Abstract
Background and purpose Cytokines play an important role in the complex process of bone formation. We have previously found an altered skeletal phenotype with reduction of cortical bone mass in mice depleted of the 2 cytokines interleukin‐4 (IL‐4) and interleukin‐13 (IL 13). The present study was performed to investigate a potential role of IL‐4 and IL‐13 in fracture healing and bone induction by demineralized xenogenic bone matrix (DXBM).
Methods Callus formation in IL‐4-/-IL‐13-/- (IL‐4/13 knockout) and wild‐type (WT) male mice was compared using a standardized fracture model. The capacity of IL‐4-/-IL‐13-/- and WT male and female mice to form heterotopic bone was compared using intramuscular implants of DXBM. Bone formation and mechanical properties were evaluated by pQCT, ash weight, 3‐point bending, radiology, and immunohistology.
Results In the fracture investigation substantial amounts of new bone formation by 5 weeks were found, but no differences in radiographical healing, callus volume, BMD, BMC, or mechanical properties were detected between IL‐4-/-IL‐13-/- and WT mice. In the DXBM investigation radiographic analysis confirmed mineralization of implants in both groups, but no difference in the amount of mineral deposition (net bone formation) between IL‐4-/-IL‐13-/- and WT mice was found. Immunohistology showed inhibition of autonomic nerves in the capsule of the IL‐4-/-IL‐13-/- group along with a lack of vascularization within the implants.
Interpretation Depletion of IL‐4 and IL‐13 does not cause any major alteration in fracture healing or heterotopic bone formation in mice. The pattern of autonomous nerve expression and expression of markers of neovascularization is, however, altered to some extent by the absence of IL‐4 and IL‐13.
In fracture healing, bone formation plays a crucial role in the repair process. The complex process of fracture healing and bone induction is characterized by a number of key events: proliferation and migration of primitive mesenchymal cells, the formation of new vessels, angiogenesis, followed by differentiation of highly specialized bone‐forming cells. This process is governed by a great number of regulatory substances such as growth factors and cytokines. Among the cytokines, those that have been attributed proinflammatory properties—such as interleukin‐1 (IL‐1) and tumor necrosis factorex (TNF-α)—have attracted most interest in bone research (Canalis et al. Citation1991, Olmedo et al. Citation1999, Kon et al. Citation2001). However, anti‐inflammatory cytokines (e.g. IL‐4 and IL‐13) also influence bone in several ways. Both cytokines have receptors on osteoblasts. The cytokines inhibit proliferation and stimulate interleukin‐6 (IL‐6) production in human osteoblasts (Frost et al. Citation2001, Silfversward et al. Citation2004). In addition, excessive expression of IL‐4 in transgenic mice results in a low‐turnover osteoporosis (Lewis et al. Citation1993). Recently, we found that depletion of IL‐4 and IL‐13 led to an isolated reduction in cortical bone mass in adult male mice (Silfversward et al. Citation2007). As this indicates that IL‐4 and IL‐13 may possibly have a role in bone modeling, we considered that it would be of interest to study their effects in other events associated with bone formation, such as fracture healing and heterotopic bone formation.
Material and methods
IL‐4-/-IL‐13-/- and WT mice were maintained on a more than 10 times backcrossed BALB/c background. In the fracture investigation, mice homozygous for the disrupted IL‐4 and IL‐13 genes were obtained by interbreeding the heterozygotes. In the DXBM investigation, homozygous mice of each type (WT and IL‐4-/-IL- 13-/ were obtained by interbreeding homozygotes (WT + WT and IL‐4-/-IL‐13-/- + IL‐4-/-IL‐13--/-, respectively). Genotyping of tail DNA was performed at 3 weeks of age using PCR as previously described (McKenzie et al. Citation1999). 3 primers with the following sequences were used: 5'‐CCT GGA TTC CCT GAC CAA CAT C‐3’, 5'‐GGC CTT GCG GTT ACA GAG GCC‐3’, and 5'‐ACC ACA CTG CTC GAC ATT GGG TG‐3’ (Thermo Electron Corporation, Ulm, Germany). Program conditions were 94°C for 2 min, and then 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 min for 30 cycles, followed by 72°C for 10 min. Animals had free access to fresh water and food pellets (R36; Lactamin, Kimstad, Sweden). Approximately 300 mice were bred to obtain the animals used in the present study.
The project was approved by the Ethics Committee of Uppsala University, Sweden (reg. no. C155/5).
