Abstract
Background and purpose Following transtibial amputation, a rigid dressing of plaster of Paris has been reported to have advantages over a soft dressing regarding wound healing and reduction of edema, but use of the former may be limited by difficulties in application and in gaining access to the wound. An easily applicable and removable vacuum‐formed rigid dressing (ORD) has recently been introduced. We compared the ORD with a conventional rigid plaster of Paris dressing with regard to wound healing, time to fitting of a prosthesis, and function with the prosthesis.
Method Patients undergoing transtibial amputation for peripheral vascular disease were randomized at surgery to receive ORD (O) or conventional rigid dressing (C) for 5 to 7 days, followed by compression therapy using silicone liner. The primary outcome measure was time to prosthetic fitting and secondary outcome measures included function with the prosthesis 3 months after amputation, measured with the Locomotor Capability Index (LCI) and the Timed “Up and Go” (TUG) test. All patients received prostheses with a total surface‐bearing socket.
Results Of 27 patients randomized to one or other dressing (15 O and 12 C), prosthetic fitting was achieved in 23 patients (mean age 76 (43-91) years; 13 (9 men) in the O group and 10 (5 men) in the C group). Wound healing was similar in both groups. Mean time to prosthetic fitting was 37 (26-54) days in the O group and 34 (21-47) days in the C group (adjusted mean difference 3, 95% CI: -3-9). At 3 months, mean LCI was 28 (6-42) in the O group and 25 (2-41) in the C group (mean difference -0.1, 95% CI: -8.5-8.2). Mean TUG was 41 (10-92) seconds and 29 (10-47) seconds, respectively (mean difference 14, 95% CI:-2-30).
Interpretation The vacuum‐formed rigid dressing appears to give results similar to those of the conventional rigid dressing regarding time to prosthetic fitting and patient's function with prosthesis.
Following transtibial amputations, various types of dressings have been used to promote uncomplicated wound healing, pain control, and shaping of the residual limb—which are the factors that facilitate rapid prosthetic fitting and rehabilitation. No consensus exists on whether this is best achieved with the use of a rigid or a soft dressing (Smith et al. Citation2003). Rigid and semi‐rigid dressings have been reported to have advantages associated with better wound healing and volume control, protection against injury during falls, lower risk of knee contracture, reduced time in hospital, and reduced time to prosthetic fitting (Nawijn et al. Citation2005). The method of applying a rigid dressing using plaster of Paris directly after surgery has been used for many years with the main aim of containing volume and preventing excessive edema (Smith et al. Citation2003). This edema has been shown to increase rapidly during the early postoperative phase (Lilja and Oberg Citation1997). In general, the practice for application of rigid dressings has been to use a cast for 1-3weeks (depending on the rehabilitation strategy used and the kind of compression therapy applied) and, if needed for more than 1 week, to replace the cast in order to accommodate volume changes (Smith et al. Citation2003).
Despite the reported advantages of rigid dressings, the most widely used type of dressing following transtibial amputation is still the soft dressing (Dormandy et al. Citation1994). The main goal of using a soft dressing is to absorb the fluid from the wound and to prevent edema with a compressive elastic bandage. Elastic bandages have the advantage of being inexpensive but may be associated with serious problems; for example, pressure levels beneath soft dressings can vary over a wide range (Isherwood et al. Citation1975) and, if incorrectly applied, they can lead to complications such as pressure sores and persistent edema (Horne and Abramowicz Citation1982). To date, there have been no studies showing that soft dressings give results superior to those achieved with rigid or semi‐rigid dressings (Smith et al. Citation2003).
Factors that limit the use of rigid dressings are believed to be related to application difficulties (surgeons may need assistance during application), time taken (time for application, prolonged anesthesia, and cleaning), difficulty in wound inspection (surgeons may prefer to have easy access to the wound, especially if complications occur) and risk of pressure ulcers or pressure on the patella (Cohen et al. Citation1974, Baumgartner and Botta Citation1995, Choudhury et al. Citation2001). Thus, a removable rigid dressing would solve some of these problems while retaining the advantages of the plaster of Paris dressing.
