The goats received 3 bone chambers on each side in the cortical bone of the proximal medial tibia. The side and position of implantation of the 6 chambers were randomized. Two concentrations of OP-1 device were tested in combination with allografts and TCP/HA. Allografts not treated with OP-1 and TCP/HA served as controls (). The observation time was 8 weeks. All procedures were approved by the Animal Ethics Committee of the Radboud University Nijmegen, the Netherlands.
Overview of experimental design. Bone conduction chambers containing allograft and TCP/HA mixed with OP-1 device, and controls without the growth factor. 12 implants in each treatment group
Bone conduction chamber
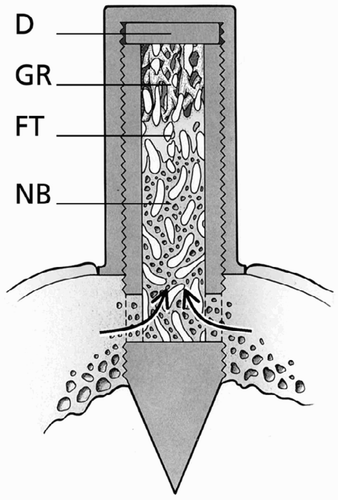
The bone conduction chamber (BCC) (Aspenberg and Wang Citation1993) consists of a threaded titanium cylinder, formed from two half-cylinders held together by a hexagonal closed screw cap. One end of the implant is screwed into the bone. The interior of the chamber has a diameter of 2 mm and a length of 7.5 mm. There are two ingrowth openings for bone ingrowth located at the bone-end of the chamber.
Thus, the ingrowing tissues enter the cylindrical space from the bone compartment. Originally developed as a rat model, the BCC was adjusted for use in goats (Van Der Donk et al. Citation2001). Since the tibial cortex in rats is thinner than that in goats, a 1 mm disk was placed in the cap of the BCC to provide for location of the ingrowth holes of the chamber to be deeper down, just at the level of the endosteum after the implant is screwed in ().
Figure 1. The bone conduction chamber. The chamber consists of two threaded half-cylinders held together by a cylindrical closed screw cap. There are ingrowth openings at the endosteal level. A 1 mm thick plate (D) was inserted into the cap to lower the ingrowth openings through the cortex. New bone (NB) and fibrous tissue (FT) are shown growing into the graft. Unremodeled graft (GR) can be seen at the top of the chamber. Modified from Jeppsson et al. (Citation2003).
Implant materials
Cancellous allografts were obtained from the sternum of six donor goats. Familial bands between donor and recepient goats were excluded. To prevent bias because of different immunologic reactions, the allografts were pooled.
Most blood and marrow was removed by rinsing the grafts with saline for approximately 1 min, leaving only a white bone structure. Rinsing was done using a high-pressure pulsatile lavage system (SurgiLav Plus; Stryker Nederland BV, Waardenburg, the Netherlands). The grafts were in a sieve during rinsing. They were stored at –80°C until use. Bacterial cultures from the grafts were negative. Before implantation, the grafts were thawed at room temperature and cut into pieces by using a rongeur.
The TCP/HA particles were composed of 20% HA (Ca10(PO4)6(OH)2) and 80% TCP (Ca3(PO4)2) (BoneSave; Stryker Howmedica Osteonics, Limerick, Ireland). We used granules with a diameter of 2–4 mm. The TCP-HA granules have a 50% non-interconnected macroporosity (range 300–600 µm), which is produced by burning sacrificial carbonaceous filler during sintering. The granules are also microporous (range 5–80 µm) (porosity values obtained from Stryker Orthopaedics). Before use, the TCP/HA particles were crushed to fit into the BCC and subsequently soaked in saline for 30 min.
Graft preparation
The rhOP-1 device (Stryker Biotech, Hopkinton, MA) was supplied sterile for implantation and consisted of 3.5 mg recombinant human osteogenic protein-1 (rhOP-1) combined with 1 g of highly purified bovine bone-derived type I collagen.
