Abstract
Background The most common complications of prosthetic hip joints are aseptic mechanical failure and infection. Delayed low-grade infections are seen most often, and they are also most difficult to distinguish from aseptic mechanical failures.
Methods We conducted a prospective study to compare inflammatory markers in patients diagnosed with aseptic or septic prosthetic loosening. The diagnostic criteria were based on the decisions of experienced orthopedic surgeons and microbiological analysis of periprosthetic tissue samples taken perioperatively.
Results Coagulase-negative staphylococci were the commonest pathogens in the infected patients. Pre- or perioperative elevation of C-reactive protein and erythrocyte sedimentation rate were significantly greater in the infection group, as were white blood cell count and levels of cytokines in synovial fluid. The patterns of infiltration of inflammatory cells in periprosthetic tissue were also significantly different between the groups.
Interpretation A combination of clinical judgment and multiple tissue samples constitutes a good platform for distinguishing between septic and aseptic loosening of prostheses. Moreover, the combined use of several laboratory and histopathological markers of inflammation, especially infiltration of polymorphonuclear cells, further helps the diagnosis.
Infections associated with in-dwelling prosthetic devices cause significant morbidity and are expensive (Crowe et al. Citation2003). The complexity of diagnosing and treating these infections has made it difficult to achieve standardized management strategies (Zimmerli et al. Citation2004). Microbial colonization of the joint space is affected by several factors such as characteristics of the prosthetic surface, occurrence of devitalized tissue, nonimmune and immune host factors, the bacterial inocula, and genetic characteristics of the species (Gallo et al. Citation2003). Prosthetic joint infections are associated with biofilms (Gristina Citation1987, Citation2004) in which bacteria are protected from both antimicrobial agents and the host response (Trampuz et al. Citation2003, Patel Citation2005).
Uniform criteria for preoperative diagnosis of prosthetic hip joint infections have not been established. Delayed, low-grade infections with less virulent bacteria are commonplace; thus, it is often difficult to discriminate between aseptic and septic loosening (Zimmerli et al. Citation2004).
Our main hypothesis was that there would be significant differences between patients with aseptic and septic loosening of prostheses regarding the expression of inflammatory markers. We conducted a prospective study focusing on differences between various markers in blood, in synovial fluid, and in histopathological specimens from peripros thetic tissue in the two groups of patients. Patients with osteoarthrosis who were hospitalized for their primary prosthesis surgery served as controls.
Patients and methods
Subjects
The 3 hospitals in the cities of Norrköping, Motala, and Linköping in Östergötland County, Sweden, participated in the study, which was approved by the Ethics Committee of Linköping University. Consecutive patients were assigned to a control group, a revision group, or an infection group. The control group comprised patients with osteoarthrosis of the hip who were undergoing primary prosthesis surgery. The revision group consisted of patients who were in need of revision surgery for radiographically confirmed loosening of hip prostheses. The criteria for inclusion in the infection group were as follows: (1) the orthopedic surgeon preoperatively classified the prosthesis loosening as being due to a deep infection of the device, based on clinical symptoms, laboratory and radiographic findings, preoperative cultures of synovial fluid aspirations, or occurrence of fistulas; and/or (2) the surgeon deemed the patient infected because significant bacterial growth was observed in cultures from tissue samples taken at revision surgery, and the surgeon decided to treat the patient as being infected. Significant bacterial growth was defined as growth of the same species in at least 3 of 5 aerobic or anaerobic cultures from tissue samples (Sanzen and Sundberg Citation1997, Atkins et al. Citation1998).
The study was conducted from 1994 to 2001. Patients were assigned to the control and revision groups during 1994 and 1995, whereas inclusion in the infection group was continued over the entire study period. 91 patients were registered as dropouts, most of them (80) belonging to the control group, and the main reason was inability to obtain synovial fluid. The final distribution of the patients was as follows: 46 (32 women) in the control group, median age 69 (43–89) years; 60 (25 women) in the revision group, median age 74 (38–86) years; 25 (10 women) in the infection group, median age 70 (30–83) years. 2 patients in the control group, 3 in the revision group, and none in the infection group had rheumatoid arthritis. Immunosuppressive treatment (peroral cortisone or chemotherapy) was given to 3 patients in the control group, to 4 in the revision group, and to 1 in the infection group. Primary arthrosis was the predominant indication for initial surgery in all groups.