A vacuum‐formed removable rigid dressing, the ORD (ÖSSUR HF, Reykjavik, Iceland), has been developed for use after transtibial amputation (Johannesson et al. Citation2004). The purpose of this randomized controlled trial was to compare the ORD with a conventional rigid plaster of Paris dressing in patients undergoing transtibial amputation because of peripheral vascular disease, regarding both time to prosthetic fitting and function with the prosthesis.
Patients and methods
Eligibility criteria
From January 2003 through July 2005, patients scheduled for transtibial amputation at one orthopedics department were screened for enrollment in this study. The department is the only medical facility where amputation surgery is performed in the region of northeastern Skåne (population 170,000) in Sweden. The inclusion criteria were transtibial amputation because of peripheral vascular disease, with or without diabetes, and willingness to participate in the study. The exclusion criteria were inability to walk, severe dementia, knee contracture, contralateral amputation, fracture, severe cerebrovascular, neurological or other medical disorders that could affect rehabilitation, severe peripheral vascular disease involving the contralateral lower limb, or inability to speak Swedish.
Patients were enrolled in the trial by the treating orthopedic surgeon. Signed informed consent was obtained from all participants. The Regional Ethics Committee of Lund University approved the study (LU 776-02).
Randomization
The patients were randomly assigned to one of the two groups, ORD (O) or conventional plaster of Paris dressing (C), according to a computer‐generated randomization list and serially numbered sealed opaque envelopes. The envelopes were opened sequentially in the operating room prior to the amputation, and the type of dressing stipulated was subsequently used.
Surgical technique
A sagittal incision (Persson Citation1974) was used in all patients except 1, in whom a transverse incision was used because of previous vascular surgery. A residual length of approximately 1.5 times the width of the knee—considered optimal for prosthetic fitting (Persson and Liedberg Citation1983)—was achieved in all patients except 3 (O group), 2 of whom had a shorter residual limb length and 1 a longer residual limb length. Good blood circulation at the incision level was observed in all patients at the time of surgery and all wounds were closed with primary suture. No drain was applied. The sutures were removed 3 weeks after surgery.
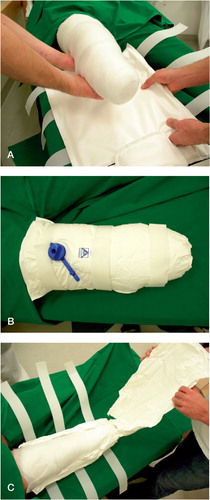
Dressings ()
The patients in the O group received the ORD dressing, applied by the surgeon immediately after surgery. The ORD was developed for fixation of the residual limb following transtibial amputation. It consists of 3 interconnected PVC‐coated bags of polyester fiber. The bags are filled with styropor granules. The rigidity is obtained by removal of air surrounding the granules within the dressing. Velcro straps secure the dressing in position. The patients in the C group received a standard dressing with circulated plaster of Paris (Murdoch and Wilson Citation1996). Both dressings extended over the knee to avoid knee contracture.
Postoperative care
The postoperative management followed a standardized protocol (Johannesson et al. Citation2004). The patients received oral or intravenous supplementary nutrition during their hospital care. The same wound‐care management (bandaging and padding) was used in both groups to ensure that no direct contact occurred between skin and outer dressings. Both dressings were worn full‐time, including at night and during physiotherapy sessions if applicable.
In the O group, the fit could be adjusted by letting air into the device and thereafter reshaping it by removal of air. The anterior shell was opened for 10-15 min twice a day for drying. The dressing could be removed by the medical team for wound inspection and dressing replacement if considered necessary ().
In the C group, wound inspection was done (if patients reported excessive pain or had any other sign of possible complications) by dividing the dressing and then replacing it.
In both groups, the dressings were used for 5-7 days. They were then removed for wound inspection, followed by standardized compression therapy using a postoperative silicone liner (ÖSSUR HF), which was continued until prosthetic fitting.