Immediately after warming to room temperature, the rhOP-1 device was mixed with a preweighed quantity of allograft chips before impaction. The BCC volume is 23.56 mm3, which allowed 0.0325 g of allograft chips and 0.0163 g of TCP/HA particles to be impacted in each implant. Two doses of rhOP-1 device were tested, a low-dose OP-1 (0.83 µg/implant) and a high-dose OP-1 (2.5 µg/implant). The high dose (2.5 µg/implant) would correspond to about one OP-1 device combined with one ordinary femoral head. According to the instructions of the manufacturer, this was the intended dose for bone impaction grafting.
Impaction procedure
Impaction was performed by gradually filling the BCC with the allograft bone/OP-1 mixtures or TCP/HA particles/OP-1 mixtures. A piston of a slightly smaller diameter was used for impaction (Van Der Donk et al. Citation2003). The piston was guided by low-friction bearings, strictly limiting it to vertical movement. The BCC was clamped into a cylindrical holder. A constant force of 40 N was kept on the free end of the piston for 2 min. During this time, fluid could escape between the piston and the wall of the bone chamber, and the ingrowth openings. The pressure applied was calculated to be 12.5 MPa. The disk was placed on the two threaded half-cylinders and the hexagonal closed screw cap was screwed on.
Surgical technique
The goats were anesthetized by intravenous administration of sodium pentobarbital (0.5 mL/kg) and maintained after intubation with nitrous oxide, oxygen and isoflurane (1.5–2%). Under aseptic conditions, a longitudinal incision was made in the skin and fascia over the medial side of the proximal tibia. After raising the periosteum, the cortical bone was observed and a hole was drilled through the medial cortex at approximately 4 cm from the joint cleft using a 3.1-mm drill. The hole was tapped and bone debris from drfilling was removed. The bone chamber was screwed in manually. The other bone chambers were placed 10 mm from the others. This was repeated for the other side. The subcutaneous layer and the skin were sutured. All animals were allowed unrestricted movement in their cages and had free access to water and food after the operation. After the implantation procedure, the animals received subcutaneous ampicillin (15 mg/kg/48 h) three times.
After 8 weeks, all goats were killed with an overdose of sodium pentobarbital. Tibias were removed, and the bone chambers with surrounding cortex were fixed in 4% buffered formalin. After 1 day, the content of the chambers was removed.
Evaluation
All specimens were fixed in 4% buffered formaldehyde, dehydrated using ethanol, and embedded in polymethylmetacrylate (PMMA). They were decalcified using 25% EDTA in 0.1 mol/L phosphate buffer (pH 7.4) before dehydration. The specimens were cut with a microtome (Leica RM 2155; Leica Microsystems Nederland BV, Rijswijk, the Netherlands) parallel to the longitudinal axis of the chamber. From each specimen, a total of 5 sections were taken at 0, 300 and 600 µm from the center of the specimen, each section being 5 µm thick. All sections within each experimental group were investigated in random order. The tests were done blind, but it was possible to see whether a specimen contained allograft or TCP/HA. The sections were stained with hematoxylin and eosin (HE), Goldner-Masson trichrome, and tartrate-resis-tant acid phosphatase (TRAP) for routine histology. Histomorphometric analysis was performed by using interactive computer-controlled image analysis (analySIS; Soft Imaging System GmbH, Münster, Germany). The bone ingrowth distance in each slide was calculated by dividing the new bone area by the width of the specimen (Aspenberg and Wang Citation1993). In all specimens, marrow cavities surrounded by bone were included in the bone area. The total tissue ingrowth distance, which is the distance from the ingrowth end to the fibrous ingrowth frontier, was measured in the same way as bone ingrowth. The mean of 5 sections yielded a value for each specimen.
Statistics
Statistical analysis was performed using a univariate analysis of variance (SPSS Inc., Chicago, IL) with factors goat, side, position and graft type. To identify the group or groups that differed from the others, Tukey's multiple comparison procedure was used. Normality and homogenity of variance were tested using the Kolmogorov-Smirnov test and the Levene test. When the assumption of normality or homogenity of variance was not met, a Friedman repeated measures ANOVA on ranks (non-paramet-ric test) was performed. This study was designed to have 80% power of detecting a difference of 0.5 mm between the means of all groups.
