Abstract
Background There is evidence of high matrix metalloproteinase (MMP) activity around sutures inserted into tendons. This probably results in tissue breakdown, allowing the suture to cut through the tendon, and thus contributes to repair-site elongation and gap formation. We therefore hypothesized that treatment with the MMP inhibitor doxycycline would improve the sutureholding capacity of tendon.
Animals, methods and results In the first sub-study, rats received a suture in the Achilles tendon. One group was treated with systemic doxycycline and the other received no treatment. At 3, 5, and 7 days, suture-holding capacity was measured mechanically. The pull-out force and energy were reduced in all tendons, at 3 days compared to freshly inserted sutures, but no further reduction was detected at later time points. Doxycycline- treated tendons showed improved suture-holding capacity as measured by higher energy uptake than in untreated tendons. Force at failure showed a trend towards improvement. The effect was most evident on day 3. In the second sub-study, sutures were coated with doxycycline. At 3 days, local doxycycline treatment caused improved suture-holding capacity—as measured by higher force at failure and higher energy uptake.
Interpretation We provide proof of a novel treatment principle. MMP inhibitor-coated sutures improve suture-holding capacity during early repair of collagenous tissues.
Although elaborate suture techniques improve tensile strength in tendon repair, they all appear to result in reduced suture-holding capacity a couple of days after the operation. The tendon tissue around the suture seems to be weakened (McDowell et al. Citation2002), which helps the suture to cut through the tendon when exposed to tensile stress, i.e. during early mobilization. Studies on flexor or Achilles tendon repair have reported a re-rupture rate of 3–6% (Harris et al. Citation1999, Lynch Citation2004). Repairsite elongation and gap formation are much more common, resulting in compromised healing and with that, poorer functional outcome (Ejeskar and Irstam Citation1981, Seradge Citation1983, Gelberman et al. Citation1999).
Implantation of a foreign material into the tendon invariably evokes a tissue reaction, and there is evidence of elevated matrix metalloproteinase (MMP) activity at the tendon-suture interface (McDowell et al. Citation2002). These enzymes degrade the extracellular matrix, allowing the suture to cut through the tendon. An analogous reduction in suture-holding capacity can be seen in animal models of intestinal anastomoses, where treatment with MMP inhibitors can totally preserve breaking strength (Ågren et al. Citation2004). Immobilization of tendons may lead to an increase in the expression of MMPs (Arnoczky et al. Citation2004, Egerbacher et al. Citation2006). Thus, the weakening of tendons after injury, unloading, and suturing should mainly be the result of increased MMP activity. Consequently, inhibition of MMP activity could have important clinical applications in tendon and ligament surgery.
We investigated the effect of the MMP inhibitor doxycycline on suture pull-out strength in the early phase of Achilles tendon healing in the rat. It is probable that reduced suture-holding capacity is the result of excessive tissue breakdown. Thus, we hypothesized that doxycycline treatment would improve suture fixation. Firstly, the effect of systemic doxycycline was investigated; then, doxycycline was applied locally, by use of a novel method to immobilize substances on the surface of a suture.
Methods
Systemic doxycycline treatment
80 male Sprague Dawley rats (Scanbur BK, Stockholm, Sweden) with a mean weight of 409 (SD 25) g were used. The rats were randomized to two groups: doxycycline treatment and untreated control. All rats received a suture in the left Achilles tendon. The treatment group received doxycycline hyclate (Sigma-Aldrich, St. Louis, MO), 100 mg/ kg/day, administered in deionized drinking water, starting 1 day before the operation. This dosage gives a serum concentration in the rat approximately corresponding to that in humans given the standard dosage of 100 mg twice a day (Pasternak et al Citation2006). Control animals received deionized drinking water. Firstly, 20 rats were randomized in an initial experiment and killed after 3 days. Then 60 rats were randomized and killed 3, 5, and 7 days after the operation. The contralateral tendons of the day-3 control group were sutured immediately after killing, to give baseline values for freshly inserted sutures.