Rehabilitation and prosthetic fitting
The patients were usually transferred to the rehabilitation clinic or to a community service home 2 weeks after surgery. All patients received training at a daycare rehabilitation center ("walking school”) throughout their rehabilitation period. The physiotherapist at the rehabilitation center and the prosthetist were blinded as to whether the patients were participants in the study and to the type of dressing they had received.
The members of the rehabilitation team (orthopedic surgeon, physiotherapist, wound‐care specialist nurse, and prosthetist) monitored wound healing and volume changes. Wound healing was recorded at the time of prosthetic fitting as “complete closure"—defined as the absence of leakage from the wound—or “incomplete closure"—defined as the presence of leakage from the wound. Complete wound healing was not mandatory for prosthetic fitting. The decision regarding prosthetic fitting was made by the members of the rehabilitation team based on the shape of the residual limb and the degree of edema, and also on the health status of the patient.
To minimize the possible influence of using different types of prostheses on functional outcome, particularly the effect of the socket (which is the most important part of the prosthesis), the ICEX prosthetic sockets (Össur Inc.) were used in all patients. The ICEX socket, which consists of carbon fiber (impregnated with polyurethane resin) is formed over a silicon liner that covers the residual limb. The total surface‐bearing socket shape is obtained with the use of a silicone bladder over the carbon fiber. By using air pressure of 80 mm Hg, the socket shape is formed (Selles et al. Citation2005). All prostheses were made by the same prosthetist and, to avoid delay in the rehabilitation process, the prostheses were delivered on the same day that the decision regarding prosthetic fitting was made. Before prosthetic fitting, a patient evaluation form based on the International Organization for Standardization (ISO) standards 8548-1:1989 and 8548-3:1993 was used for registration of limb shape and wound status. In this study, we defined the prosthetic fitting to have occurred when the patient received a custom‐made prosthesis. Before receiving the prosthesis, no loading was applied to the stump.
Follow‐up evaluation
3 months after the amputation, a follow‐up evaluation was performed according to a standardized protocol (Johannesson et al. Citation2004) and functional outcome was measured using the Locomotor Capability Index (LCI) and the Timed “Up and Go” (TUG) test. The LCI is a questionnaire designed to measure the capabilities of patients with pros‐theses. It is composed of 14 items divided into two subscales; basic (7 items) and advanced (7 items) (Grise et al. Citation1993, Franchignoni et al. Citation2004). The items inquire about the ability to perform activities and the level of independence while performing these activities. Each of the 14 items is graded on a 4-point scale from 0 (unable to) to 3 (yes, independently). The total LCI score can range from 0 to 42 and total subscale scores of 21 may be achieved for basic and advanced capabilities with the prosthesis. In this study, a Swedish version of the LCI that has already been assessed for reliability and validity (Larsson Citation2005) was used. In a patient population with vascular lower limb amputation, the Swedish version has shown a high degree of reliability and construct validity and a high degree of correlation with the Timed “Up and Go” test (unpublished data).
In the TUG test the patients wearing their pros‐theses were observed and timed while rising from an armchair, walking 3 meters, and returning to the chair (Schoppen et al. Citation1999). Both tests were conducted by the physiotherapists of the rehabilitation team, who had no knowledge of the type of dressing used after surgery.
Any changes of prosthetic sockets that occurred during the first year were documented. How each patient was living after 1 year was recorded.
Outcome measures
The primary outcome measure was the number of days from amputation to prosthetic fitting. The secondary outcome measures were the wound healing rate, functional outcome as measured with the LCI and TUG test at 3 months, the need for socket changes during the first year, and the proportion who had returned to their previous living conditions at 1 year.