Results
Clinical
No intraoperative complications occurred during surgery. All goats recovered fully after surgery, were standing within one day, and had a normal gait pattern within three days after surgery. There were no signs of inflammation, skin ulceration, or wound-healing problems. All bone chambers were strongly fixed into the tibia. In most cases the bone chambers were surrounded by a layer of callus and covered with fibrous tissue, irrespective of the contents of the chamber. No new bone formation was seen at the endosteal surface of the tibial cortex.
Histology
A layer of necrotic, non-vascularized graft remnant was present at the top of the chamber—either with fibrous tissue infiltration or only graft material as it was inserted. Ffibrous tissue was present between the new bone at the bottom of the chamber and the graft remnant at the top of the chamber (. This fibrous tissue preceded the front of bone ingrowth. A loose mesenchymal-like tissue with blood sinusoids and capillaries penetrated the graft material.
Figure 2. A. Typical examples of the bone chamber content of allograft control (left) and allograft with high-dose OP-1 (right). Graft remnants (GR) are still present in the top of the specimen. New bone (NB) formation takes place from the ingrowth openings upward to the top of the chamber. Ffibrous tissue (FT) precedes the front of bone ingrowth. The line demarcates this front. Hematoxylin-eosin stained section. Magnification: 12.5×. B. Detail of allograft with high-dose OP-1 specimen showing newly formed bone at the bottom of the chamber. There were no differences in appearance of newly formed bone between the controls and either low-dose or high-dose OP-1 specimens. Hematoxylin-eosin stained section. Magnification: ×100. C. Typical examples of the bone chamber content of TCP/HA control (left) and TCP/HA (TCP) with high-dose OP-1 (right). There are TCP/HA granules throughout the entire specimen. New bone (NB) formation takes place from the ingrowth openings upward to the top of the chamber. Ffibrous tissue precedes the front of bone ingrowth. New bone is apposited on the TCP/HA granules at the bottom of the chamber. The front is again indicated by a line. Hematoxylin-eosin stained section. Magnification: ×12.5. D. Detail of TCP/HA specimen with high-dose OP-1 showing new bone (NB) apposited on the TCP/HA granules (at the bottom of the chamber). There were no differences in appearance of newly formed bone between the controls and either low-dose or high-dose OP-1 specimens. Hematoxylin-eosin stained section. Magnification: ×100.
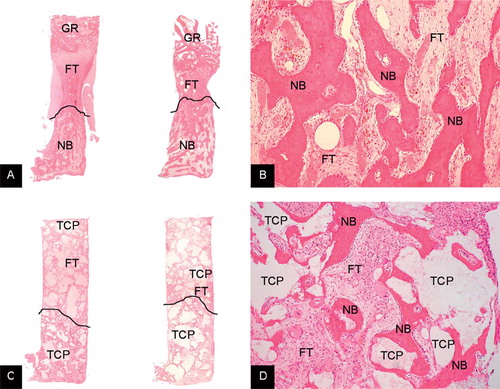
Newly formed bone was present in all bone chambers. New bone was formed by intramembranous ossification, with growth upward into the chamber. The amount and appearance of the new bone varied between specimens—from young, woven bone surrounded by active osteoclasts to more mature lamellar bone with fatty marrow and trabeculae (. No resorption of the graft material was observed in the TCP/HA-filled chambers (. Apposition of new bone on the TCP/HA was observed in all TCP/HA-filled chambers (. No collagen carrier material could be seen filling up the spaces between the impacted graft materials.
Histomorphometry
Neither side (p = 0.6 and p = 0.1) nor position (p = 0.9 and p = 0.2) of the chamber affected bone or total tissue ingrowth, respectively. There was no (p = 0.02). interaction between factors. The addition of OP-1device to allografts at either low dose (p = 0.04) relative to the controls for both allografts and or high dose (p = 0.02) gave increased total tissue ingrowth distances compared to the allograft control. The use of TCP/HA either with or without the addition of OP-1 device did not result in any differences in total tissue ingrowth. However, total tissue ingrowth distance was significantly less in the allograft control than in the TCP/HA control (p = 0.02)
OP-1 had no effect on bone ingrowth distance relative to the controls for both allografts and TCP/HA (). However, high-dose OP-1 mixed with allograft gave significantly more bone ingrowth than the TCP/HA control (p = 0.004).