Local doxycycline treatment
Male Sprague Dawley rats with a mean weight of 374 (SD 20) g were used. All rats received a suture in the left Achilles tendon. The treatment group (n = 17) received doxycycline-coated sutures, while control animals (n = 16) received uncoated sutures. The rats were killed 3 days after the operation. Another group (n = 10) served as “freshly inserted” controls. In addition, the experiment was repeated. This time, control animals received carrier (fibrinogen)-coated sutures (n = 24), while the treatment group received doxycycline-coated sutures (n = 24). The rats were killed 3 days after the operation. Another group (n = 10) served as “freshly inserted” controls.
Suture coating procedure
Sterile 3-0 polybutester monofilament sutures (Novafil; Tyco Healthcare, Schaffhausen, Switzerland) were activated for 10 seconds on each side in a radiofrequency plasma chamber (Plasmaprep 100; Nanotech, Sweden). Thus, the sutures were exposed to a reactive gas plasma containing free electrons, gas radicals, and ions. This causes the surface polymer chains to become cleaved to shorter units, ionized, and radicalized, i.e. chemically activated. The activated sutures were incubated for 30 min in 6% glutaraldehyde in phosphate-buffered saline (PBS) at pH 9. The surfaces were rinsed extensively in PBS at pH 9. 10 layers of fibrinogen (Hyphen BioMed, Neuville-sur-Oise, France; molecular weight: 340 kDa, clotability 98%) were prepared as follows (Tengvall et al. Citation2003): the glutaraldehyde- coated sutures were incubated for 30 min in 1 mg/mL fibrinogen dissolved in PBS at pH 7.4. The sutures were rinsed extensively in PBS, followed by incubation for 30 min in PBS (pH 5.5) containing 0.2 M N-(3-dimethylaminopropyl)- N'-ethylcarbodiimide (EDC; Sigma-Aldrich) and 0.05 M N-hydroxy succinimide (NHS) (Sigma-Aldrich). Then a new 1 mg/mL fibrinogen solution was prepared in PBS buffer at pH 5.5, and the sutures were incubated in this solution for 30 min, rinsed in PBS buffer, and again incubated in the EDC/NHS solution. As the EDC solution is unstable under ambient conditions, new solutions were prepared every second hour. This procedure was repeated until 9 (experiment 1) or 11 (experiment 2) fibrinogen layers had been immobilized. The cross-linked fibrinogen surface was subsequently incubated in EDC/NHS as above, and for 3 h in a solution of 1 mg/mL doxycycline hyclate for 3 h in PBS (carrier-coated control sutures), and finally rinsed in distilled water.
The thicknesses of the fibrinogen and doxycycline layers on the sutures were measured by null ellipsometry (Auto-Ell III; Rudolph Research, Flanders, NJ) on a reference silicone surface in air, calculated according to the McCrackin (Citation1969) evaluation algorithm and converted into an approximate adsorbed amount per unit area by de Feijter's (Citation1978) formula. The assumed refractive index of 682 Acta Orthopaedica 2007; 78 (5): 680–686 the protein and immobilized doxycycline film (nf) was 1.465 (Benesch et al. Citation2000). During the measurements, a 1-nm-thick layer of adsorbed proteins was equivalent to 120 ng/cm2 approximately (Stenberg and Nygren Citation1983).
These aseptically prepared sutures were stored at room temperature in the dark, in PBS (pH 5.5) containing 1 mg/mL doxycycline, until they were used (within 1 week). Fibrinogen-coated control sutures were stored in PBS (pH 5.5) under identical conditions.
In vitro drug release from the suture
Doxycycline-coated polybutester sutures prepared as above were immersed in sterile physiological saline solution. The doxycycline concentrations in the solution at 24 h and 72 h were determined by way of an agar well diffusion assay (Smittskyddsinstitutet, Solna, Sweden) using Bacillus cereus ATCC 11778 as the test organism (Klassen and Edberg Citation1996). Measurements were made in triplicate. The results are expressed as percentage of the expected total amount of doxycycline on the thread. The total amount was calculated from ellipsometric measurements on similarly treated silicon surfaces.