Fracture healing
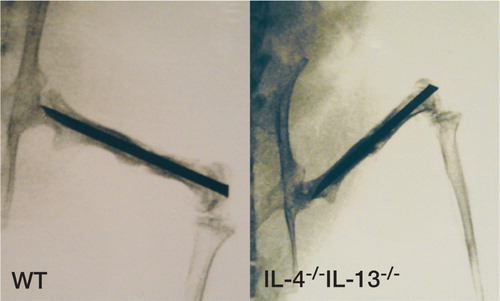
We used a modified version of a mouse fracture model as previously described (Skoglund et al. Citation2002). Adult male mice, IL‐4-/-IL‐13-/- and WT, were anesthetized by intraperitoneal injection with 0.2 mL of a mixture of Dormitor Vet. (1 mL at 1 mg/mL; Orion Pharma Animal Health Care, Sollentuna, Sweden) and Ketalar (1.5 mL at 50 mg/mL; Phizer AB, Täby, Sweden) suspended in 2.5 mL of NaCl. Using sterile conditions, the distal part of the left femur was exposed by a lateral incision. The patella was dislocated medially to expose the femur condyles. A cannulated needle (0.6 mm diameter) was drilled into the bone marrow cavity to the level of the major trochanter. The needle was backed 1 mm, cut off, and reinserted to minimize damage to the knee. A standardized diaphysial fracture of the femur was then created using specially constructed tongs with semi‐lunar cutting edges, sparing the intramedullarly placed needle precisely. After repositioning of the patella muscles covered the fracture and the muscles on the lateral aspect of the femur, mostly bluntly dissected, spontaneously fall back in position to cover the fracture giving no need for adaptive muscular sutures. Postoperatively, the mice showing slow recovery were given a wake‐up dose (0.1 mL) of Antisedan Vet. (5 mg/mL; Orion Pharma Animal Health Care, Sollentuna, Sweden) suspended in NaCl in a 1:4 ratio. They were initially left to recover in separate heated cages. The mice started to use their operated leg within 1-3 days postoperatively, whereafter they—apart from initial limping—showed normal behavior. 5 weeks postoperatively, the animals were killed by CO2 inhalation, examined by faxitrone, and dissected for removal of the femora. Before further analysis, femurs were fixed by immersion in Zamboni's solution for 24 h and the specimens were then incubated for 2 days in 20% sucrose with Sörensen phosphate buffer containing 0.01% sodium azid and 0.02% bacitracin (Sigma), and then kept in the same solution until further analysis ().
Table 1. Protocol for animals (all male) in the fracture study
Heterotopic bone
In a parallel investigation, adult male and female mice (IL‐4-/- IL‐13-/- and WT) were operated with implantation of demineralized, xenogenic bone matrix (DXBM) prepared from long bones of Sprague‐Dawley rats (Nilsson et al. Citation1986). The mice were anesthetized as described above. Under sterile conditions, a small incision was made bilaterally on the lateral proximal thigh and a pouch was created in the rectus femoris muscle by blunt dissection. One DXBM implant (2 mg) was placed in each muscle pouch, and then the fascia and skin were closed with sutures. The above‐mentioned postoperative regime was used and most of the animals started to use their operated leg within 1-2 days. 5 weeks postoperatively, the animals were killed by CO2 inhalation and examined by faxitron radiography. The implants were dissected free and removed for further analysis ().
Table 2. Protocol for animals in the DXBM study
Radiography by faxitron
In both investigations, radiographs of animals were taken immediately after killing and prior to dissection using faxitron (Faxitron Series Cabinet X‐ray System; Hewlet Packard, Palo Alto, CA). Voltage and duration were optimized separately for animals included in the fracture experiment (40 V and 40 seconds, respectively) and for animals in the DXBM experiment (46 V and 40 seconds).
Peripheral quantitative computerized tomography (pQCT)
Dissected fracture specimens, IL‐4-/-IL‐13-/- and WT (n = 8 + 8), were analyzed by pQCT using Stratec peripheral quantitative computerized tomography (pQCT) equipment (XCT Research M with software version 5.4B; Norland Medical Systems/CooperSurgical, Trumbull, CT) operating at a resolution of 70 μm as previously described (Windahl et al. Citation1999). The callus was measured by performing 5 consecutive pQCT sections, starting from the center of the callus, each with a thickness of 5 |im. Analysis of these 5 sections was used to determine the average cross‐sectional area, calculation of callus volume, and for determination of bone mineral density (BMD) and bone mineral content (BMC) in each fracture.