Discussion
Within impacted bone chips, the migration of cells into the impacted allograft is compromised and vascularization is delayed for several weeks. It seems, however, that delayed or reduced new bone ingrowth seen in experiments with impaction is less important and may even be beneficial, as long as the graft/fibrous tissue composite remains strong enough to withstand forces acting on it during remodeling (Tägil and Aspenberg Citation2001). In a previous study, impaction of cancellous bone grafts and a TCP/HA bone substitute mixed with OP-1 device led to reduced fibrous tissue ingrowth as seen after 4 weeks (Hannink et al. Citation2006). Using higher doses of the OP-1 device, more collagen carrier material was impacted between the graft material, filling up the space between the impacted material and thereby delaying tissue ingrowth and remodeling. Similar observations, although not in the context of the impaction grafting setting, have been described previously by Franke Stenport and coworkers (Citation2003). In addition, the volume available for tissue ingrowth was reduced by the impaction of TCP/HA particles, creating small TCP/HA particles between the larger ones.
From our present findings, the previously observed lack of ingrowth after 4 weeks appears to have been a delay rather than an inhibition. After 8 weeks, however, the delay was only partially overcome. Similarly to our results after 4 weeks, no differences in bone ingrowth between OP-1 device groups and their controls were observed. However, after 8 weeks significantly more fibrous tissue ingrowth was measured in allografts mixed with OP-1 device compared to the allograft control. In contrast, after 4 weeks significantly less fibrous tissue was measured when comparing the allografts mixed with OP-1 to the allograft control (Hannink et al. Citation2006). In the TCP/HA groups, no differences in fibrous tissue ingrowth between OP1 device groups and their controls were observed, where there was a highly significant dose-depen-dent decrease in fibrous tissue ingrowth after 4 weeks. An observation time of 8 weeks might still be too short; however, no remnants of collagen carrier could be seen between the impacted allografts or TCP/HA granules, suggesting that all the OP-1 had been released from the carrier, thus no effect of the growth factor could be expected.
The data from both our previous study and the present work demonstrate the difficulty of using a biological enhancer of bone healing, such as an osteoinductive growth factor, in a situation where access to the blood supply and stem cells is limited and where bone healing is not optimal. Recently, Kärrholm et al. (Citation2006) showed that mixing of OP1 with morselized allograft did not improve early fixation of either the acetabular or femoral component in revision surgery of the hip. In contrast, the influence of OP-1 on impacted allograft implants was recently investigated experimentally in loaded, primary, and impaired revision settings (Soballe et al. Citation2004). Under primary conditions, OP-1 reduced mean implant fixation; in contrast, under the impaired healing conditions of the revision setting, OP-1 increased incorporation and fixation of the implants. However, an OP-1 solution (without the collagen carrier) was used in that study.
The collagenous extracellular matrix of bone is considered an optimal delivery system for osteogenic proteins (Cook et al. Citation2005). However, probably the collagen type-I carrier is not the optimal carrier to use in impaction grafting. Ideally, a carrier should perform several important functions in addition to binding the protein (Pinholt et al. Citation1991); it should not, however, obstruct the migration of cells into the impacted graft or vascularization. This makes it interesting to compare the results of the present study with the results of studies in which OP-1 was combined with graft without a carrier system. In a similar bone chamber model in rats, using a dose similar to our lowest dose without a collagen carrier (OP-1 solution) in impaction grafting, Tägil et al. (Citation2000) found an increase in bone formation. In contrast, a study using an OP-1 device (with collagen carrier) in combination with impaction grafting showed only very moderate effects on bone ingrowth (Lind et al. Citation2001). Moreover, using an OP-1 solution in a weight-bearing rabbit knee impaction grafting model, Tägil et al. (Citation2003) showed no augmentation of morselized impacted bone graft incorporation.