Surgical procedure
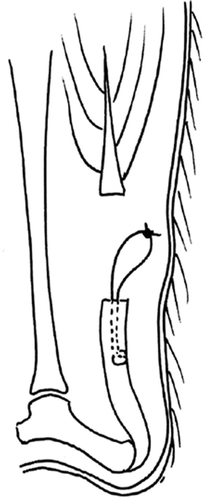
The surgeon was blinded as to treatment. Animals were anesthetized with isoflurane gas and given subcutaneous injections of 40 mg/kg body weight trimetoprim-sulfadoxine and 0.09 mg/kg body weight buprenorphine preoperatively. The skin was shaved and, under aseptic conditions, an oblique 1-cm skin incision was made over the left Achilles tendon. The tendon was dissected free and the polybutester suture with a tapered needle was inserted into the intact tendon to make a modified (one-sided) Kessler stitch spanning 1 cm longitudinally, starting at the tendon's proximal end 2 mm from the musculotendinous junction. Thereafter, the free ends of the thread were approximated with a double knot, leaving a 1-cm free loop for attachment during pull-out testing. The Achilles tendon was then cut transversely just proximal to the suture, to unload the tendon. Thus, the Kessler stitch was only inserted into the distal part of the Achilles tendon in order to specifically evaluate suture-holding capacity (). The suture was inserted into the tendon before transection to avoid unnecessary damage during handling. The plantaris tendon was cut and the skin was sutured. Animals were allowed free activity in their cages immediately after operation. The study was approved by the regional ethics committee for animal experimentation, and followed established guidelines.
Mechanical testing
Animals were killed by asphyxiation with CO2. The tendon with the attaching calcaneus was removed and dissected clean of surrounding tissue. The calcaneus was fixed in a custom-made clamp, while the suture loop was attached to a hook via a freely movable metal device to allow a straight pull. The complex was mounted in a materials testing machine (100 R; DDL Inc. Eden Praire, MN) and pulled at a constant speed of 0.1 mm/s until pull-out. Peak force and energy at a 10% drop of the curve were recorded.
Statistics
The hypothesis tested was that there would be a difference between the doxycycline-coated sutures and controls. The results of systemic and local treatment were analyzed separately using a twoway ANOVA, with doxycycline versus control as one independent factor. For systemic treatment, the other independent factor was the 4 groups (the first 3-day experiment, the second 3-day experiment, and the 5- and 7-day experiments). For local administration, the other independent factor was the first or the repeat experiment.
Results
Systemic treatment
Doxycycline-treated rats lost slightly more weight than control animals: mean 9 g and 4 g, respectively (p = 0.2). This was probably a result of drinking less because the water tasted of doxycycline. 5 doxycycline rats lost over 2 SD more weight than the average loss (i.e. they lost more than 37 g). One rat died postoperatively in the 5-day control group. In addition, 3 rats in the doxycycline group and 2 rats in the control group were excluded due to technical failure or due to the rats chewing on their wound.
There was no statistically significant difference in suture pull-out force between the time groups, but there was a trend towards improved fixation with doxycycline (). If the 5 rats with a large weight loss had been excluded from the analysis, the effect of doxycycline would have been significant (p = 0.02), but this exclusion was not done. The pull-out strength of freshly inserted control (day 0) sutures was 18 (SD 3.5) N. Mean overall pull-out strength in the 3- to 7-day controls was 3.5 N lower than in the day-0 controls.
Table 1. Systemic treatment with doxycycline. Force (N) at suture pull-out and energy uptake (Nmm) 3, 5, and 7 days after tendon suture. One 3 day-group was operated on at a separate occasion (a). The p-values refer to two-way ANOVA for the effect of treatment (b) and time (c).
Energy uptake was increased by doxycycline and there was a trend towards increased energy uptake over time (). Mean energy uptake of freshly inserted control (day 0) sutures was 106 (SD 28) Nmm. Mean overall energy uptake in the 3- to 7-day controls was 24 Nmm lower than in the day-0 controls.
