Abstract
Background Aseptic loosening is the most important complication after total hip arthroplasty (THA). The nervous system has been implicated in the etiology and pathogenesis of joint diseases.
Methods We compared levels of substance P (SP) and calcitonin gene‐related peptide (CGRP) in pseudosynovial fluid from patients with aseptic loosening after THA with those in synovial fluid from patients undergoing primary THA for osteoarthritis, who served as controls. Levels of SP and CGRP were measured using an enzyme immunoassay.
Results We found that SP and CGRP levels were significantly higher in the pseudosynovial fluid of loose artificial joints than in the synovial fluid of controls.
Interpretation SP and CGRP may have a role in aseptic loosening.
The most frequent mode of failure after arthroplasty is aseptic loosening due to periprosthetic osteolysis. The pathogenesis of prosthetic loosening is still unknown. Wear debris has been extensively studied as a cause of osteolysis. Prior work has shown a relationship between the generation of particles and aseptic loosening. Cytokines that have been demonstrated in the periprosthetic tissues include tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6, and receptor activator of nuclear factor kappa B ligand (RANKL) (Haynes et al. Citation2001). These factors are involved in complex signaling pathways for osteoclast activation.
Synovial membrane‐like interface tissue develops between implants and periprosthetic reactive cancellous bone‐like cortex. This fibrous tissue mainly consists of fibroblasts, macrophages, and a vascular component. Pseudosynovial fluid probably contributes to the development of the interface tissue. As a result of fluid pressure waves during cyclic loading, the fluid gradually penetrates between the implant and the host bone. Pseudosynovial fluid has already been recognized to act as a liquid transport medium for particulate ultra‐high molecular weight polyethylene debris (Robertsson et al. Citation1997). The pseudosynovial fluid contains cytokines such as IL-1, IL-6, TNF, and RANKL (Nivbrant et al. Citation1999, Mandelin et al. Citation2005). It may thus constitute a transport vehicle for potent biologically active osteoclastogenic factors.
Pseudosynovial fluid from a loosened total hip prosthesis can induce osteoclast formation (Mandelin et al. Citation2005). The nervous system has been implicated in the etiology and pathogenesis of joint diseases. SP and calcitonin gene‐related peptide (CGRP) are sensory neuropeptides. CGRP has been found to increase IL-6 and IL-8 secretion in RA fibroblasts and SP has been found to stimulate IL-8 secretion in OA fibroblasts (Raap et al. Citation2000). It is widely accepted that SP is a proinflammatory neurotransmitter. For example, SP stimulates expression of IL-1, TNF (Lotz et al.Citation1988), IL-2 (Rameshwar et al. Citation1993), and nuclear factor kB (Lieb et al. Citation1997) in various cell types. This indicates that it is a potent proinflammatory agent. There is only limited information regarding the role of SP and CGRP in asceptic loosening. SP‐and CGRP‐immunoreactive nerve fibers have been detected in the pseudocapsular tissues (Niissalo et al. Citation2002) and interface membranes (Ahmed et al. Citation1998) of aseptically loosened hip prostheses. In this study, we investigated the levels of SP and CGRP in the pseudosynovial fluid from patients with aseptic loosening after total hip arthroplasty (THA), and compared them with the levels in synovial fluid of patients undergoing primary THA for osteoarthritis.
Patients and methods
Samples of pseudosynovial fluid were collected from 10 patients (6 women) undergoing revision operation due to aseptic loosening of THA. All hips had radiographic osteolysis and had a loose implant at revision. The indication for primary THA had been osteoarthritis in all cases. There were no clinical or laboratory signs of infection in any patient. The mean time from primary arthroplasty to revision was 10 (3-19) years. The type of prostheses was Charnley and all of them were cemented. The mean age at revision was 70 (62-81) years.
The fluid samples were aspirated with a syringe and needle before incision of the joint capsule. Control synovial fluid samples were collected by the same method from 12 patients (mean age 58 (50-72) years, 7 women) undergoing primary THA for osteoarthritis of the hip. All patients had advanced disease radiographically. Patients with rheumatoid arthritis or other forms of inflammatory arthritis were excluded from serological examination and clinical features.