The effects of BMPs have been shown to be con-centration-dependent. The local concentration will be greatly determined by the release kinematics of the carrier (Takita et al. Citation2004). The collagen carriers that have been used in clinical studies effect an initial bulk release. Such a release may result in excessive bone formation at locations with favorable conditions. At locations with less favorable conditions, however, such as within impacted bone grafts, the BMP clearance might be faster than the bone induction response of the host (Groeneveld and Burger Citation2000).
Our data again indicate that the lack of effect when OP-1 is used in different clinical situations could be related to the problem that different biological environments require different dosages of growth factors and carriers for optimal stimulation. The challenge is to find ways to apply these drugs with consistent success in various applications in humans.
Contributions of authors
GH: did most of the work. BWS: supervised the study from a surgical perspective. PB: supervised the study from a biological perspective.
This study was sponsored by Stryker Orthopaedics, Limerick, Ireland.
- Aspenberg P., Åstrand J. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand 2002; 73: 20–3
- Aspenberg P., Wang J. S. A new bone chamber used for measuring osteoconduction in rats. Eur J Exp Musculoskel Res 1993; 2: 69–74
- Åstrand J., Aspenberg P. Reduction of instability-induced bone resorption using bisphosphonates: high doses are needed in rats. Acta Orthop Scand 2002; 73: 24–30
- Buma P., Lamerigts N., Schreurs B. W., Gardeniers J., Versleyen D., Slooff T. J. Impacted graft incorporation after cemented acetabular revision. Histological evaluation in 8 patients. Acta Orthop Scand 1996; 67: 536–40
- Cook S. D., Salkeld S. L., Brinker M. R., Wolfe M. W., Rueger D. C. Use of an osteoinductive biomaterial (rhOP-1) in healing large segmental bone defects. J Orthop Trauma 1998; 12: 407–12
- Cook S. D., Barrack R. L., Santman M., Patron L. P., Salkeld S. L., Whitecloud T. S. The Otto Aufranc Award. Strut allograft healing to the femur with recombinant human osteogenic protein-1. Clin Orthop 2000; 381: 47–57
- Cook S. D., Salkeld S. L., Patron L. P. Bone defect healing with an osteogenic protein-1 device combined with carboxymethylcellulose. J Biomed Mater Res B Appl Biomater 2005; 75: 137–45
- Franke Stenport V., Johansson C., Joo Heo S., Albrektsson T. Titanium implants and BMP-7 in bone: an experimental model in the rabbit. J Mat Sci Mat Med 2003; 14: 247–54
- Groeneveld E. H., Burger E. H. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 2000; 142: 9–21
- Hannink G., Aspenberg P., Schreurs B. W., Buma P. High doses of OP-1 inhibit fibrous tissue ingrowth in impaction grafting. Clin Orthop 2006; 452: 250–9
- Jensen T. B., Overgaard S., Lind M., Rahbek O., Bunger C., Soballe K. Osteogenic protein 1 device increases bone formation and bone graft resorption around cementless implants. Acta Orthop Scand 2002; 73: 31–9
- Jeppsson C., Astrand J., Tagil M., Aspenberg P. A combination of bisphosphonate and BMP additives in impacted bone allografts. Acta Orthop Scand 2003; 74: 483–9
- Kanatani M., Sugimoto T., Kaji H., Kobayashi T., Nishiyama K., Fukase M., Kumegawa M., Chihara K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res 1995; 10: 1681–90
- Kaneko H., Arakawa T., Mano H., Kaneda T., Ogasawara A., Nakagawa M., Toyama Y., Yabe Y., Kumegawa M., Hakeda Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000; 27: 479–86
