Abstract
Background Giant cell tumors of bone rarely metasta-size but often recur locally after surgery. There is limited knowledge about the risk of recurrence related to different types of treatment.
Patients and methods We analyzed factors affecting the local recurrence rate in 294 patients with giant cell tumors of the extremities using prospectively collected material from 13 centers. The median follow-up time was 5 (0.2–18) years.
Results A local recurrence was diagnosed in 57 of 294 patients (19%). The overall 5-year local recurrence rate was 0.22. Univariate analysis identified young age and intralesional surgery to be associated with a higher risk of recurrence. Based on multivariate analysis, the relative risk was 2.4-fold for intralesional surgery compared to more extensive operative methods. There was no correlation between tumor size, tumor extension, sex of the patient, tumor location, or fracture at diagnosis and outcome. In the subgroup of 200 patients treated with intralesional surgery, the method of filling (cement or bone) was known for 194 patients and was statistically highly significant in favor of the use of cement.
Interpretation Intralesional surgery should be the first choice in most giant cell tumors, even in the presence of a pathological fracture. After thorough evacuation, the cavity should be filled with cement.
Giant cell tumors of bone (GCT) are locally aggressive tumors with unpredictable behavior. They tend to recur locally and they can even metastasize, although rarely. Different classifications based on histology (Jaffe et al. Citation1940) and clinical and radiological appearance (Campanacci et al. Citation1987, Enneking et al. Citation2003) have been put forward, but they provide little prognostic information regarding the risk of local recurrence.
GCTs are usually lytic lesions located in the epiphysis of long bones in young adults, mostly around the knee. The tumor may involve the sub-chondral bone but rarely the joint cartilage. The tumors are often complicated by a pathological fracture, which can involve the articular surface. Intralesional curettage has been the standard method of treatment, but is associated with a relatively high risk of local recurrence. Different means of improving the effect of curettage are known, e.g. phenol, freezing, and using a highspeed burr to remove peritumoral bone. Filling of the cavity with bone cement allows immediate weight bearing and the heat induced during the setting of the cement has been claimed to kill the remaining tumor cells, thus reducing the risk of recurrence. En bloc resections with a marginal or wide margin entail a lower risk of local recurrence but—except for the proximal fibula and distal ulna—necessitate major articular reconstruction with loss of function and the risk of subsequent revision of the reconstruction (McDonald et al. Citation1986, Mankin and Hornicek Citation2005).
Basic textbook information is that after evacuation, the cavity can be filled either with (allograft) bone or cement (Heck Citation2003, Szendroi Citation2004, Turcotte Citation2006). Recommendations for treatment are based on results from retrospective analyses of non-randomized series from single or multiple institutions. This study was based on a non-randomized, prospectively collected series of GCT patients reported to the Scandinavian Sarcoma Group (SSG) Register from 1986 through 2003. The aim was to correlate clinical features and features of treatment to local recurrence, to form a basis for treatment recommendations.
Patients and methods
304 patients with GCTs of the extremities were reported to the Register from 1986 through 2003. After excluding 10 patients due to missing follow-up data, there remained 294 patients from 13 Scandinavian hospitals: 42 from Denmark, 39 from Finland, 65 from Norway, and 148 from Sweden, with a median follow-up time of 5 (0.2–18) years.
To ensure accuracy, the data reported were verified and corrected retrospectively as required, by one of the orthopedic authors after review of patient files at the participating hospitals. The histological and radiological signs were interpreted by experts in bone tumor diagnostics in individual centers.
The standard method of classifying resection margins in bone involves 4 types: intralesional, marginal, wide, and radical. This classification was used initially because the SSG data forms were used. Basically, in GCT one chooses between intralesional/intracavital surgery or wider surgery with the purpose of removing the tumor en bloc. With malignant bone tumors, the real problem is to define the difference between marginal and wide. Thus, in this study all the other 3 groups (i.e. marginal, wide, and radical) were regrouped into one single group termed “wider”. “Wider” or “all other” in this study means basically en bloc removal.