Mechanical testing
Fracture specimens, IL‐4-/-IL‐13-/- (n = 6) and WT (n = 10), were tested to failure by three‐point bending at room temperature on an electromechanical testing machine (Avalon Technologies, Rochester, MN) at a rate of 1 mm/second. The distance between the end‐supports was 8 mm. The specimens were placed in such a way that the load was applied in an anteroposterior direction with the center of the callus in the middle between the two supports. An axial load cell with range 0-25 lb was used (Transducer Techniques Inc., Temecula, CA). Values for load and displacement were collected 50 times per second until failure, using software provided with the testing machine (Testware II; Avalon Technologies). The data collected were stored as data files including the variables time, displacement, and load. Based on the collected data load at failure, displacement at failure, stiffness at failure, maximal stiffness, and energy to failure were calculated. The bending stiffness was calculated by using the load and displacement data to define a slope, after which the tangent of the maximal slope for each sample was used. Energy to failure was defined by the area under the load‐displacement curve.
Measurement of mineralization
Retrieved (left-side) DXBM implants from IL‐4-/-IL‐13-/- and WT male (n = 10 + 8) and female (n = 11 + 12) mice were ashed in a muffle furnace (type 48000; Barnstead/Thermolyne, Essex, UK) at 600°C for 24 h and weighed. Mean values of ash weights in the 2 groups (WT and IL‐4-/-IL‐13-/-) were compared, for male and female animals separately.
Histological analysis
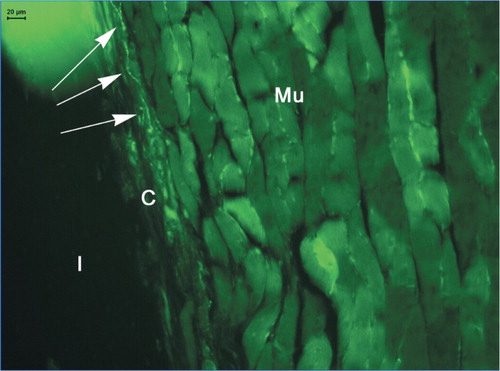
Fracture model. In the fracture experiment, histological analysis was performed in mechanically tested callus specimens from 6 IL‐4-/-IL‐13-/- male and 5 WT male animals. Also, 4 intact calluses, 2 IL‐4-/-IL‐13-/- and 2 WT, were examined. For his-tochemistry, the specimens were demineralized in EDTA at 4°C for 4 weeks before being sectioned longitudinally on a Leica cryostat at a thickness of 14 urn, and then kept at -70°C. Frozen sections were chosen consecutively for hematoxylin‐eosin (H&E) staining, for morphological analysis, and indirect immunofluorescence. Antibodies (rabbit) to protein gene product 9.5 (PGP 9.5) (Dako, Glostrup, Denmark) and growth‐associated protein 43 (GAP‐43) (Chemicon) were used as markers of mature and newly formed immature nerve fibers, respectively. Antibodies (rabbit) to calcitonin gene‐related peptide (CGRP) (Peninsula Laboratories/Bachem, Torrance, CA) and neuropep‐tide Y (NPY) (Peninsula Laboratories/Bachem) were used as markers of sensory and autonomic nerves. For identification of newly formed vessels, antibodies (goat) to platelet endothelial cell adhesion molecule‐1 (PECAM‐1/CD31) (Santa Cruz Biotechnology, Santa Cruz, CA) were used. The sections were initially incubated with 10% normal NSS (normal swine serum), followed by incubation with the primary antibody (one of the 5 mentioned above) at a dilution of 1:200, for 12 h at 4°C. The secondary antibody, fluorescein isothiocyanate (FITC)‐conjugated antibodies (swine anti‐rabbit was used for the neuropeptides. Donkey anti‐goat was used for detection of pecam‐1/CD‐31) to the appropriate species of primary antibody and diluted 1:20 was applied for 30 min at 37°C. Vectashield (Vector Laboratories, Burlingame, CA) was used as anti‐fade mounting medium.
The sections were analyzed by 2 independent observers using a Nikon and a Leica epifluorescence microscope at a wavelength of 490 nm. Morphology and occurrence of positive immunoreactive staining for nerve fibers and vessels were considered. A finding was regarded as positive when confirmed by both observers in at least one‐third of the sections, and in the majority of the implants. Thus, a positive finding required positive immu‐nostaining for the specific antibody in 5 specimens out of 8 in male IL‐4-/-IL‐13-/-mice and in 4 specimens out of 7 in male WT mice.