Tissue samples
Isolation of bacteria
Multiple tissue samples proximal to the prosthesis were taken perioperatively under aseptic conditions from all but 1 of the patients in the revision group (i.e. 59 patients) and from all patients in the infection group (i.e. 25 patients). 6 to18 tissue samples for culture were taken from each patient, with a mean value of 10 cultures per patient, 5 aerobic and 5 anaerobic. In all, 718 tissue samples were collected from the revision group and 249 from the infection group. The tissue samples were incubated in thioglycolate broth for either 5 days under aerobic conditions or 10 days under anaerobic conditions, according to standard procedures (Augustinsson et al. Citation2001). Coagulase-negative staphylococci (CoNS) were further characterized to species level using API Staph kits (BioMérieux, Marcy l'Etoile, France).
Tissue cell infiltration
Tissue samples for assessment of cell infiltration around the prostheses were collected from patients in the revision group and the infection group. Tissue sections were fixed in 4% buffered formaldehyde before histopathological and immunohistochemical analysis. Fixed biopsies were embedded in paraffin and cut into 3–5-μm-thick sections. Routine staining with the hematoxylin-eosin-saffran method was done, and samples with a distinct synovial or periprosthetic surface were selected for further studies. Areas exhibiting inflammatory reactions, foreign body reactions, and fibrous tissue were identified. Foreign body reactions, characterized by multinucleate giant cells, were estimated on an equivalent arbitrary scale of 0–3. Fibrosis was defined as occurrence of thick, keloid-like collagen bundles and was arbitrarily estimated on a scale of 0–3 (representing none, mild, moderate, and substantial, respectively). Material from 26 patients in the revision group and from 21 from the infection group was suitable for further analysis. Sections were examined in a blind fashion. The same pathologist evaluated all samples without any knowledge of clinical or laboratory data concerning the patients. Hematoxylin-eosin-saffran stained sections were examined for polymorphonuclear (PMN) cell infiltration, foreign body reactions, and fibrous tissue. Surface areas with the most florid inflammatory changes were evaluated for infiltration of PMN cells (PMNs). PMNs were counted in 10 consecutive high-power fields (HPF; 0.152 mm2).
Immunostaining was performed using the following monoclonal antibodies (mAbs): anti-CD 20 (1:1,000; Dakocytomation, Glostrup, Denmark) to detect mature B-lymphocytes; anti-CD 79a (1:1,000; Dakocytomation) for plasma cells; anti-CD 4 (1:10; Novocastra, Newcastle upon Tyne, UK) for T-helper lymphocytes; anti-CD 8 (1:10; Novocastra) for T-cytotoxic lymphocytes; anti- CD 68 (1:200; Dakocytomation) for macrophages. ChemMate Envision detection (Dakocytomation, Glostrup, Denmark) was used to visualize the staining. As with PMNs, immunostained cells from a particular surface area were counted in 10 consecutive HPFs.
Blood samples
Blood samples for determination of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cell (WBC) count were collected on day 0 (before operation). CRP values of > 10 mg/L, ESR > 30 mm/h, and WBC > 11 × 109/L were considered to represent elevated values (Li et al. Citation2004, Sanzen and Sund berg 1997). Samples for measurement of serum levels of TNF-α, IL-1β, and IL-6 were taken on day 0 from patients in the revision and infection groups at one of the participating hospitals to allow comparison of serum levels of these cytokines with local production in synovial fluid. Blood samples for detection of serum cytokines were collected in tubes with endotoxin-free heparin as anticoagulant (Endotube ET). The tubes were immediately centrifuged at 4°C, after which the contents were divided into aliquots in endotoxin-free tubes and stored at –70°C until used. We used the following reference intervals in serum suggested by the manufacturer of the enzyme-linked immunosorbent assays (ELISAs): < 20 pg/mL for TNF-α, < 15 pg/ mL for IL1-β, and 3–8.5 pg/mL for IL-6.