Statistics
The relationship between local recurrence and the clinical features and features of treatment was assessed by univariate analysis. Factors with significant correlation (p < 0.05) to the outcome were entered into a multivariate Cox regression analysis. Patient age and tumor size were used as continuous variables; sex, tumor location, fracture at diagnosis, margin (intralesional/larger), method of reconstruction (cement vs. other), and tumor extension were used as dichotomous variables. No lower age limit was set as the age distribution was a regular bell-shaped distribution. Local recurrence-free survival was estimated with the Kaplan-Meier method, which also takes into account those patients whose follow-up time, for any reason, remained short. All analyses were done with the SPSS version 11 software for Macintosh OS X.
Results
The mean age at diagnosis was 35 (9–83) years. There was a slight predominance of male patients (53%). More than two-thirds of the tumors were in the lower extremity. 109 tumors were in the femur, 75 in the tibia, 24 in the fibula, 17 in the humerus or scapula, 42 in the radius or ulna, and 27 in the small bones of the hand or foot.
The median tumor size as determined from radiographs or MR images was 5 (1–16) cm. 43% of the tumors had an extraosseus extension. Tumor extension was graded as extraosseous when there was no cortex left in the bulging area, so that the tumor was in direct contact with soft tissue such as periosteum or fascia adjacent to the original bone. Fracture at presentation was reported in 21% of the patients.
All patients were treated operatively. The reported surgical margin was intralesional in 68% of cases, and wider in 31%. In 2 patients, the margin could not be assessed.
The most common techniques of reconstruction were cementation in 52% of cases (), autologous bone in 17%, and allograft bone in 5% ().
Figure 1. A. Giant cell tumor of the distal femur, treated with evacuation and cement filling. B. After 11 years, there were no signs of recurrence and no deleterious effects of the cement.
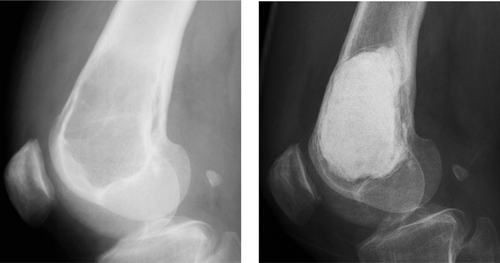
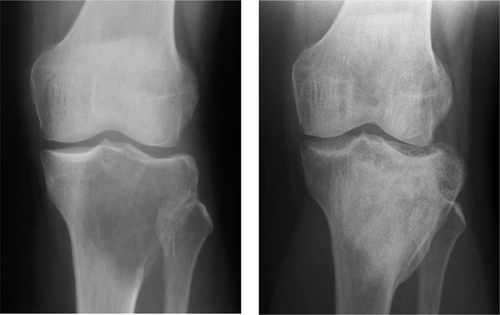
Figure 2. A. Giant cell tumor of the proximal tibia filled with autologous and allogeneous bone. B. After 6 years, there were no signs of recurrence and there was good consolidation.
Reconstruction with endoprosthesis was performed in 8% of cases. In 14% of the cases no reconstruction was done, e.g. after resection of small bones of the hand and foot, fibula and ulna, and in 4% of cases the method was unknown.
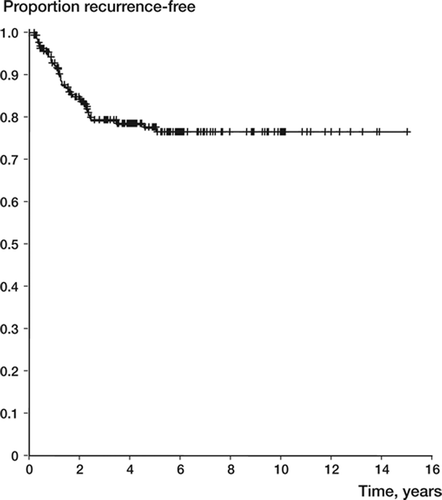
57 of the 294 patients (19%) had a local recurrence diagnosed between 2 months and 5 years after surgery. Three-quarters of the recurrences appeared within 2 years after surgery, and none after 5 years (). No patient below the age of 20 years or over 40 years had a recurrence after two years. By univariate analysis, patient age and surgical margin were prognostic of local recurrence (). Younger patients had a slightly increased risk of local recurrence (with an annual reduction of around 2%). Intralesional surgery was associated with a local recurrence rate of 0.27, as compared to 0.12 (p = 0.007) for wider surgery ().