Heterotopic bone model. Retrieved (right-side) DXBM implants fromIL‐4-/-IL‐13-/- and WT male (n = 10 + 8) and female (n = 11 + 12) mice were harvested 5 weeks after implantation, as described above. 27 specimens of the total (41) were handled and processed for histochemistry, H&E staining, and indirect immunofluorescence in accordance with the technique given for the fractures previously mentioned, except that no demineralization was required. After fixation and rinsing, the sections were kept at -70°C before staining. Specimens were prepared for histological and immunohistological analysis of nerve ingrowth and vascularization as specified above. The DXBM implants were analyzed separately for DXBM, bone marrow formed within the implant, capsule around the implant, and surrounding muscle. A finding was regarded as positive when confirmed by both observers in at least one‐third of the sections, and in the majority of the implants. Thus, a positive finding required a positive immunostaining for the specific antibody in 4 specimens out of 7 in male and female IL‐4-/--IL‐13-/- mice and male WT mice, and in 4 specimens out of 6 in female WT mice.
Statistics
For statistical evaluation of pQCT data, callus volume, BMD, BMC, and ash weight, Student's 2‐tailed t‐test for independent groups was used. Mechanical data were statistically evaluated using the Mann‐Whitney U-test. We used Statistica software (Scand AB, Uppsala, Sweden).
Results
Fracture investigation
3 mice in the IL‐4-/-IL‐13-/- group died during surgery or in the recovery period (within 24 h of surgery). No heavy bleeding was detected and we believe that an overdose of anesthesia was the cause of death. The remaining mice sustained the treatment without complications ().
Radiography
All animals showed healing by radiography, with mineralized callus formation at the fractures. There were no visual differences in callus development between the 2 groups ().
Callus bone parameters as determined by pQCT
A slight decrease in callus volume and bone mineral content (BMC) as determined by pQCT was detected between IL‐4-/-IL‐13-/- male mice and WT male mice (n = 8 in both cases). This difference was not statistically significant, however. No difference in BMD was found between the IL‐4-/-IL‐13-/-group and the WT group ().
Table 3. Callus bone parameters in dissected femora 5 weeks after fracture. Measurement by 5 consecutive pQCT scans over the center of the callus in adult male WT (n = 8) and KO (n = 8) mice. Values are mean (SEM)
Mechanical strength
No differences in the parameters load, displacement, stiffness, or energy to failure were detected between IL‐4-/-IL‐13-/- and WT mice (n = 6 and n = 10, respectively). ()
Table 4. Mechanical strength of callus as measured by three‐point bending 5 weeks after femur fracture in adult male WT (n = 10) and KO (n = 6) mice. Values are mean (SEM)
Histology
4 animals (2 WT and 2 IL‐4-/-IL‐13-/-) with intact fracture callus, and mechanically tested callus specimens from 6 IL‐4-/-IL‐13-/- and 5 WT males were analyzed histologically. H&E staining revealed a sparse fracture callus in all femora. Close to the fracture site, inclusions of fibrous tissue resembling Volkmann's canals were found. In both knockout mice (IL‐4-/-IL‐13-/-) and WT mice, vascular endothelial staining (by PECAM1) was positive only in the Volkmann's canals. In the callus tissue, no positive immunoreactivity was found for any of the four primary neuronal antibodies in use (against GAP‐43, PGP 9.5, CGRP, and NPY). In the marrow of IL‐4-/-IL‐13-/- mice, immature nerve fibers (GAP‐43) and sensory (CGRP) and autonomic fibers (NPY) were found while only immature fibers (GAP‐43) were found in the marrow of WT mice. Nerve fibers in the marrow were often adjacent to vessels, but were also represented by single branching fibers with varicosities and without sprouting. In the cortical bone, no positive immunoreactivity to nerves was found in either IL‐4-/--IL‐13-/- or WT mice. In the periosteum and surrounding muscular tissue, positive immunoreactivity to all four neuronal antibodies was found in all mice (IL‐4-/-IL‐13-/- and WT). Nerve fibers in these tissues were often long and branching, and rich in varicosities—or accompanied newly formed vessels. No histological differences were detected between broken and unbroken calluses ().
Table 5. Histology fracture model
Heterotopic bone investigation
Three mice in the IL‐4-/-IL‐13-/- group and four in the WT group died after surgery. 5 of these animals died in the recovery period within 24 h of surgery. Similarly to the fracture study, no heavy bleeding was detected and we believe that an overdose of anesthesia was the cause of death. 2 of the animals died after 5 and 7 days, respectively, probably as a result of bite injuries detected at autopsy. The remainder sustained the treatment without complications ().