Synovial fluid samples
Samples of synovial fluid for WBC counts and analyses of lactate and the cytokines TNF-α, IL-1β, and IL-6 were collected from all 3 groups perioperatively. Samples for WBC counts and lactate analysis were processed on a routine basis at the Clinical Chemistry laboratory. The following reference levels were used for assessment of WBCs (values × 109/L, levels given by the laboratory): <0.2, normal level; 0.2–15, mild inflammation; > 15–50, moderate inflammation; > 50, suspicion of infection. The reference level for lactic acid was 0.6−3.1 mmol/L (normal value). Samples of synovial fluid for cytokine detection were collected in endotoxin-free tubes and then transported on ice to where they were immediately centrifuged at 4°C, and stored thereafter at –70°C until use. TNF-α, IL-1β, and IL-6 were quantified using commercially available ELISAs (Medgenix Diagnostics EASIA; BioSource Europe S.A., Fleurus, Belgium) according to the instructions of the manufacturer. The absorbance values measured are proportional to the cytokine concentrations in the samples. Standards were used to create a standard curve, and the concentration of TNF-α in the patient samples was determined by interpolation from the standard curve. In our experiments, some samples had to be diluted to obtain a sufficient sample volume (200 μL).
Statistics
We used descriptive and interference methods for statistical analysis of the data. Significance was determined using Chi-square tests and, where appropriate, nonparametric tests (Mann-Whitney U-test and the Kruskal-Wallis test for multiple comparisons). Bonferroni's correction was used for multiple comparisons.
Results
Clinical data
The prostheses had been in place for a shorter amount of time in the infection group than in the revision group (p < 0.001). The median interval between the primary or previous surgery and the present revision was 9 (1–22) years in the revision group and 3 (0.2–16) years in the infection group. In the infection group only one patient (designated L333 in ) underwent prosthesis revision within 3 months, 6 patients underwent revision between 3 months and 1 year after previous surgery (2 with CoNS, 1 with CoNS and Propionibacterium, 1 with enterococci, and 2 culture negative in tissue samples), and the remaining 18 patients underwent their present revision more than 1 year after previous surgery. Only 1 patient in the revision group had a history of postoperative infection at the site of previous hip surgery, which contrasts with the results in the infection group (p < 0.001) in which 9 of the patients showed infection after an earlier hip operation.
Table 1. Culture results in the infection group
All patients in the control and revision groups experienced pain whereas 4 in the infection group did not report pain. In the revision group, 52 of the patients had a cemented prosthesis, and 55 patients received a cemented prosthesis at the current revision; the corresponding values for the infected group were 24 and 15, respectively. In the infected patients, antibiotic-loaded cement was used at revision, if cement was used at all (15 subjects out of 25).
Culture results ( and )
The surgeon suggested a clinical diagnosis of infection in 23 of the 25 subjects in the infection group, and 2 additional patients (L330 and L332 in ) were classified as being infected on the basis of significant growth of bacteria from tissue samples. 4 of 5 anaerobic cultures for patients L330 and L332 were positive for Propionibacterium and CoNS, respectively. Interestingly, the CoNS isolated from patient L332 grew only in anaerobic cultures. Tissue bacteriology was negative in 3 patients who had a clinical diagnosis of infection. In addition, 2 patients had no determined etiology according to our definition (L338 and N310). Hence, the etiology was not ascertained for 5 of 25 patients in the infection group.
Table 2. Bacterial etiology in infected patients
CoNS constituted the most common pathogens, being found in 14 of 25 patients and representing the only pathogen in 8 patients (). The second most common bacterium was Propionibacterium, which was found in 4 patients and was the sole pathogen in 3. Other pathogens in descending frequency were Staphylococcus aureus, enterococci, β-hemolytic streptococci group B, and one untypable anaerobic gram-positive coccal species (ANGPC). 14 of the 25 patients had only 1 pathogen, 6 had mixed infections, and 5 had infections of unknown etiology.
Microbiological culture from periprosthetic tissue samples was performed for 59 of the 60 patients in the revision group, in the same way as for the infected patients. In 36 of these patients all cultures from each patient were negative. Of the rest of the subjects in that group (23 patients), all but 1 were microbiologically negative according to our definition of significant bacterial growth. The most common bacteria in that context were Propionibacterium, followed by CoNS, α-streptococcus, Bacillus cereus, diphtheroids, and ANGPC. None of these subjects showed signs of deep infection at follow-up, and 1 of them had been revised 3 years postoperatively due to cup loosening, at which time cultures from tissue samples were negative. The unexpected significant bacterial growth seen from 1 patient was identified as Propionibacterium, and the surgeon and the specialist in infectious diseases were not certain whether the culture results were representative. This patient was not given antibiotics against Propionibacterium, but clinical outcome was nonetheless satisfactory at 1- year follow-up.