Sample size
A pre‐trial estimation of sample size was performed based on the results from 15 patients who underwent transtibial amputation and rehabilitation with prosthesis over a 2-year period prior to the study, and who would have met the inclusion criteria for this study. In that group, a rigid plaster of Paris dressing was used and the mean number of days from amputation to prosthetic fitting was 36 days (SD 11). In a previous randomized study comparing a soft dressing with a semi‐rigid dressing, a 40% difference in the number of days from admission to the rehabilitation unit until readiness for prosthetic fitting was found (Wong and Edelstein Citation2000). Working on the assumption that the ORD would perform similarly to the soft dressing, testing the hypothesis that the rigid dressing results in a shorter time to prosthetic fitting than the ORD using a two‐sided test with a 0.05 level of significance and 80% power to detect a difference of 15 days would require 10 patients in each group to complete rehabilitation with their prosthesis. To account for dropouts, it was estimated that 27 patients would be needed.
Statistics
The chi‐squared test was used to compare the O and C groups with regard to sex, smoking status, and presence of other medical conditions. We used the Mann‐Whitney test to compare them regarding patient age. Multiple linear regression analyses were performed to calculate age- and sex‐adjusted mean differences and 95% confidence intervals between the groups regarding time from amputation to prosthetic fitting (both LCI and TUG results). As a number of patients did not undergo prosthetic fitting for various reasons including death, we tested two other models to compare the groups regarding time to prosthetic fitting: a conventional proportional hazards (Cox) model to include the censored observations and a Fine and Gray proportional hazards model to account also for competing risk (Fine and Gray Citation1999). All statistical analyses were 2-sided and p‐values of < 0.05 were taken to indicate statistical significance. Calculations were performed using SPSS version 15.0 and R version 2.4.1.
Results
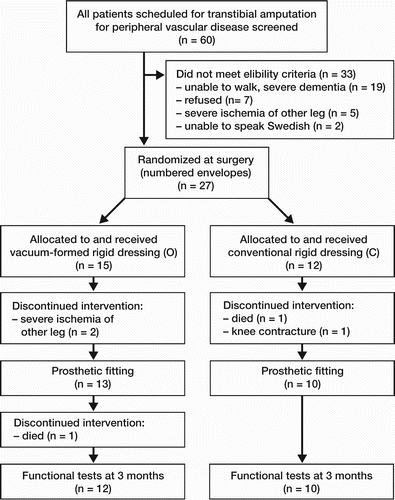
Between January 2003 and July 2005, 60 patients were screened for enrollment in the trial (). 27 patients fulfilled the eligibility criteria and accepted to participate in the study; 15 patients were randomized to the O group and 12 patients to the C group. In the O group, 1 patient (a man aged 69 years) died 11 days after surgery and in the C group 2 patients (women aged 48 and 77) died 72 and 121 days after surgery. Both of these women were not fitted with prostheses because of severe ischemic pain and wound problems in the contra‐lateral leg. In the C group, 1 patient (a man aged 84) developed a 45-degree contracture in the knee and hip of the amputated limb during the early postoperative period, which precluded prosthetic fitting.
Of the 23 patients in whom prosthetic fitting was achieved, 1 patient in the O group (a man of 84 years) died after receiving the prosthesis 84 days after amputation and the remaining 22 patients (12 O and 10 C) were included in the comparison of the functional tests (). Age, sex, proportion with diabetes, and smoking status were similar in the 2 groups. 12 patients were diagnosed with diabetes at the time of amputation. All patients except 1 had other medical conditions, mainly cardiac diseases, and 5 patients (3 O and 2 C) had recovered from previous minor cerebrovascular accidents (). None of the patients had undergone lower‐level amputation prior to the amputation in question. Before surgery, 20 patients lived in their own homes and 3 patients lived in community service homes.
Table 1. Characteristics of the patients in the two groups
Wound healing and limb shape
No wound complications were seen in the O group. In the C group, 1 patient had excessive bleeding and 2 patients had severe pain that necessitated change of dressing. When patients received the first definitive prosthesis, wound status was complete closure in 6 of 13 patients in the O group and in 4 of 10 patients in the C group (p = 0.6). The shape of the residual limb was described as cylindrical in 11 patients and bulbous in 2 patients in the O group, and cylindrical in 9 patients and conical in 1 patient in the C group. 1 patient in each group had an adherent scar at the time of prosthetic fitting.