- Kärrholm J., Hourigan P., Timperley J., Razaznejad R. Mixing bone graft with OP-1 does not improve cup or stem fixation in revision surgery of the hip: 5-year follow-up of 10 acetabular and 11 femoral study cases and 40 control cases. Acta Orthop 2006; 77: 39–48
- Laursen M., Hoy K., Hansen E. S., Gelineck J., Christensen F. B., Bunger C. E. Recombinant bone morphogenetic pro-tein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J 1999; 8: 485–90
- Lind M., Overgaard S., Jensen T. B., Song Y., Goodman S. B., Bunger C., Soballe K. Effect of osteogenic protein 1/colla-gen composite combined with impacted allograft around hydroxyapatite-coated titanium alloy implants is moderate. J Biomed Mater Res 2001; 55: 89–95
- Ling R. S., Timperley A. J., Linder L. Histology of cancellous impaction grafting in the femur. A case report. J Bone Joint Surg (Br) 1993; 75: 693–6
- McGee M. A., Findlay D. M., Howie D. W., Carbone A., Ward P., Stamenkov R., Page T. T., Bruce W. J., Wildenauer C. I., Toth C. The use of OP-1 in femoral impaction grafting in a sheep model. J Orthop Res 2004; 22: 1008–15
- Oonishi H. Orthopaedic applications of hydroxyapatite. Biomaterials 1991; 12: 171–8
- Oonishi H., Iwaki Y., Kin N., Kushitani S., Murata N., Wakitani S., Imoto K. Hydroxyapatite in revision of total hip replacements with massive acetabular defects: 4 to 10 year clinical results. J Bone Joint Surg (Br) 1997; 79: 87–92
- Pinholt E. M., Solheim E., Bang G., Sudmann E. Bone induction by composite of bioerodible polyorthoester and demineralized bone matrix in rats. Acta Orthop Scand 1991; 62: 476–80
- Schreurs B. W., Huiskes R., Buma P., Slooff T. J. Biomechanical and histological evaluation of a hydroxyapatite-coated titanium femoral stem fixed with an intramedullary morsellized bone grafting technique: an animal experiment on goats. Biomaterials. 1996; 17: 1177–86
- Soballe K., Jensen T. B., Mouzin O., Kidder L., Bechtold J. E. Differential effect of a bone morphogenetic protein-7 (OP-1) on primary and revision loaded, stable implants with allograft. J Biomed Mater Res A 2004; 71: 569–76
- Tägil M., Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop 1998; 352: 231–8
- Tägil M., Aspenberg P. Fibrous tissue armoring increases the mechanical strength of an impacted bone graft. Acta Orthop Scand 2001; 72: 78–82
- Tägil M., Jeppsson C., Aspenberg P. Bone graft incorporation. Effects of osteogenic protein-1 and impaction. Clin Orthop 2000; 371: 240–5
- Tägil M., Jeppsson C., Wang J. S., Aspenberg P. No augmentation of morselized and impacted bone graft by OP-1 in a weight-bearing model. Acta Orthop Scand 2003; 74: 742–8
- Takita H., Vehof J. W., Jansen J. A., Yamamoto M., Tabata Y., Tamura M., Kuboki Y. Carrier dependent cell differentiation of bone morphogenetic protein-2 induced osteogenesis and chondrogenesis during the early implantation stage in rats. J Biomed Mater Res A 2004; 71: 181–9
- Van Der Donk S., Buma P., Aspenberg P., Schreurs B. W. Similarity of bone ingrowth in rats and goats: a bone chamber study. Comp Med 2001; 51: 336–40
- Van Der Donk S., Weernink T., Buma P., Aspenberg P., Slooff T. J., Schreurs B. W. Rinsing morselized allografts improves bone and tissue ingrowth. Clin Orthop 2003; 408: 302–10
- Wang J. S., Tagil M., Aspenberg P. Load-bearing increases new bone formation in impacted and morselized allografts. Clin Orthop 2000; 378: 274–81
![Figure 3. Ingrowth distances of fibrous tissue and bone for each treatment group, depicted in a box plot. The doses of OP-1 device included: [1] 0.83 µg OP-1 combined with 0.24 mg collagen carrier per implant, and [2] 2.5 µg OP-1 combined with 0.72 mg collagen carrier per implant.](/cms/asset/b9849366-df3f-438c-acdc-cb3c20ba560f/iort_a_11327335_f0003_b.jpg)