Cell infiltration in tissue ( and )
The infection group showed significantly more infiltration of all the types of inflammatory cells that were considered, except macrophages (). None of the patients in the revision group had PMN infiltration greater than 5 cells per HPF. 4 subjects in the infection group had <5 PMNs/HPF, and they had infection with Propionibacterium (patient L330), CoNS (patients N342 and L353), and both S. aureus and CoNS (patient N345). B-cells were seen in only 1 revision patient, whereas 18 of the infected patients showed such infiltration. No infiltrating plasma cells were found in tissue from any of the revised patients. T-helper cells above the cut-off level (i.e. ≥ 5 cells/HPF) were observed in 15 of 21 of the infected patients, but in only 2 of 26 patients in the revised group.
Table 3. Cell infiltration in periprosthetic tissue in the revision and infection groups. Values are median (range) of cells/HPF
Table 4. Data on arbitrary fibrosis and foreign body reactions in the revision and infection groups
All patients in both groups showed some degree of fibrosis, and there were no obvious differences between the groups. Most infected patients had no foreign body reactions at all, and more reactions were seen in the revision group than in the infection group ().
Analysis of blood and synovial fluid
WBC count, CRP, and ESR in blood ()
Median counts of WBCs in blood on day 0 (before surgery) were all within the normal range and there were no statistically significant differences between the groups (not shown). Both CRP and ESR levels were significantly higher in the infection group than in the revision group on day 0 ().
Table 5. Preoperative C-reactive protein (CRP; mg/L) and erythrocyte sedimentation rate (ESR; mm/h) for the different groups
WBC counts and lactate in synovial fluid ()
Synovial fluid for WBC counts was obtained from only 7 of the 25 infected patients. However, the counts were significantly higher in the infection group than in the revision group, and all but 1 of the infected patients had values greater than 2 × 109/L. In contrast, there was no significant difference in lactate levels between the revised and infected patients, and the median values for this variable were elevated in all groups (even the control group, in which the subjects had osteoarthritis but no prostheses) compared to the normal reference interval for synovial fluid.
Table 6. White blood cell (WBC) count and lactate levels in synovial fluid
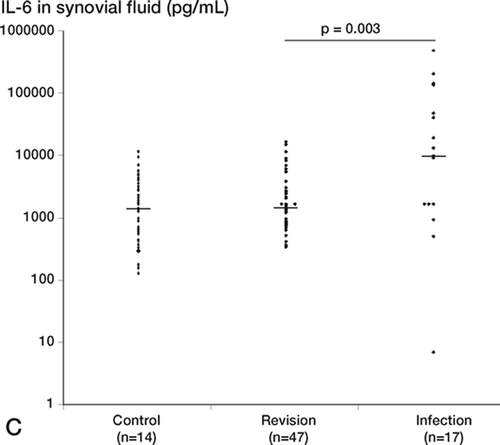
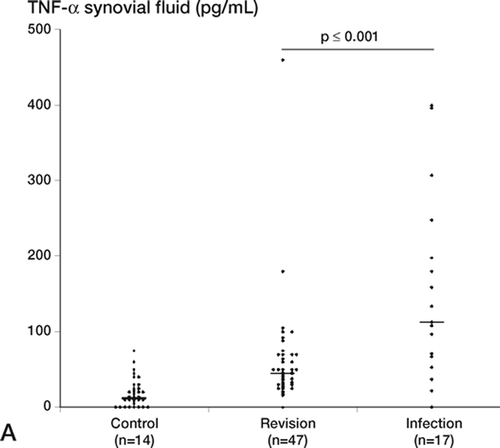
Cytokines in synovial fluid and blood (Figure)
The levels of TNF-α, IL-1β, and IL-6 in synovial fluid were significantly higher in the infection group than in the revision group. Despite that, the positive predictive values were low for the infected patients because there were also patients in the revision group with levels above cut-off. This trend was seen for all 3 cytokines. In the revision group, the outlier for TNF-α was also the outlier for IL-1β: this patient was being treated with prednisolon for polymyalgia rheumatica. We compared the cytokine levels in the subgroup of revision patients who yielded 1 or 2 positive cultures (but were not classified as having significant bacterial infection) with the majority of the revision patients, who were all culture negative. We found no significant differences between these 2 groups, which supports the hypothesis of bacterial contamination. In the infection group, the TNF-α (p = 0.002), IL-1β(p = 0.002), and IL-6 (p = 0.002) levels were signifi- cantly higher in synovial fluid than in serum (not shown). In the revision group, this trend was seen only for IL-6.