Local treatment
There was no difference in average weight loss between doxycycline-treated rats and controls: 5.5 and 4 g, respectively (p = 0.4). 3 rats in the control group died postoperatively. 3 rats in the doxycycline group, 1 rat in the control group, and 1 rat in the immediate control group were excluded due to technical problems.
Force at suture pull-out was higher with doxycycline () and there was no difference between the two experiments (p = 0.3). Energy uptake was increased with doxycycline (), and showed a trend of a difference between the two experiments (p = 0.07). Controls in the first and repeat experiment were similar: the mean pull-out strength of freshly inserted control (day 0) sutures was 19 (SD 1.4) N and 18 (SD 3.8) N in the first and the repeat experiment, respectively. Mean energy uptake in freshly inserted control (day 0) sutures was 120 (SD 30) Nmm and 101 (SD 23) Nmm in the two experiments.
Table 2. Local doxycycline treatment using drug-coated sutures. Force (N) at suture pull-out and energy uptake (Nmm) 3 days after tendon suture. The p-values refer to the effect of treatment in a two-way ANOVA where the other factor was experiment 1 vs. experiment 2. The results were not significantly different between the two experiments. Controls were uncoated in experiment 1 and carrier (fibrinogen)-coated in experiment 2
Suture coating
By ellipsometry, we estimated that the total thickness of the fibrinogen layer was approximately 28 nm in the first experiment and approximately 50 nm in the repeat experiment. The thickness of the doxycycline layer was 4.5 and 2.5 nm, respectively. Thus, the amount of immobilized doxycycline was 300–540 ng/cm2. The diameter of the uncoated suture threads was 0.21 mm. Thus, 1 cm of the doxycycline-coated sutures carried about 20–36 ng of doxycycline.
In vitro drug release from the suture
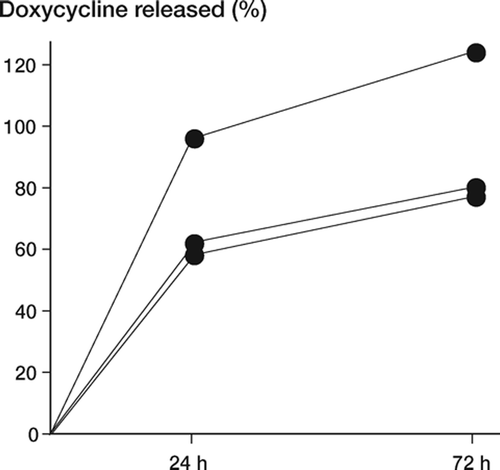
Three-quarters of the doxycycline dose delivered on the surface of the suture had been released after 24 h ().
Discussion
A decrease in suture-holding capacity increases the risk of gap formation after tendon suturing, which may result in poorer healing—or even rupture (Ejeskar and Irstam Citation1981, Seradge Citation1983, Gelberman et al. Citation1999). Good suture fixation would allow early rehabilitation programs with positive effects in terms of mechanical stimulation, leading to quicker restoration of tendon tensile strength (Murrell et al. Citation1994) and prevention of adhesion formation (Zhao et al. Citation2002). Our study indicates that tendon suture fixation can be improved by treatment with an MMP inhibitor. In our rat model, there is little spontaneous deterioration in the properties of tendon. This means a small margin for improvement, and thus low statistical power. Even so, we have been able to demonstrate that in our rat model the MMP inhibitor doxycycline improves suture holding capacity, even when delivered locally. This shows two important principles, both of which may have clinical implications. Firstly, inhibition of degradative processes might serve as a way of optimizing surgical repair when biomaterials are used. Previous studies using bisphophonates have suggested that this approach can be successful in improving bone screw fixation (Tengvall et al. Citation2004). Secondly, only minute doses of enzyme inhibitors are needed. These inhibitors can be applied locally via the suture, avoiding unnecessary systemic effects.
1 cm of doxycycline-coated 3-0 suture carries about 30 ng of doxycycline. This dose seems to be sufficient to affect suture-holding capacity. Thus, it appears as if the tissue degradation leading to reduced suture-holding capacity is a localized phenomenon, restricted to the immediate vicinity of the sutures.