Radiography
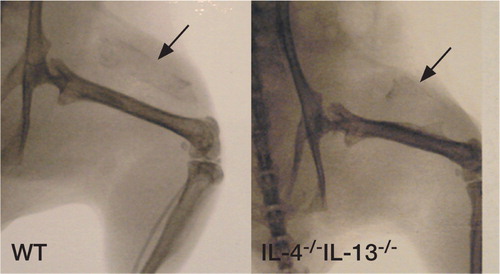
Animals of both sexes in both groups, (IL‐4-/-IL‐13-/- and WT), were examined by radiography of the implant sites after being killed at 5 weeks. Radiographic signs of mineralization were evident in most, but not all, animals (). Repeated radiographs of dissected implants did, however, confirm mineralization in all specimens (data not shown).
Mineralization as determined by ash weight of the DXBM implants
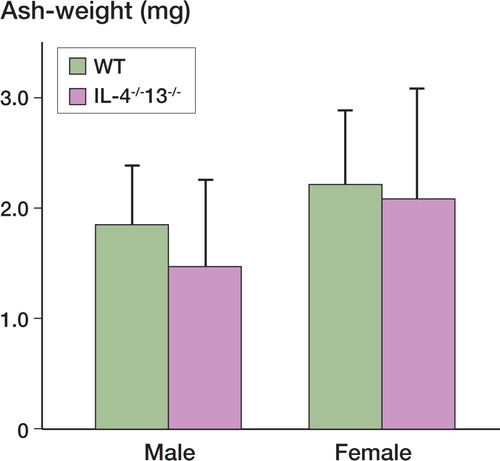
When mean values of ash weight at 5 weeks in IL‐4-/-IL‐13-/- and WT mice of both sexes were compared, no statistically significant differences in the amount of mineralization were detected. The mean values were: male WT mice, 1.85 mg (n = 10); male IL‐4-/-IL‐13-/- mice, 1.48 mg (n = 8); female WT mice, 2.2 mg (n = 12); female IL‐4-/-IL‐13-/-mice, 2.08 mg (n = 11). Thus, there was a tendency to be less bone in the IL‐4-/-IL‐13-/- mice (), but this was not statistically significant.
Histology
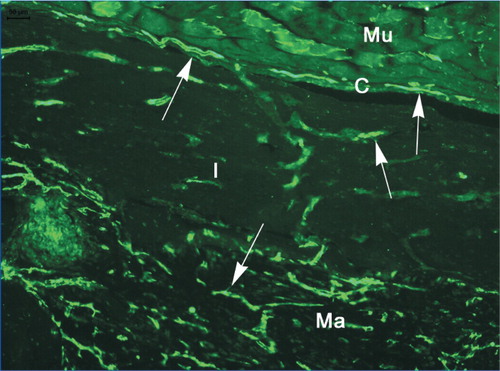
H&E staining revealed bone formation in 13/14 implants in IL‐4-/-IL‐13-/- mice and in 13/13 implants in WT mice. Thus, there was no difference between wild‐type and knockout mice, female or male, in occurrence of new bone formation. In all the implants, an ossicle was found with a surrounding periosteum‐like capsule of connective tissue. Extensions of this fibrous tissue were found in the implants as finger‐like taps and islands, and they showed a floating transition to the bone marrow. A vascular endothelial network was found (PECAM‐1) in the implanted matrix and newly formed marrow. Within the implant matrix, positive PECAM‐1 staining was found but only in WT males and females. In the capsule and surrounding muscular tissue, the vessels resembled a dense network in both IL‐4-/-IL‐13-/- and WT mice regardless of gender ( and ). No positive immunoreactivity to nerve fibers was found in the DXBM matrix or marrow in any type of mouse, while the muscle showed immunoreactivity to all types of nerve fibers. In the capsule and muscular tissue, immature nerve fibers (as detected by GAP‐43 staining) and mature nerve fibers (as detected by PGP 9.5 staining) were present in all mice. These were seen as a network surrounding blood vessels or as single nerve fibers rich in varicosi‐ties and windings. Autonomic (NPY staining) and sensory (CGRP staining) nerve fibers were present in the surrounding muscular tissue in all mice. However, autonomic nerve fibers (NPY staining) were found in the capsules of WT male and female mice () but not in the capsules of IL‐4-/-IL‐13-/- mice, and sensory fibers (CGRP staining) were only found in the capsules of WT males but not in those of WT females ().