Figure 4. Recurrence-free survival by margin in 292 giant cell tumors of the extremities (upper line: wider; lower line: intralesional surgery).
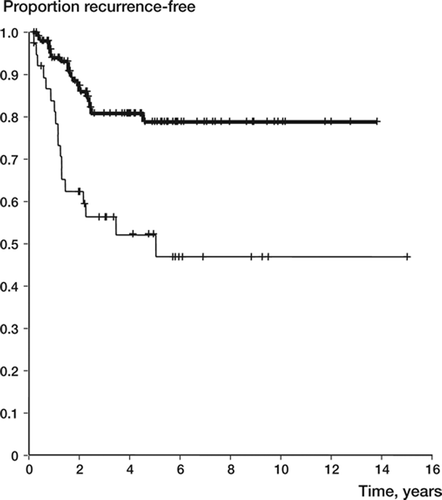
Table 1. Comparison of clinical features and features of treatment with local recurrence rates
In order to remove the effect of possible con-founders, we performed a multivariate analysis including the two factors that were statistically significant in univariate analysis and also fracture, which was close to significance. The risk ratios for age and surgical margin did not change substantially, indicating that these associations were probably not due to confounding factors (). The most significant factor, intralesional surgical margin, implied a 2.5-fold risk of local recurrence.
Table 2. Univariate and multivariate analyses of risk factors for recurrence (n = 294)
In 194 patients treated with intralesional surgery, the method of filling the cavity was known. The recurrence rate was 0.20 for filling with cement and 0.56 for intralesional surgery without cementation (p = 0.001) ( and ).
Figure 5. Recurrence-free survival after intralesional surgery (filling with cement or bone) in 194 giant cell tumors of the extremities (upper line: with cement; lower line: without cement).
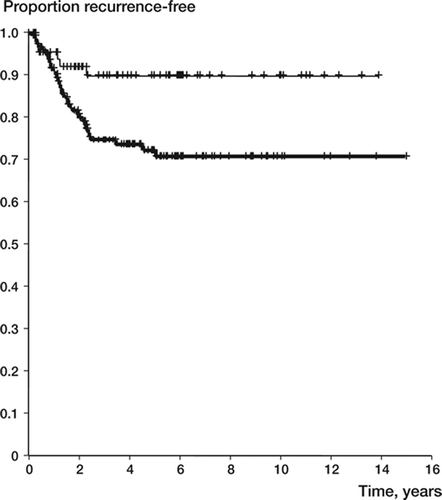
Table 3. Intralesional surgery: univariate analysis of cavity filling with cement vs. other
The local recurrence rate in the 27 patients who were operated with intralesional margin and filling of the cavity with bone was 0.61 (p < 0.001 as compared to cement).
Discussion
Giant cell tumors rarely metastasize but are locally aggressive. For that reason, the absence of local recurrence—rather than patient survival—is the major criterion used to assess the adequacy of treatment. Few clinical, radiographic, histological, or biological factors have been identified to date that are predictive of the clinical course in patients with GCT.
In this series, the prognostic effects of age and of the operative margin were the most important in the whole series. In the subgroup of patients treated with intralesional surgery, the method of cavity filling was the most important. The results are comparable to those of the few large, published series. Our observations on the influence of age and method of filling the cavity are new.
Campanacci et al. (Citation1987) reported a local recurrence rate of 22% after intralesional excisions and 8% after marginal excisions in 273 procedures, and 0% in 58 wide or radical procedures in a material of 280 patients with 331 surgical procedures. Ghert et al. (Citation2002) found a recurrence rate of 13% in their series of 75 patients who were followed up for at least 2 years and treated mostly with intralesional curettage, high-speed burring, and adjuvant. In their series no patient factor, tumor characteristic, or surgical practice variable showed any correlation to local recurrence.