Figure 1. Proinflammatory cytokine levels in synovial fluid (pg/mL), shown for individual patients in the control, revision, and infection groups. For each group, the number of patients is indicated and the median is marked with a horizontal bar. A. Median levels of TNF-α: control group, 12 pg/mL; revision group, 45 pg/mL; infection group, 113 pg/mL. The difference between the revision group and the infected group was statistically significant (p = 0.001). B. Median levels of IL-1β: control group, 22 pg/mL; revision group, 14 pg/mL; infection group, 63 pg/mL. The difference between the revision group and the infected group was statistically significant (p < 0.001). C. Median levels of IL-6: control group, 1450 pg/mL; revision group, 1490 pg/mL; infection group, 10,140 pg/mL. Note log scale. The difference between the revision group and the infected group was statistically significant (p = 0.003).
Statistical summary of the test results ()
Sensitivity, specificity, and positive and negative predictive values provided by the pre- and perioperative tests were summarized for the inflammatory markers that differed significantly on comparison of the revision and infection groups (). As shown, sensitivity and specificity varied between the tests. We found no single marker that detected all infected patients. However, PMN infiltration of ≥ 5 cells/HPF appeared to be highly indicative of infection since none of the patients in the revision group showed such an infiltration.
Table 7. Statistical summary of the results of pre- and perioperative tests used to diagnose hip prosthesis infection
Discussion
The culture results showed that most of the patients in the infection group were infected by low-gradevirulent bacterial species. The predominance of such microbes is also reflected by the fact that the median lifetime of the prostheses in the infection group was relatively long (3 years). Hence, according to the Coventry (Citation1975) classification, the absolute majority of the infected patients in our study had late chronic infections. CoNS were the most common pathogens, which is in agreement with a review published by Lentino (Citation2004). Notably, we also observed that conventional bacterial culture on tissue samples revealed polymicrobial infections in 6 of the patients in the infection group. However, that level may be an underestimation, because it has been shown that detection of polymicrobial growth increases from up to 20% by conventional culture methods to 63% by extensive microbiological culture from an explanted prosthesis (Neut et al. Citation2003, Lentino Citation2004). As proposed by Gallo et al. (Citation2003), a symbiotic relationship between several bacterial species may be advantageous for the development of biofilms.
A surgical site infection after primary or revision hip replacement is a risk factor for development of a deep prosthesis infection (Wymenga et al. Citation1992), which is supported by our data. Nevertheless, it is a challenge to distinguish aseptic loosening of a hip prosthesis from septic loosening caused by lowvirulence bacteria, since these two conditions have similar symptoms. Contamination of the samples is most often caused by the same species as those infecting the prosthesis, as we found, which demonstrates how difficult it is to accurately interpret culture results. Accordingly, we recommend using 10 tissue samples for microbial culture: 5 aerobic and 5 anaerobic. The anaerobic culture is important not only to detect anaerobic microbes such as Propionibacterium, but also because anaerobic conditions seem to encourage the growth of some CoNS from periprosthetic tissue.
Preoperative tests that are consistently sensitive to and specific for low-grade hip infections are not easy to find, but El Esper et al. (2004) have reported on the good diagnostic value of bone marrow scintigraphy combined with leukocyte scintigraphy for detection of infected joint prostheses. Unfortunately, these techniques were not available during our study.
Significantly positive cultures together with the clinical evaluations by orthopedic surgeons were regarded as the “gold standard” for diagnosis of prosthetic joint infection in our study. Bernard et al. (Citation2004) retrospectively reviewed 230 patients with suspected prosthetic joint infections (hip or knee) and reviewed 33 articles dealing with preoperative evaluation of infection in prosthetic joints. They concluded that CRP and joint aspiration are the most useful tools to diagnose prosthetic joint infections, even in situations of chronic infection. We would have missed 4 of the 22 patients investigated in the infection group, if elevated CRP (> 10 mg/L) had been used as the only marker of infection. This CRP cut-off level gives a specificity of only 71%, which agrees with the results of Sanzén and Sundberg (1997), who stated that the diagnostic usefulness of CRP and ESR is limited in low-grade periprosthetic hip infections. 6 of the 56 evaluable patients in the revision group in our study had CRP > 10 mg/L.