Time to prosthetic fitting
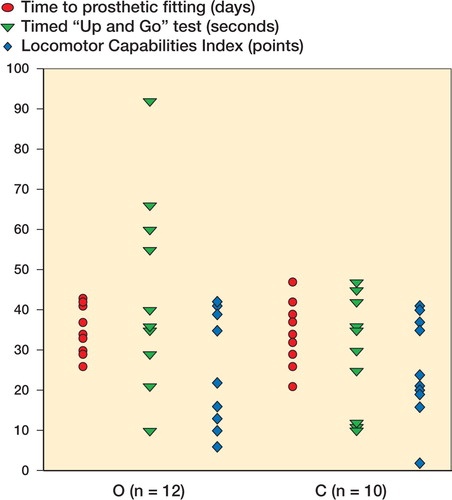
The mean time from amputation to prosthetic fitting was 37 (26-54) days in the O group and 34 (2157) days in the C group (p = 0.4) (). The alternative statistical models did not show significant differences between the groups (sex- and age‐adjusted, p = 0.4 for the Cox regression model and p = 0.4 for the Fine and Gray model).
Table 2. Outcome regarding days to prosthetic fitting and function with prosthesis
Functional tests at 3 months
No statistically significant differences in the results of the functional tests were found between the two groups at the 3-month follow‐up evaluation (). A wide range of LCI and TUG values were observed in both groups ().
Socket changes during the first year
No socket change was needed during the first year in 4 patients in the O group and 2 patients in the C group. In the patients who had a socket change, the mean time to socket change was 185 (95-269) days in the O group and 206 (95-267) days in the C group (p = 0.9).
Return to previous living conditions at 1 year
All patients had returned to their previous living conditions by 1 year, except for 2 patients (in the C group) who had moved from their own home to a community service home.
Discussion
The findings of this randomized controlled study suggest that a vacuum‐formed removable rigid dressing that is easy to apply can give results similar to those achieved with a conventional rigid plaster of Paris dressing. Wound‐healing status at the time of prosthetic fitting was similar in the two groups. Factors that may affect wound healing are patient age and early start of prosthetic rehabilitation, the latter being important for achievement of better mobility and general health. The main factors that have been found to predict successful rehabilitation outcome following transtibial amputation are walking ability prior to the amputation and preservation of the knee joint (Hermodsson et al. Citation1998). This amputation level has also been shown to be associated with the highest rate of prosthetic fitting and subsequently restoration of function and quality of life. Uncomplicated wound healing following amputation has become increaseingly important in view of the many medical disorders commonly found in this patient group, and the increasing emphasis that hospitals are placing on early discharge (Wong and Edelstein Citation2000). Apart from prevention of infection, the main aim in wound management following transtibial amputation is control of edema—as this is one of the major factors in wound healing, and reducing edema without compromising perfusion is critical (Murdoch Citation1975, Murdoch and Wilson Citation1996).
Postoperatively, early mobilization is essential in order to avoid the deleterious effects of immobility in older persons (Cutson and Bongiorni Citation1996). The main outcome measure in this study was time to prosthetic fitting. If edema is controlled in the early phase of the rehabilitation, the time to prosthetic fitting can be shortened and can thus be used as an indicator of the rehabilitation outcome (Wong and Edelstein Citation2000). However, the length of time to prosthetic fitting does not show how well the patients are actually able to use the prosthesis. Thus, as a secondary outcome measure, we also evaluated function using the first prosthesis, measured with the Locomotor Capability Index (LCI) and the Timed “Up and Go” test (TUG) test. The wide range of values for the LCI and the TUG test demonstrates that other medical conditions may also affect patients’ walking ability with the prosthesis. The goal of regaining walking ability with a prosthesis is realistic after transtibial amputation, even in elderly people, and the need to load only on one leg is thus avoided (Cutson and Bongiorni Citation1996).