In the Canadian multicenter study by Turcotte et al. (Citation2002) involving 186 patients, displaced fracture was shown to be a prognostic factor. 30% of the patients presented with a pathological fracture and the overall recurrence rate was 17% after a minimum follow-up of 2 years. The adjuvant method (e.g. burring) or the filling material was not statistically significantly associated with the risk of recurrence. Functional results were poorer in patients who sustained a displaced fracture.
In the series of McGough et al. (Citation2005) involving 183 patients, all of whom were treated with intralesional surgery, the recurrence rate was 25% (45/183). Incomplete initial surgery, a delay in diagnosis of the recurrence of greater than 6 months, and subchondral recurrence of the tumor were contributory factors in failure to salvage the joint.
In the classic series of 53 giant cell tumors, most of them treated with curettage and autologous bone transplantation, the recurrence rate was 42% and the tumors around the knee (distal femur and proximal tibia) were highly recurrent (Larsson et al. Citation1975). It is generally agreed that there is a high risk of local recurrence with curettage only—with or without bone grafting (Larsson et al. Citation1975, Campanacci et al. Citation1987, Blackley et al. Citation1999). However, some authors have found curettage without adjuvant treatment or filling material to be the method of choice in intraosseous giant cell tumors (Prosser et al. Citation2005).
The overall risk of local recurrence of all giant cell tumors is 25–35% in older series and 10–20% in recent series (Sung et al. Citation1982, McDonald et al. Citation1986, O'Donnellet al. Citation1994, Blackley et al. Citation1999, Ghert et al. Citation2002, Turcotte et al. Citation2002, Saiz et al. Citation2004, Lackman et al. Citation2005). In our series the observed recurrence rate was 0.22, with 19% of patients having a recurrence. Better results have been reported from single institutions, mainly after cryotreatment (Malawer et al. Citation1999) or high-speed-burring and bone grafting (Blackley et al. Citation1999). Cryotreatment is demanding and complicated with fractures. In the high-speed-burring series by Blackley, the thoroughness of curettage is emphasized as being a main factor for a low local recurrence risk. Yip et al. (Citation1996) had a recurrence rate of 5% in their series of 44 patients, and no adjuvant therapy was used. Bini et al. (Citation1995) reported 8% recurrence in a series of 38 patients.
The recurrence rate of 19% found in the present study is not the lowest rate reported in the literature. This is a long-term series from different institutions, and the best results reported in the literature are usually from one center and taken over a limited time period. There is a trend toward centralizing the treatment to fewer centers.
According to the literature, the frequency of pathological fracture is 9–30% (Campanacci et al. Citation1987, Blackley et al. Citation1999, Ghert et al. Citation2002, Turcotte et al. Citation2002, Jeys et al. Citation2006). Campanacci had 9% fractures in 1987, as assessed by plain radiographs. In our series the higher value of 21% could be due to a more accurate level of diagnosis with MR imaging. The risk of local recurrence was increased in the series of Dreinhofer et al. (Citation1995) when there was a fracture, and many authors have recommended refraining from intralesional surgery in cases of fracture. In our series, the probability of a fracture being a negative prognostic sign was close to being statistically significant. There might also be a difference between fracture line and dislocated fracture, which is a topic for further study.
En bloc resection is indicated for the proximal fibula and distal ulna because of dispensable bones, and for the distal radius because of a high risk of recurrence (Szendroi Citation2004). In primary lesions of the distal radius, good results have also been achieved with intralesional procedures in selected cases (Cheng et al. Citation2001). In cases where the articular surface is grossly damaged, initial resection should be considered—e.g. when there is a dislocated intraarticular fracture (Ghert et al. Citation2002, Ward and Li Citation2002). A second or severalfold recurrence often necessitates en bloc resection.
Several local adjuvants have been proposed to kill the remaining tumor cells in the cavity after tumor curettage, e.g. phenol, alcohol, sterile water, heat, freezing, zinc, and hydrogen peroxide (Nicholson et al. Citation1998, Malawer et al. Citation1999, Trieb et al. Citation2001, Ghert et al. Citation2002, Ward and Li Citation2002, Haskell et al. Citation2003, Zhen et al. Citation2004). Recently, argon laser therapy has been added to the list (Lewis et al. Citation2007). None of these methods were used extensively in the present series and their indications remain unclear.