All samples were immediately centrifuged at 3,000 rpm for 15 min to remove cellular debris, and the supernatants were frozen and stored at -80°C until analysis. The concentrations of SP in synovial fluid and pseudosynovial fluid were measured using enzyme immunoassay (EIA) kits (Cayman Chemical, Ann Arbor, MI) in accordance with the directions of the supplier. The detection limit was 8 pg/mL. CGRP was measured using EIA kits (SPIbio, city, France) in accordance with the instructions of the manufacturer. The detection limit was 7.81 pg/mL.
The study was conducted with the informed consent of patients and was approved by the local Ethics Committee (2005-12-03).
Statistics
All values are expressed as mean (SD). The Mann‐Whitney U‐test was used to compare values in patients with prosthesis loosening to those in patients with OA. We considered p‐values of < 0.05 to be statistically significant.
Results
The mean concentration of SP was 42 (10) pg/mL in pseudosynovial fluid from patients with aseptic loosening and 27 (12) pg/mL in synovial fluid from patients with OA who were undergoing primary THA (p = 0.01) ().
Box plots showing SP (left) and CGRP (right) concentrations in pseudosynovial fluid from patients with aseptic loosening (AS) after THA, and in synovial fluid from patients with OA undergoing primary THA. The box plots show the tenth and ninetieth percentile, the twenty‐fifth and seventy‐fifth percentile, and the median. The concentrations of SP and CGRP in pseudosynovial fluid were higher than in synovial fluid (p = 0.01 and p = 0.02).
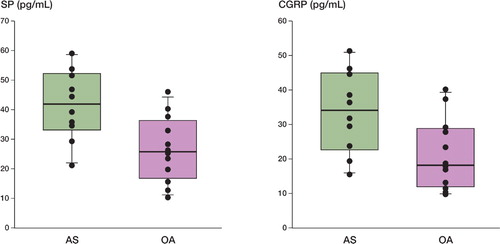
The mean concentration of CGRP was 33 (10) pg/mL in pseudosynovial fluid from patients with aseptic loosening and 21 (12) pg/mL in synovial fluid from patients with OA undergoing primary THA (p = 0.02) ().
Discussion
Relevant studies have focused mainly on the role of cellular mediators in aseptic loosening of prostheses and the presence of sensory neuropeptides in the pseudosynovial fluid has not been investigated. However, the nervous system has been implicated in the etiology and pathogenesis of joint diseases. The involvement of neurogenic inflammation in adjuvant‐induced experimental arthritis has been demonstrated in rats (Levine et al. Citation1984). SP- and CGRP‐immunopositive nerve fibers in the joints of patients with painful osteoarthritis of the hip have been found in the soft tissue of the fossa acetabuli, as well as in the synovial layer of the hip joint capsule (Saxler et al. Citation2007). Joints with severe arthritis have a dense innervation of SP‐containing sensory neurons and a higher SP content than joints that develop mild arthritis (Weidler et al. Citation2005). Infusion of SP into the knee exacerbates the severity of experimental arthritis (Levine et al. Citation1984, Citation1985). There are increased levels of SP in the synovial fluid and serum of patients with RA (Marshall et al. Citation1990, Menkes et al. Citation1993). These findings support the possibility of SP and CGRP having a role in bone lesion development. In this study, we found that there were higher levels of SP and CGRP in the pseudosynovial fluid. SP- and CGRP‐immunoreactive nerve fibers have been detected in the pseudocapsular tissues (Niissalo et al. Citation2002) and in the interface membranes (Ahmed et al. Citation1998) of aseptically loosened hip prostheses. SP- and CGRP‐immunoreactive axons have been shown to be localized in bone (Bjurholm et al. Citation1991). In the pathogenesis of aseptic loosening, loose implants, wear particles, and cytokines may stimulate the surrouding nerves and promote the release of sensory neuropeptides.