Previous studies have used different follow‐up times to assess outcomes after major amputations. We believe that a follow‐up time of 3 months after transtibial amputation is appropriate when comparing results of different postoperative treatments in terms of the functional status of the patient. A 1-year follow‐up evaluation is also a reasonable time to assess the need for socket replacement (volume changes) and to measure changes in functional status. Although 17/23 of our patients had sockets changed during the first year, only 5 had sockets changed within 6 months. The reason for this might be our use of a sagittal incision in combination with silicone liner as a compression therapy.
Other types of dressings can be used to minimize postoperative edema, such as different types of air splints (Ivanic et al. Citation2002) or dressings of other materials such as Unna paste or fiber glass, both with and without a prosthetic foot attachment. In a review of postoperative dressing and management after transtibial amputation, Smith et al. (Citation2003) suggested the need for further studies that would accurately document and control for variables such as amputation technique, healing potential, co‐morbidity, nutrition, and functional ability. Cost is also a relevant factor to consider when choosing between various dressings. In this study, no cost analysis was performed. The cost of CRD is mostly related to the time required for application in the operating room (approx. 15 min at approx. 10 euros/min) (Sjöli and Sundberg Citation2002) and for its subsequent removal or replacement. The cost of the ORD (approx. 180 euros) would depend on whether it is used for one patient only (as recommended by the manufacturer) or re‐used. In our hospital the ORD was cleaned and disinfected with alcohol and re‐used (except in patients found to be carriers of MRSA or other antibiotic‐resistant bacteria).
The number of patients in our study was small, which raises the possibility of type-2 error being a cause of not finding statistically significant differences. We have, however, shown the 95% confidence intervals for the differences between the two groups regarding days to prosthetic fitting and results of the function tests, and these suggest that any possible difference in outcomes in favor of the conventional rigid dressing may not be large. Generalizability of our findings is supported by the screening of all patients for eligibility from a relatively large general population, the random allocation of participants, and the blinded assessment.
In conclusion, this randomized trial comparing a vacuum‐formed removable rigid dressing with a conventional plaster of Paris rigid dressing after transtibial amputation has shown that the two dressings give similar results. Thus, the vacuum‐formed rigid dressing—which is easily applied and which can easily be removed—can be used as an alternative to a conventional cast rigid dressing after transtibial amputation.
Contributions of authors
AJ, TÖ, and GUL conceived and designed the study. AJ, TÖ, and IA analyzed and interpreted the data. AJ, GUL, and IA wrote the manuscript.
This work was supported by a research grant from Hässle‐holm Hospital, Hässleholm, Sweden. The authors thank Jonas Ranstam of the Swedish National Competence Center for Musculoskeletal Disorders, Department of Orthopedics, Lund University Hospital, and Ulf Strömberg, Department of Epidemiological Methods, Lund University, Lund, Sweden, for statistical advice. The ORDs used in the study were provided by the manufacturing company ÖSSUR HF, Reykjavik, Iceland. The sponsor had no role in the study.
No competing interests declared.