Considering ESR, several investigators have used > 30 mm/h as the pathological cut-off, and at that level we would have missed 8 infected patients. Furthermore, 7 of the 55 evaluable patients in the revision group had ESR levels of > 30 mm/h. A combination of CRP and ESR analysis might help identify all infected patients, and that concept has already been tested by Sanzén and Sundberg (1997) and Spangehl et al. (Citation1999). In the latter study, all patients determined to have a periprosthetic infection (35 patients) had elevated levels of ESR (> 30 mm/h) or CRP (> 10 mg/L). That was not the case in our study, and we would have missed 2 patients even if we had used increased CRP (> 10 mg/L) or ESR (> 20 mm/h) as the criterion for suspected infection. However, ESR was elevated in all patients in the infection group who had negative cultures. We conclude that determination of levels of both CRP and ESR provides a valuable tool for detection of most, but not all, low-virulence periprosthetic infections.
The synovial fluid WBC count was significantly higher in patients with prosthetic joint infection than in those with aseptic failure, which is in accordance with the results of a recent study by Trampuz et al. (Citation2004). Using the same cut-off as these authors, we observed better sensitivity and specificity for WBC counts in synovial fluid than for measurement of CRP and ESR. Lactate dehydrogenase, or lactate, is an indicator of cell death and lysis, and elevated levels of these substances in synovial fluid can be due to infectious disorders or to inflammatory conditions caused by particulate cement debris, metallosis, polyethylene wear, or hypersensitivity allergic reactions (Messieh Citation1996). These findings explain our data showing increased lactate levels in all three groups of patients and no significant differences between the groups. We conclude that WBC counts in synovial fluid represent a valuable marker of infection, but that analysis of lactate levels is not suitable for distinguishing between aseptic and septic loosening of prostheses.
We found that PMN infiltration in tissue at a rate of ≥ 5 cells/HPF was highly indicative of infection, and Mirra et al. (Citation1976) and Spangehl et al. (Citation1999) have also used that level as a cut-off. Furthermore, we obtained positive results when we analyzed PMN infiltration in 3 of our infected patients with negative cultures. We conclude that histopathological analysis of PMN infiltration in periprosthetic tissue is the best method to distinguish between aseptic and septic loosening of hip prostheses. The usefulness of PMN infiltration is supported by the work of Pandey et al. (Citation1999) and Musso et al. (Citation2003). The latter authors recommended histology for all suspected cases of total joint infections.
Proinflammatory cytokines induce osteoclast activation and consequent bone resorption, osteolysis, and finally prosthetic loosening. It is believed that these cytokines are produced mainly by macrophages activated by phagocytosed particles and/ or wear debris and cyclic pressure (Glant et al. Citation1993, Horowitz and Gonzales Citation1996, McEvoy et al. Citation2002, Hirakawa et al. Citation2004, Wang et al. Citation2004). Different mechanisms of cell activation in aseptic loosening were recently reviewed by Sundfeldt et al. (Citation2006). Several research groups have detected substantial production of TNF-α, IL-1β, and IL-6 in interface tissue from patients with loosening of prostheses but no infection (Al-Saffar and Revell Citation1994, Chiba et al. Citation1994, Goodman et al. Citation1998). In agreement with this, we observed significantly higher levels of TNF-α in the group with loosened prostheses than in the subjects with osteoarthritis but no prosthesis, but we found no significant differences in the levels of IL-1β or IL-6 level. The results regarding TNF-α and IL-6 correspond well with the observations of Nivbrant et al. (Citation1999).