SP and CGRP may be transported in the pseudosynovial fluid and act on the different cells they contact, such as fibroblasts, macrophages, lymphocytes, and osteoclasts. SP promotes osteoclastogenesis through both upregulation of RANKL expression and downregulation of OPG expression in synovial fibroblastic cells, and induction of the proliferation of synovial fibroblastic cells (Matayoshia, et al. Citation2005). SP has been found to stimulate the production of prostaglandin (PG) E2 and RANKL in fibroblast‐like cells of human dental pulp, and to promote bone resorption (Kojima et al. Citation2006). Fibroblasts, lymphocytes, and macrophages are equipped with SP receptors and SP has been shown to induce the release of IL-1, IL-6, and TNF from these cells (Yamaguchi et al. Citation2004). SP receptors have also been found on the plasma membrane and in the cytoplasm of osteoclasts (Goto et al. Citation1992). In an experiment using mouse calvaria, SP caused increased bone resorption (Sherman et al. Citation1995). The addition of SP was also found to increase the bone resorption activity of cultured rabbit osteoclasts (Mori et al. Citation1999). These findings suggest that the neuropeptide SP is one of the risk factors in bone resorption, and that it may participate in the periprosthetic osteolysis. The role of CGRP is ambiguous. CGRP stimulates lymphocyte proliferation (Casini et al. Citation1989). There have been few reports documenting an effect of CGRP on the production of proinflammatory factors by macrophages. Feng et al. (Citation1997) reported that CGRP reduces TNF production in response to lipopoly‐saccharide (LPS). According to Torii et al. (Citation1997), IL-1β production of macrophages in response to LPS diminishes in the presence of CGRP. However, Cuesta et al. (Citation2002) reported an enhancement effect of CGRP on the basal secretion of IL-1β, TNF, and IL-6 from human peripheral blood mononuclear cells. Yaraee et al. (Citation2003) suggested that CGRP has the ability to induce production of TNF and IL-1 β. We found that the levels of CGRP in pseudosynovial fluid were increased in this study.
In conclusion, we have found elevated levels of SP and CGRP in the pseudosynovial fluid of patients with aseptic loosening. As SP and CGRP may be implicated in bone resorption and subsequent aseptic loosening of joint implants, these findings are certainly worthy of further study.
Contributions of authors
YQ designed the study and performed the research, collected and analyzed the data, and prepared the manuscript. BZ designed the study, analyzed the data, and also prepared the manuscript. XZ and YJ collected the data, and prepared the manuscript.
No competing interests declared.
- Ahmed M, Bergström J, Lundblad H, Gillespie W J, Kreicbergs A. Sensory nerves in the interface membrane of aseptic loose hip prostheses. J Bone Joint Surg (Br) 1998; 80: 151–5
- Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides 1991; 9: 165–71
- Casini A, Geppetti P, Maggi C A, Surrenti C. Effects of calcitonin gene-related peptide (CGRP), neurokinin A and neurokinin A (4-10) on the mitogenic response of human peripheral blood mononuclear cells. Naunyn-Schmiedeberg's Arch Pharmacol 1989; 339: 354–8
- Cuesta M C, Quintero L, Pons H, Suarz-Roca H. Substance P and calcitonin gene-related peptide increase IL-1 beta, IL-6 and TNF alpha secretion from human peripheral blood mononuclear cells. Neurochem Int 2002; 40: 306–10
- Feng Y, Tang Y, Guo J, Wang X. Inhibition of LPS-induced TNF-alpha production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci 1997; 61: 281–7
- Goto T, Tsukuba T, Ayasaka N, Yamamoto K, Tanaka T. Immunocytochemical localization of cathepsin D in the rat osteoclast. Histochemistry 1992; 97: 13–8