- Baumgartner R, Botta P. Amputation und Prothesenversor-gung der unteren extremität: indikationsstellung, operative technik, nachbehandlung, prothesenversorgung, gangschulung, rehabilitation. 2nd Edition. Ferdinand Enke, Stuttgart 1995
- Choudhury S R, Reiber G E, Pecoraro J A, Czerniecki J M, Smith D G, Sangeorzan B J. Postoperative management of transtibial amputations in VA hospitals. J Rehabil Res Dev 2001; 38: 293–8
- Cohen S I, Goldman L D, Salzman E W, Glotzer D J. The deleterious effect of immediate postoperative prothesis in below-knee amputation for ischemic disease. Surgery 1974; 76: 992–1001
- Cutson T M, Bongiorni D R. Rehabilitation of the older lower limb amputee: a brief review. J Am Geriatr Soc 1996; 44: 1388–93
- Dormandy J, Belcher G, Broos P, Eikelboom B, Laszlo G, Konrad P, Moggi L, Mueller U. Prospective study of 713 below-knee amputations for ischaemia and the effect of a prostacyclin analogue on healing: Hawaii Study Group. BrJSurg 1994; 81: 33–7
- Fine J P, Gray R J. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509
- Franchignoni F, Orlandini D, Ferriero G, Moscato T A. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil 2004; 85: 743–8
- Grise M C, Gauthier-Gagnon C, Martineau G G. Prosthetic profile of people with lower extremity amputation: conception and design of a follow-up questionnaire. Arch Phys Med Rehabil 1993; 74: 862–70
- Hermodsson Y, Ekdahl C, Persson B M. Outcome after trans-tibial amputation for vascular disease: a follow-up after eight years. Scand J Caring Sci 1998; 12: 73–80
- Horne G, Abramowicz J. The management of healing problems in the dysvascular amputee. Prosthet Orthot Int 1982; 6: 38–40
- Isherwood P A, Robertson J C, Rossi A. Pressure measurements beneath below-knee amputation stump bandages: elastic bandaging, the Puddifoot dressing and a pneumatic bandaging technique compared. Br J Surg 1975; 62: 982–6
- Ivanic G M, Schon L C, Badekas T, Badekas O, Homann N C. Trnka H J.Airlimb: initial experiences with a new immediate early management prosthesis with individually adjustable air chambers. Chirurg 2002; 73: 360–5
- Johannesson A, Larsson G U, Oberg T. From major amputation to prosthetic outcome: a prospective study of 190 patients in a defined population. Prosthet Orthot Int 2004; 28: 9–21
- Larsson B. Locomotor Capabilities Index: validation of the Swedish version. Presented at: The SMART seminar (Evidence-based practice in prosthetics and orthotics). Växjö, Sweden 2005
- Lilja M, Oberg T. International forum: proper time for permanent prosthetic fitting. J Prosthet Orthot 1997; 9: 90–5
- Murdoch G. Research and development within surgical amputee management. Acta Orthop Scand 1975; 46: 526–47
- Murdoch G, Wilson A B. Amputation: surgical practice and patient management. Butterworth-Heinemann. Oxford, Boston 1996
- Nawijn S E, van der Linde H, Emmelot C H, Hofstad C J. Stump management after trans-tibial amputation: a systematic review. Prosthet Orthot Int 2005; 29: 13–26
- Persson B M. Sagittal incision for below-knee amputation in ischaemic gangrene. J Bone Joint Surg (Br) 1974; 56: 110–4
- Persson B M, Liedberg E. A clinical standard of stump measurement and classification in lower limb amputees. Prosthet Orthot Int 1983; 7: 17–24
- Schoppen T, Boonstra A, Groothoff J W, de Vries J, Goeken L N, Eisma W H. The Timed “up and go” test: reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil 1999; 80: 825–8
- Selles R W, Janssens P J, Jongenengel C D, Bussmann J B. A randomized controlled trial comparing functional outcome and cost efficiency of a total surface-bearing socket versus a conventional patellar tendon-bearing socket in transtibial amputees. Arch Phys Med Rehabil 2005; 86: 154–61
- Sjöli P, Sundberg L. KPP - kostnad per patient i västra Götalandsregionen. 2002; 52–3, http:\\www.vgregion. se\upload\Regionkanslierna\hsskansli\Analys\TEK\KPP\ KPPgrVGR%20slutrapport%20030811.pdf
- Smith D G, McFarland L V, Sangeorzan B J, Reiber G E, Czerniecki J M. Postoperative dressing and management strategies for transtibial amputations: a critical review. J Rehabil Res Dev 2003; 40: 213–24
- Wong C K, Edelstein J E. Unna and elastic postoperative dressings: comparison of their effects on function of adults with amputation and vascular disease. Arch Phys Med Rehabil 2000; 81: 1191–8