We immobilized doxycycline onto a relatively thick cross-linked fibrinogen matrix. This protein can be replaced by other suitable carriers, such as other proteins, proteoglycans, and synthetic polymers. Fibrinogen is advantageous due to its low immunogenicity and the ease of EDC/NHS activation of its side-groups carrying –COOH groups.
We used the MMP inhibitor doxycycline, which is the most potent amongst the clinically approved tetracyclines. Some synthetic MMP inhibitors are more powerful, and may be of further benefit. No analysis was performed to confirm that the doxycycline acted through MMP inhibition. This is a main weakness of our study. However, it is now well established that doxycycline functions as an MMP inhibitor. Until proven otherwise, the improvement of suture fixation can be considered an effect of MMP inhibition, since this is regarded as the main non-antibiotic mechanism of action of doxycycline. This is supported by the notions that MMP concentrations are elevated at the tendonsuture interface and that the pull-out strength of sutures is concomitantly reduced during the early phase of healing (Hatanaka et al. Citation2000, Wada et al. Citation2001, McDowell et al. Citation2002). Further support can be found in animal models of colon anastomoses, where breaking strength is thought to be a measure of suture-holding capacity. Treatment with MMP inhibitors such as doxycycline diminishes the reduction in colon breaking strength, which is most evident 3 days postoperatively (Syk et al. Citation2001, Siemonsma et al. Citation2003). Nonetheless, further studies are required to establish the exact mechanism of action and the most efficacious drug to be delivered locally at the tendon-suture interface.
Most studies evaluating tendon suture fixation in vivo have been performed on large animals. Here, we have presented a simple model for measurement of tendon suture fixation in the rat. After 3 days, the suture pull-out strength was reduced by a quarter. This is slightly earlier than what has been shown previously in dog flexor tendons with passive mobilization, but with similar magnitude (Hatanaka et al. Citation2000). The dogs showed a 10% decrease at 3 days and an 18% decrease at 7 days, with no further deterioration. Another study performed on immobilized dog tendons showed a 45% decrease in ultimate strength and a 41% reduction in gap strength after 7 days (Wada et al. Citation2001). In addition, a recent report concerning unloaded Achilles tendons in rabbits showed a 70% reduction in ultimate strength at 7 days (Yildrim et al. Citation2006). Since the tendons are unloaded in our model, we would have expected a greater decrease in mechanical parameters, but this did not happen. This highlights another weakness of this study: the low statistical power. Nevertheless, using groups of sufficient size, differences were apparent. It was not practical to use side comparisons, because each side difference would carry the variability of two measurements. The model was limited to evaluation of mechanical parameters in suture pull-out testing and was not used to address the issue of gapping or tendon callus elongation.
This work was supported by the strategic research programme Materials in Medicine, Linköping, and by a grant from the Swedish Research Council (2031). The authors are grateful to Mårten Fellenius for technical assistance and to Helen Lundqvist Gustafsson for invaluable help during suture coating method development.
Contributions of authors
BP: planned the study, did the laboratory work, and prepared the manuscript. AM: helped plan the study, did the laboratory work, and helped write the manuscript. AA: did some of the laboratory work and planning. PT: invented the immobilization technique, planned the study, and helped write the manuscript. PA: planned the study, analyzed the data, prepared the manuscript, and supervised the study.
PA, PT, and BP have sold the idea of immobilization of drugs on biomaterials to an independent biomedical investment company. This company has now patented the idea.