Table 6. Histology DXBM model
Discussion
Here we studied the effect of IL‐4 and IL‐13 depletion on bone regeneration in mice. We found recently that there was a selective reduction of cortical bone mass in adult male mice depeleted of these Th2‐related anti‐inflammatory cytokines (Silfversward et al. Citation2007). This finding prompted us to perform the present investigation, which was designed to investigate the role of IL‐4 and IL‐13 in bone formation. There are a number of essential events involved in fracture healing and bone induction, each of which may be influenced by alterations in cytokine levels. Injury affects the immuno‐logical system, with alterations in T‐cell response and cytokine production (Zellweger et al. Citation1995). Suppression of T‐cell proliferation is seen in the early stages after trauma. Earlier studies on Th2 type cytokines have been inconclusive. However, there is some evidence for a possible shift towards Th2 cytokine production following trauma (Mack et al. Citation1996, Meert et al. Citation1998, Kang et al. Citation2004). A number of important events regulate fracture healing, to restore the strength and integrity of the bone (Bolander Citation1992). A key event is the formation of new blood vessels—angiogenesis—which is in fact the hallmark of all types of wound healing. Callus is characterized by rapidly developing new vessels that are necessary to provide nutrients to the new, growing bone tissue (Folkman and Shing Citation1992, Street et al. Citation2000). Recruitment of pleuripotent cells, and their proliferation and differentiation into bone‐forming cells in response to trauma, or bone loss, is also crucial. Bone progenitor cells are recruited from the periosteum and endosteum by chemotaxis. They differentiate to become osteoblasts producing components of the extracellular matrix, most importantly type I collagen. The matrix is mineralized by hydroxyapatite crystals deposited around the collagen fibrils (Ducy et al. Citation2000, Szczesny Citation2002). This process is orchestrated by a variety of factors in the microenvironment of bone formation where growth factors, cytokines, and neuropeptides have important roles in regulating chemotaxis, proliferation, differentiation, and communication (Canalis et al. Citation1989, Sisask et al. Citation1996, Reddi 1998, Barnes et al. Citation1999).
In this context, we have now studied the possible roles of IL‐4 and IL‐13 in fracture healing and in heterotopic bone formation. These two experimental models were chosen since they both reflect physiological processes. Fracture healing has been studied earlier in different rodent models (Hiltunen et al. Citation1993, Olmedo et al. Citation1999, Bhandari and Shaughnessy Citation2001). Also, studies on treatment with growth factors have been published that have shown a positive effect of growth hormone (GH), insulin‐like growth factor (IGF‐1), and transforming growth factor-β1 (TGF-β1) on fracture healing. (Linkhart et al. Citation1996, Schmidmaier et al. Citation2002, Wildemann et al. Citation2003).
Here we adopted a modified version of a mouse femoral fracture model described by Skoglund et al. (Citation2002). In the fracture part of the study, we chose only adult male mice in which we had previously detected a selective cortical bone pheno type. Comparing IL‐4-/-IL‐13-/- and WT mice, we found no differences in fracture healing as evaluated by radiology, pQCT, or mechanics. In contrast to what was found in WT mice, however, autonomous and sensory nerve fibers could be detected in the marrow cavities of the fractured bones of male IL‐4/IL‐13 depleted mice by immunohisto chemistry, but this difference did not result in any difference in fracture healing or gross morphology of the calluses. We conclude that the reduction in cortical bone mass seen in the absence of the two Th2‐derived cytokines IL‐4 and IL‐13 was not reflected by alteration in fracture healing in adult male mice.
Bone formation may occur heterotopically, outside normal bone tissue borders, e.g. in some hereditary disorders (Shore et al. Citation2000) or as a complication of endoprosthetic surgery or soft tissue trauma. Heterotopic bone formation is made possible by chemotaxis of undifferentiated mesenchymal cells that become primed to differentiation along the chondroblastic and osteoblastic pathways of bone formation. This process of osteoin‐duction is also dependent on cytokine influences in the microenvironment, with bone morphogenetic proteins (BMPs) as important regulators (Urist et al. Citation1983, Linkhart et al. Citation1996, Wozney and Rosen Citation1998, Croteau et al. Citation1999).
In the second part of this study, we investigated the capacity to form heterotopic bone by induction of DXBM in IL‐4 and IL‐13 depleted adult mice of both sexes. Analysis of net bone formation at 5 weeks by radiography and ash weight measurements revealed no differences between IL‐4-/-IL‐13-/- and WT mice, although a tendency of less bone in the IL‐deficient mice was noted. By histology, we found an absence of autonomous nerves (by NPY staining) in the capsule of IL‐4-/-IL‐13-/-mice of both sexes. This finding is interesting since osteoblasts have been shown to express receptors for NPY, indicating that this neuropeptide may have a role in bone formation (Bjurholm et al. Citation1992). In addition, NPY has been shown to inhibit the effects of PTH in osteoblastic cells (Bjurholm et al. Citation1988). Thus, the lack of expression of NPY in the IL‐4 and IL‐13 deficient mice indicates one possible mechanism by which the reduced cortical bone in these mice may have been produced. In both groups, there was abundant vascularization of all parts of the implants, but no differences were detected except for less labeling within the inductive implant matrix of IL‐4 and IL‐13 deficient mice of both genders relative to WT mice. The importance of these histological findings is not clear at the moment since the effects of induction on net bone formation seem limited. They indicate another possible mechanism for inhibition of bone formation since vascularization of the inductive implant matrix is one prerequisite for the induction of cartilage and bone (Urist et al. Citation1983). However, the results also show that IL‐4 and IL‐13 have no major net effects on fracture healing or on heterotopic bone formation in mice. This is fully compatible with the rather subtle effect on bone noted in adult male mice only.