- Haynes D R, Crotti T N, Potter A E, Loric M, Atkins G J, Howie D W, Findlay D M. The osteoclastogenic molecules RANKL and RANK are associated with periprosthetic osteolysis. J Bone J Surg (Br) 2001; 83: 902–11
- Kojima T, Yamaguchi M, Kasai K. Substance P stimulates release of RANKL via COX-2 expression in human dental pulp cells. Inflamm Res 2006; 55: 78–84
- Levine J D, Clark R, Devor M, Helms C, Moskowitz M A, Basbaum A I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science 1984; 226: 547–9
- Levine J D, Moskowitz M A, Basbaum A I. The contribution of neurogenic inflammation in experimental arthritis. J Immunol (2 Suppl) 1985; 135: 843–7
- Lieb K, Fiebich B L, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol 1997; 159: 4952–8
- Lotz M, Vaughan J H, Carson D A. Effect of neuropep tides on production of inflammatory cytokines by human monocytes. Science 1988; 241: 1218–21
- Mandelin J, Liljeström M, Li T F, Ainola M, Hukkanen M, Salo J, Santavirta S, Konttinen Y T. Pseudosynovial fluid from loosened total hip prosthesis induces osteoclast formation. J Biomed Mater Res B Appl Biomater 2005; 74: 582–8
- Marshall K W, Chiu B, Inman R D. Substance P and arthritis: Analysis of plasma and synovial fluid levels. Arthritis Rheum 1990; 3: 87–90
- Matayoshia T, Gotob T, Fukuharaa E, Takanoa H, Kobayashib S, Takahashi T. Neuropeptide substance P stimulates the formation of osteoclasts via synovial fibroblastic cells. Biochem Biophys Res Commun 2005; 327: 756–64
- Menkes C J, Renoux M, Laoussadi S, Mauborgne A, Bruxelle J, Cesselin F. Substance P levels in the synovium and synovial fluid from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 1993; 20: 714–7
- Mori T, Ogata T, Okumura H, Shibata T, Nakamura Y, Kataoka K. Substance P regulates the function of rabbit cultured osteoclast; increase of intracellular free calcium concentration and enhancement of bone. resorption. Biochem Biophys Res Commun 1999; 262: 418–22
- Niissalo S, Li T F, Santavirta S, Takagi M, Hietanen J, Konttinen Y T. Dense innervation in pseudocapsular tissue compared to aneural interface tissue in loose totally replaced hips. J Rheumatol 2002; 29: 796–803
- Nivbrant B, Karlsson K, Kärrholm J. Cytokine levels in synovial fluid from hips with well-functioning or loose prostheses. J Bone Joint Surg (Br) 1999; 81: 163–6
- Raap T, Justen H P, Miller L, E,Cutolo M, Scholmerich J, Straub R H. Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol 2000; 27: 2558–65
- Rameshwar P, Gascon P, Ganea D. Stimulation of IL-2 production in murine lymphocytes by substance P and related tachykinins. J Immunol 1993; 151: 2484–96
- Robertsson O, Wingstrand H, Kesteris U, Jonsson K, Onnerfalt R. Intracapsular pressure and loosening of hip prostheses. Preoperative measurements in 18 hips. Acta Orthop Scand 1997; 68: 231–4
- Saxler G, Löer F, Skumavc M, Pförtner J, Hanesch U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain 2007; 11: 67–74
- Sherman B E, Chole R A. A mechanism for sympathecto-myinduced bone resorption in the middle ear. Otolaryngol Head Neck Surg 1995; 113: 569–81
- Torii H, Hosoi J, Biessert S, Xu S, Fox F E, Asahina A, et al. Regulation of cytokine expression in macrophages and the Langerhans cell-like line XS52 by calcitoningenerelated peptide. J Leukoc Biol 1997; 61: 216–23
- Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Schölmerich J, Straub R H. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis 2005; 64: 13–20
- Yamaguchi M, Kojima T, Kanekawa M, Aihara N, Nogimura A, Kasai K. Neuropeptides stimulate production of interleukin-1β, interleukin-6, and tumor necrosis factor-a in human dental pulp cells. Inflamm Res 2004; 53: 199–204
- Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F. Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. International Immunopharmacology 2003; 3: 1883–7