In the present study, the levels of all 3 cytokines analyzed (TNF-α, IL-1β, and IL-6) in synovial fluid were significantly higher in infected patients than in patients with aseptic loosening, although we consider the majority of infecting bacteria to be of low virulence. There were high levels of proin- flammatory cytokines in 2 of 3 tested patients with negative cultures and in 3 of 4 patients with low levels of both CRP and ESR. In as much as proin- flammatory cytokines such as TNF-α are critical components of osteoclastogenesis and bone resorption during osteolysis, the high cytokine levels we detected in infected patients can probably explain why loosening of prostheses progresses faster in such subjects than in patients with aseptic loosening. The generally higher level of proinflammatory cytokines in the infected patients might have been due to the fact that the bacteria-activated macrophages are able to upregulate their cytokine production even though they have already been stimulated by the loosened prosthesis. Another explanation might be the differences we observed in the types of cells infiltrating the periprosthetic tissue in the infected patients; or perhaps bacteria-neutrophil- macrophage interactions played a role (Zheng et al. Citation2004).
The limited systemic inflammatory response in infections of hip prostheses appears not to be due to failure of inflammatory cells to reach the infected area, but is more likely caused by an inadequate response to the infecting organisms. It is not known how long an occult bacterial infection on the surface of a prosthesis can last without provoking a host response, although a period of several years seems possible (Nguyen et al. Citation2002, Tunney et al. Citation1998), during which the process of periprosthetic osteolysis might maintain immunoincompetence—and also facilitate expansion of biofilm growth (Gallo et al. Citation2003).
We conclude that a combination of clinical judgment and carefully collected multiple tissue samples constitutes a good platform for distinguishing between septic and aseptic loosening of prostheses. Moreover, the combined use of several laboratory and histopathological markers of inflammation will significantly support clinicians in making adequate diagnoses. At present, the patterns of the cytokines considered in our study are primarily of interest for clarification of the pathogenesis of hip prosthesis infections, rather than for use as a discriminating test, although high levels of these substances suggest ongoing infection.
We are very grateful to the research nurses Lise-Lott Lindvall and Inger Eriksson for excellent technical assistance throughout the study, and to the staff of the medical record archives in Motala, Norrköping, and Linköping. Furthermore, we are indebted to Liv Gröntoft and Birgitta Frånlund for preparing the fixed biopsies for histopathological and immunohistochemical evaluation, and to Dr Florence Sjögren for the cytokine analyses. We also greatly appreciate the statistical advice given by Mats Fredriksson, Department of Occupational and Environmental Medicine. This work was supported by the County Council of Östergötland and the Faculty of Health Sciences of Linköping University.
No competing interests declared.
Contributions of authors
ÅN-A: study design, coordination of study, collection and analysis of data, putting data and references together, and writing of the manuscript; corresponding author. GB: study design, clinical aspects from the field of infectious diseases, and critical review of the manuscript. AH: histopathological study design, histopathological analysis and evaluation of specimens, and critical review. OL: expert in the field of cytokines, analysis of data, and critical review. OW: study design, clinical aspects from the field of orthopedic surgery, and critical review. LÖ: supervisor, initiation of the study, study design, and critical review.
- Al-Saffar N, Revell P A. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol 1994; 33: 309–16
- Atkins B L, Athanasou N, Deeks J J, Crook D W, Simpson H, Peto T E, McLardy-Smith P, Berendt A R. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol 1998; 36: 2932–9
- Augustinsson Å, Frydén A, Lindgren P-E, Stendahl O, Öhman L. Interaction of Staphylococcus epidermidis from infected hip prostheses with neutrophil granulocytes. Scand J Infect Dis 2001; 33: 408–12
- Bernard L, Lubbeke A, Stern R, Bru J P, Feron J M, Peyramond D, Denormandie P, Arvieux C, Chirouze C, Perronne C, Hoffmeyer P. Groupe D'Etude Sur L'Osteite. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review. Scand J Infect Dis 2004; 36: 410–6