In conclusion, we could not identify any major effect of IL‐4 and IL‐13 depletion on fracture healing or heterotopic bone formation in mice, although this alteration results in reduced cortical bone mass in male mice. We detected altered expression of autonomous and sensory nerves in the bone marrow of fractured bone, and in the capsule surrounding induced heterotopic bone along with reduced angiogenesis within the implants. The reason for this lack of effect on bone regeneration can only be speculated upon. Experimental conditions with a strong signal for new bone formation or a possible alteration in immunological response secondary to the operative trauma might have influenced the results. In this way, the relatively weak effects of IL‐4 and IL‐13 in bone can be hidden by the effects of more powerful factors such as growth hormone (GH), insulin‐like growth factor (IGF‐1), bone morphogenetic proteins (BMPs), and transforming growth factor-β1 (TGF-β1).
Contributions of authors
CJS, GS (contributed equally to this study): planning of the study, animal breeding including genotyping, surgery, radiological evaluations, animal dissections, evaluation of mineralization by ash weight, statistics, and writing. SL: carried out all biomechanical work including calculations, statistics, and evaluation of results. CO: carried out all pQCT measurements together with calculations, statistics, and evaluation of pQCT results. AF: initiated the study, planning of animal breeding, surgery, and dissections of animals. ÖL: consultant in all aspects of bone biology and metabolism, and critical analysis of the manuscript. ON: conception and planning of the study, planning of animal breeding, surgery, histological evaluations, consultant in all aspects of the study, creation and critical analysis of the manuscript.
Acknowledgement
This work was supported by grants from the Swedish Cancer Society, the Swedish Rheumatism Association, the Swedish Medical Research Council (Grant No. K2001-73X-13512-028), the Swedish Foundation for Strategic Research, the European Commission, the Lundberg Foundation, the Torsten and Ragnar Söderberg Foundation, the Emil and Vera Cornell Foundation, and the Petrus and Augusta Hedlund Foundation. We express our sincere gratitude to Dr A.N. McKenzie, Laboratory of Molecular Biology, Medical Research Council, Cambridge, UK, for supplying the animals used in this study. We are grateful to Anna‐Lena Johansson and Anette Hansevi for skillful technical support. We also thank the SWEGENE Center for Bio‐Imaging (CBI) for technical support regarding image analysis.
- Barnes G L, Kostenuik P J, Gerstenfeld L C, Einhorn T A. Growth factor regulation of fracture repair. J Bone Miner Res 1999; 14(11)1805–15
- Bhandari M, Shaughnessy S. A minimally invasive percutaneous technique of intramedullary nail insertion in an animal model of fracture healing. Arch Orthop Trauma Surg2001; 2001; 121(10)591–3
- Bjurholm A, Kreicbergs A, Schultzberg M, Lerner U H. Parathyroid hormone and noradrenaline-induced enhancement of cyclic AMP in a cloned osteogenic sarcoma cell line (UMR 106) is inhibited by neuropeptide Y. Acta Physiol Scand 1988; 134(3)451–2
- Bjurholm A, Kreicbergs A, Schultzberg M, Lerner U H. Neuroendocrine regulation of cyclic AMP formation in osteo blastic cell lines (UMR-106-01, ROS 17/2.8, MC3T3-E1, and Saos-2) and primary bone cells. J Bone Miner Res 1992; 7(9)1011–9
- Bolander M E. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 1992; 200(2)165–70
- Canalis E, McCarthy T L, Centrella M. The role of growth factors in skeletal remodeling. Endocrinol Metab Clin North Am 1989; 18(4)903–18
- Canalis E, McCarthy T L, Centrella M. Growth factors and cytokines in bone cell metabolism. Annu Rev Med 1991; 42: 17–24
- Croteau S, Rauch F, Silvestri A, Hamdy R C. Bone mor-phogenetic proteins in orthopedics: from basic science to clinical practice. Orthopedics 1999; 22(7)685–695, quiz 696-7
- Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science 2000; 289(5484)1501–4
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267(16)10931–4
- Frost A, Jonsson K B, Brandstrom H, Ljunghall S, Nilsson O, Ljunggren O. Interleukin (IL)-13 and IL‐4 inhibit proliferation and stimulate IL‐6 formation in human osteo blasts: evidence for involvement of receptor subunits IL-13R, IL-13Ralpha, and IL-4Ralpha. Bone 2001; 28(3)268–74
- Hiltunen A, Vuorio E, Aro H T. A standardized experimental fracture in the mouse tibia. J Orthop Res 1993; 11(2)305–12
- Kang S C, Matsutani T, Choudhry M A, Schwacha M G, Rue L W, Bland K I. Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage?. Cytokine 2004; 26(5)223–30
- Kon T, Cho T J, Aizawa T, Yamazaki M, Nooh N, Graves D. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 2001; 16(6)1004–14
- Lewis D B, Liggitt H D, Effmann E L, Motley S T, Teitel-baum S L, Jepsen K J. Osteoporosis induced in mice by overproduction of interleukin 4. Proc Natl Acad Sci U S A 1993; 90(24)11618–22
- Linkhart T A, Mohan S, Baylink D J. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone (1 Suppl) 1996; 19: 1S–12S
- Mack V E, McCarter M D, Naama H A, Calvano E S, Daly J M. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg 1996; 131(12)1303–1308, discussion 1308-9
- McKenzie G J, Fallon P G, Emson C L, Grencis R K, McKenzie A N. Simultaneous disruption of interleukin (IL)-4 and IL‐13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med 1999; 189(10)1565–72
- Meert K L, Ofenstein P J, ,Sarnaik A P. Altered T cell cytokine production following mechanical trauma. Ann Clin Lab Sci 1998; 28(5)283–8
- Nilsson O S, Bauer H C, Brosjo O, Tornkvist H. Influence of indomethacin on induced heterotopic bone formation in rats. Importance of length of treatment and of age. Clin Orthop 1986, 207: 239–45
- Olmedo M L, Landry P S, Sadasivan K K, Albright J A, Meek W D, Routh R. Regulation of osteoblast levels during bone healing. J Orthop Trauma 1999; 13(5)356–62
- Reddi A H. Initiation of fracture repair by bone morphoge-netic proteins. Clin Orthop 1998, 355 Suppl: S66–72
- Schmidmaier G, Wildemann B, Heeger J, Gabelein T, Flyvbjerg A, Bail H J. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor‐1 and transforming growth factor-beta1. Bone 2002; 31(1)165–72
- Shore E M, Glaser D L, Gannon F H. Osteogenic induction in hereditary disorders of heterotopic ossification. Clin Orthop 2000, 374: 303–316
- Silfversward C J, Frost A, Brandstrom H, Nilsson O, Ljunggren O. Interleukin-4 and interleukin‐13 potentiate interleukin‐1 induced secretion of interleukin‐6 in human osteoblast-like cells. J Orthop Res 2004; 22(5)1058–62
- Silfversward C J, Larsson S, Ohlsson C, Frost A, Nilsson O. Reduced cortical bone mass in mice with inactivation of interleukin‐4 and interleukin-13. J Orthop Res 2007; 25(6)725–31
- Sisask G, Bjurholm A, Ahmed M, Kreicbergs A. The development of autonomic innervation in bone and joints of the rat. J Auton Nerve Syst 1996; 59(1-2)27–33
- Skoglund B, Forslund C, Aspenberg R. Simvastatin improves fracture healing in mice. J Bone Miner Res 2002; 17(11)2004–8
- Street J, Winter D, Wang J H, Wakai A, McGuinness A, Redmond H R. Is human fracture hematoma inherently angiogenic?. Clin Orthop 2000, 378: 224–237
- Szczesny G. Molecular aspects of bone healing and remodeling. Pol J Pathol 2002; 53(3)145–53
- Urist M R, DeLange R J, Finerman G A. Bone cell differentiation and growth factors. Science 1983; 220(4598)680–6
- Wildemann B, Schmidmaier G, Ordel S, Stange R, Haas N P, Raschke M. Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater 2003; 65(1)150–156
- Windahl S H, Vidal O, Andersson G, Gustafsson J A, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest 1999; 104(7)895–901
- Wozney J M, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop 1998, 346: 26–37
- Zellweger R, Ayala A, DeMaso C M, Chaudry I H. Trauma-hemorrhage causes prolonged depression in cellular immunity. Shock 1995; 4(2)149–53