- Ågren M S, Jorgensen L N, Delaisse J M. Matrix metalloproteinases and colon anastomosis repair: a new indication for pharmacological inhibition?. Mini Rev Med Chem 2004; 4: 769–78
- Arnoczky S P, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res 2004; 22: 328–33
- Benesch J, Askendal A, Tengvall P. Quantification of adsorbed human serum albumin at solid interfaces: a comparison between radioimmunoassay (RIA) and simple null ellipsometry. Coll Surf B: Biointerfaces 2000; 18: 71–81
- de Feijter J A, Benjamins J, Veer F A. Ellipsometry as a tool to study the adsorption of synthetic and biopolymers at the air-water interface. Biopolymers 1978; 17: 1759–73
- Egerbacher M, Arnoczky S P, Gardner K, Caballero O, Gartner J. Stress-deprivation of tendons results in alterations in the integrin profile and pericellular matrix of tendon cells. Transactions of the ORS 2006, Paper No 1100
- Ejeskar A, Irstam L. Elongation in profundus tendon repair. A clinical and radiological study. Scand J Plast Reconstr Surg 1981; 15: 61–8
- Gelberman R H, Boyer M I, Brodt M D, Winters S C, Silva M J. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons: an experimental study on the early stages of tendon healing in dogs. J Bone Joint Surg (Am) 1999; 81: 975–82
- Harris S B, Harris D, Foster A J, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg Br 1999; 24: 275–80
- Hatanaka H, Zhang J, Manske P R. An in vivo study of locking and grasping techniques using a passive mobilization protocol in experimental animals. J Hand Surg Am 2000; 25: 260–9
- Klassen M, Edberg S C. Measurement of antibiotics in human body fluids: Techniques and significance. Antibiotics in Laboratory Medicine 4th, V Lorian. Williams & Wilkins, New York 1996
- Lynch R M. Achilles tendon rupture: surgical versus nonsurgical treatment. Accid Emerg Nurs 2004; 12: 149–58
- McCrackin F L. A FORTAN Program for the Analysis of Ellipsometer Measurements. NBS Technical No. Washington DC, 1969; 479
- McDowell C L, Marqueen T J, Yager D, Owen J, Wayne J S. Characterization of the tensile properties and histologic/ biochemical changes in normal chicken tendon at the site of suture insertion. J Hand Surg Am 2002; 27: 605–14
- Murrell G A, Lilly E G, 3rd, Goldner R D, Seaber A V, Best T M. Effects of immobilization on Achilles tendon healing in a rat model. J Orthop Res 1994; 12: 582–91
- Pasternak B, Fellenius M, Aspenberg P. Doxycycline impairs tendon repair in rats. Acta Orthop Belg 2006; 72: 756–60
- Seradge H. Elongation of the repair configuration following flexor tendon repair. J Hand Surg Am 1983; 8: 182–5
- Siemonsma M A, de Hingh I H, de Man B M, Lomme R M, Verhofstad A A, Hendriks T. Doxycycline improves wound strength after intestinal anastomosis in the rat. Surgery 2003; 133: 268–76
- Stenberg M, Nygren H. The use of the isoscope ellipsometer in the study of adsorbed proteins and biospecific binding reactions. J dé Physique 1983; C10(S12)83–6
- Syk I, Agren M S, Adawi D, Jeppsson B. Inhibition of matrix metalloproteinases enhances breaking strength of colonic anastomoses in an experimental model. Br J Surg 2001; 88: 228–34
- Tengvall P, Jansson E, Askendal A, Thomsen P, Gretzer C. Preparation of multilayer plasma protein films on silicon by EDC/NHS coupling chemistry. Colloids Surf B Biointerfaces 2003; 28: 261–72
- Tengvall P, Skoglund B, Askendal A, Aspenberg P. Surface immobilized bisphosphonate improves stainless-steel screw fixation in rats. Biomaterials 2004; 25: 2133–8
- Wada A, Kubota H, Miyanishi K, Hatanaka H, Miura H, Iwamoto Y. Comparison of postoperative early active mobilization and immobilization in vivo utilising a four-strand flexor tendon repair. J Hand Surg Br 2001; 26: 301–6
- Yildirim Y, Kara H, Cabukoglu C, Esemenli T. Suture holding capacity of the Achilles tendon during the healing period: an in vivo experimental study in rabbits. Foot Ankle Int 2006; 27: 121–4
- Zhao C, Amadio P C, Momose T, Couvreur P, Zobitz M E, An K N. Effect of synergistic wrist motion on adhesion formation after repair of partial flexor profundus tendon lacerations in a canine model in vivo. J Bone Joint Surg (Am) 2002; 84: 78–84