- Chiba J, Schwendeman L J, Booth R E, Jr, Crossett L S, Rubash H E. A biochemical, histologic, and immunohistologic analysis of membranes obtained from failed cemented and cementless total knee arthroplasty. Clin Orthop 1994, 299: 114–24
- Coventry M B. Treatment of infections occurring in total hip surgery. Orthop Clin North Am 1975; 6: 991–1003
- Crowe J F, Sculco T P, Kahn B. Revision total hip arthroplasty: Hospital cost and reimbursement analysis. Clin Orthop 2003, 413: 175–82
- El Esper I, Blondet C, Moullart V, Saidi L, Havet E, Mertl P, Canarelli B, Schmit J-L, Meyer M-E. The usefulness of 99mTc sulfur colloid bone marrow scintigraphy combined with 111 In leucocyte scintigraphy in prosthetic joint infection. Nucl Med Commun 2004; 25: 171–5
- Gallo J, Kolar M, Novotny R, Rihakova P, Ticha V. Pathogenesis of prosthesis-related infection. Biomed Papers 2003; 147: 27–35
- Glant T T, Jacobs J J, Molnar G, Shanbhag A S, Valyon M, Galante J O. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res 1993; 8: 1071–9
- Goodman S B, Huie P, Song Y, Schurman D, Maloney W, Woolson S, Sibley R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg (Br) 1998; 80: 531–9
- Gristina A G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 1987; 237: 1588–95
- Gristina A G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Clin Orthop 2004, 427: 4–11, (Reprint)
- Hirakawa K, Jacobs J J, Urban R, Saito T. Mechanisms of failure of total hip replacements: lessons learned from retrieval studies. Clin Orthop 2004, 420: 10–7
- Horowitz S M, Gonzales J B. Inflammatory response to implant particulates in a macrophage/osteoblast coculture model. Calcif Tissue Int 1996; 59: 392–6
- Lentino J R. Infections associated with prosthetic knee and prosthetic hip. Curr Infect Dis Rep 2004; 6: 388–92
- Li S F, Henderson J, Dickman E, Darzynkiewicz R. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint?. Acad Emerg Med 2004; 11: 276–80
- McEvoy A, Jeyam M, Ferrier G, Evans C E, Andrew J G. Synergistic effect of particles and cyclic pressure on cytokine production in human monocyte/macrophages: proposed role in periprosthetic osteolysis. Bone 2002; 30: 171–7
- Messieh M. Synovial fluid levels of lactate dehydrogenase in patients with total knee arthroplasty. J Arthroplasty 1996; 11: 484–6
- Mirra J M, Amstutz H C, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop 1976, 117: 221–40
- Musso A D, Mohanty K, Spencer-Jones R. Role of frozen section histology in diagnosis of infection during revision arthroplasty. Postgrad Med J 2003; 79: 590–3
- Neut D, van Horn J R, van Kooten T G, van der Mei H C, Busscher H J. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop 2003, 413: 261–8
- Nguyen L L, Nelson C L, Saccente M, Smeltzer M S, Wassell D L, McLaren S G. Detecting bacterial colonization of implanted orthopaedic devices by ultrasonication. Clin Orthop 2002, 403: 29–37
- Nivbrant B, Karlsson K, Karrholm J. Cytokine levels in synovial fluid from hips with well-functioning or loose prostheses. J Bone Joint Surg (Br) 1999; 81: 163–6
- Pandey R, Drakoulakis E, Athanasou N A. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol 1999; 52: 118–23
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop 2005, 437: 41–7
- Sanzen L, Sundberg M. Periprosthetic low-grade hip infections. Erythrocyte sedimentation rate and C-reactive protein in 23 cases. Acta Orthop Scand 1997; 68: 461–5
- Spangehl M J, Maesri B A, O'Connell J X, Duncan C P. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg (Am) 1999; 81: 672–83
- Sundfeldt M, Carlsson L V, Johansson C B, Thomsen P, Gretzer C. Aspetic loosening, not only a question of wear. Acta Orthop 2006; 77: 177–97
- Trampuz A, Osmon D R, Hanssen A D, Steckelberg J M, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection. Clin Orthop 2003, 414: 69–88
- Trampuz A, Hanssen A D, Osmon D R, Mandrekar J, Steckelberg J M, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 2004; 117: 556–62
- Tunney M M, Patrick S, Gorman S P, Nixon J R, Anderson N, Davis R I, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem. J Bone Joint Surg (Br) 1998; 80: 568–72
- Wang M L, Sharkey P F, Tuan R S. Particle bioreactivity and wear-mediated osteolysis. J Arthroplasty 2004; 19: 1028–38
- Wymenga A B, van Horn J R, Theeuwes A, Muytjens H L, Slooff T J. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthop Scand 1992; 63: 665–71
- Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen- induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol 2004; 173: 6319–26
- Zimmerli W, Trampuz A, Ochsner P E. Prosthetic-joint infections. N Engl J Med 2004; 351: 1645–54