After tumor evacuation, the cavity can be left unfilled (Prosser et al. Citation2005) or it can be filled with cement, bone, or bone substitute. Cement has several advantages: it hardens quickly, the heating effect may destroy remaining cells in the cavity walls, and recurrences are easier to detect early, which makes local recurettage and recementation possible. The sign that has been described is lysis or nondevelopment of the sclerotic rim adjacent to the radiolucent zone between the cement and the can-cellous bone (Pettersson et al. Citation1986, Remedios et al. Citation1997). Cement can be combined with bone, which is first impacted right under the bare cartilaginous surface to increase the endurance of the cartilage. The rationale is to reduce the risk of damage to the cartilage, but this has not been shown (Blackley et al. Citation1999). However, there seems to be a minor risk of cartilage damage even with cement close to the cartilage (Vult von Steyern et al. Citation2007).
To our knowledge, there have been no studies showing the effects of different methods of filling the cavity. In our study, cement had a significant advantage compared to bone grafting after intralesional surgery.
The vast majority of local recurrences in this series occurred within 3 years, and there were none after five years. Similar results have already been published (Ghert et al. Citation2002, Haskell et al. Citation2003). Thus, we do not advocate patient follow-up after 5 years of recurrence-free observation.
GCTs can also produce metastases, the risk being around 3% (Turcotte et al. Citation2002, Siebenrock et al. Citation1998). In this material the number of metastases was 5 (1.7%). The occurrence of multicentric tumors is less than 1% of all giant cell tumor cases (Hoch et al. Citation2006). Surprisingly, no correlation could be found between the size or the extension of the tumor and the outcome, a correlation so often encountered with malignant tumors.
En bloc resections, the use of adjuvant therapies, and bone cement have been associated with reduced risk of local recurrence in selected series (Ghert et al. Citation2002). In our study, surgical margins were the most significant factors predicting eventual recurrence.
The inherently greater risk of local recurrence after curettage should be weighed against the possibility to treat a recurrence successfully, and the functional deficiency and later risks associated with large resections. As recurrences can usually be treated successfully (Vult von Steyern et al. Citation2006), intralesional surgery is preferable. In intralesional surgery, we strongly recommend the use of cement. As seen from , cement appears to be a long-lasting substitute for bone—even after 11 years of follow-up.
Contributions of authors
All the authors were involved in the treatment and analysis of the patients in individual centers. AK performed data analysis and wrote the paper, CB also performed data analysis, and GF reviewed the accuracy of the data.
- Bini S A, Gill K, Johnston J O. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop 1995, 321: 245–50
- Blackley H R, Wunder J S, Davis A M. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg (Am) 1999; 81: 811–20
- Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg (Am) 1987; 69: 104–14
- Cheng C Y, Shih H N, Hsu K Y, Hsu R W. Treatment of giant cell tumor of the distal radius. Clin Orthop 2001, 383: 221–8
- Dreinhofer K E, Rydholm A, Bauer H C, Kreicbergs A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. J Bone Joint Surg (Br) 1995; 77: 183–93
- Enneking W F, Spanier S S, Goodman M A. The classic. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 2003, 415: 4–18
- Ghert M A, Rizzo M, Harrelson J M, Scully S P. Giant-cell tumor of the appendicular skeleton. Clin Orthop 2002, 400: 201–10
- Haskell A, Wodowoz O, Johnston J O. Metachronous mul-ticentric giant cell tumor: a case report and literature review. Clin Orthop 2003, 412: 162–8
- Heck R K. Benign occasionally agrressive tumors of bone. Campbell's operative orthopedics, S Terry Canale, 2003; 813–7
- Hoch B, Inwards C, Sundaram M, Rosenberg A E. Multi-centric giant cell tumor of bone. Clinicopathologic analysis of thirty cases. J Bone Joint Surg (Am) 2006; 88(9)1998–2008
- Jaffe H L, Lichtenstein L, Portis R B. Giant cell tumor of bone: Its pathologic appearance, grading, supposed variants and treatment. Arch Pathol 1940; 30: 993–1031
- Jeys L M, Suneja R, Chami G, Grimer R J, Carter S R, Till-man R M. Impending fractures in giant cell tumours of the distal femur: incidence and outcome. Int Orthop 2006; 30(2)135–8
- Lackman R D, Hosalkar H S, Ogilvie C M, Torbert J T, Fox E J. Intralesional curettage for grades II and III giant cell tumors of bone. Clin Orthop 2005, 438: 123–7
- Larsson S E, Lorentzon R, Boquist L. Giant-cell tumor of bone. A demographic, clinical and histopahtological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg (Am) 1975; 57: 167–73
- Lewis V O, Wei A, Mendoza T, Primus F, Peabody T, Simon M A. Argon beam coagulation as an adjuvant for local ontrol of giant cell tumor. Clin Orthop 2007, 454: 192–7
- Malawer M M, Bickels J, Meller I. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin Orthop 1999, 359: 176–88
- Mankin H J, Hornicek F J. Treatment of giant cell tumors with allograft transplants: A 30-year study. Clin Orthop 2005, 439: 144–50
- McDonald D J, Sim F H, McLeod R A, Dahlin D C. Giant-cell tumor of bone. J Bone Joint Surg (Am) 1986; 68: 235–42
- McGough R L, Rutledge J, Lewis V O, Lin P P, Yasko A W. Impact severity of local recurrence in giant cell tumor of bone. Clin Orthop 2005, 438: 116–22
- Nicholson N C, Ramp W K, Kneisl J S, Kaysinger K K. Hydrogen peroxide inhibits giant cell tumor and osteo-blast metabolism in vitro. Clin Orthop 1998, 347: 250–60
- O'Donnell R J, Springfield D S, Motwani H K. Recurrence of giant cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg (Am) 1994; 76: 1827–33
- Pettersson H, Rydholm A, Persson B. Early radiologic detection of local recurrence after curettage and acrylic cementation of giant cell tumours. Eur J Radiol 1986; 6: 1–4
- Prosser G H, Baloch K G, Tillman R M, Carter S R, Grimer R J. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone?. Clin Orthop 2005, 435: 211–8
- Remedios D, Saifuddin A, Pringle J. Radiological and clinical recurrence of giant-cell tumour of bone after the use of cement. J Bone Joint Surg (Br) 1997; 79: 26–30
- Saiz P, Virkus W, Piasecki P. Results of giant cell tumor of bone treated with intralesional excision. Clin Orthop 2004, 424: 221–6
- Siebenrock K A, Unni K K, Rock M G. Giant-cell tumor of bone metastasizing to the lungs. A long-term follow-up. J Bone Joint Surg (Br) 1998; 80: 43–7
- Sung H W, Kuo D P, Shu W P. Giant-cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg (Am) 1982; 64: 755–61
- Szendroi M. Giant-cell tumour of bone. Review. J Bone Joint Surg (Br) 2004; 86(1)5–12
- Trieb K, Bitzan P, Lang S, Dominkus M, Kotz R. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur J Surg Oncol 2001; 27: 200–2
- Turcotte R E. Giant cell tumor of bone. Review. Orthop Clin North Am 2006; 37(1)35–51
- Turcotte R E, Wunder J S, Isler M H. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop 2002, 397: 248–58
- Ward W G, Li G. Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop 2002, 397: 259–70
- Vult von Steyern F, Bauer H C, Trovik C, Kivioja A, Bergh P, Holmberg Jorgensen P, Folleras G, Rydholm A. Scandinavian Sarcoma Group. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing. A Scandinavian Sarcoma Group study. J Bone Joint Surg (Br) 2006; 88: 531–5
- Vult von Steyern F, Kristiansson I, Jonsson K, Mannfolk P, Heinegård D, Rydholm A. Giant-cell tumour of the knee: The condition of the cartilage after treatment by curettage and cementing. J Bone Joint Surg (Br) 2007; 89: 361–5
- Yip K M H, Leung P C, Kumta S M. Giant cell tumor of bone. Clin Orthop 1996, 323: 60–64
- Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J Bone Joint Surg (Br) 2004; 86(2)212–6