Abstract
Background and purpose For early detection of postoperative infections, the level of C‐reactive protein (CRP) may be useful. We analyzed baseline and time‐dependent reference values for the postoperative use of CRP as an indicator of infection.
Methods We studied the kinetics of CRP levels after fracture surgery in 1,418 patients. In 787 cases the operative fracture treatment was uneventful; in 17 of the other cases a deep wound infection occurred.
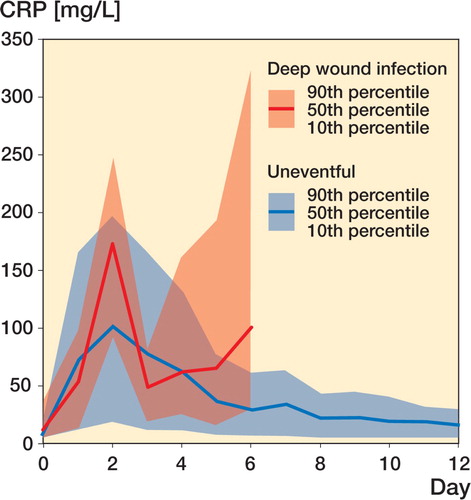
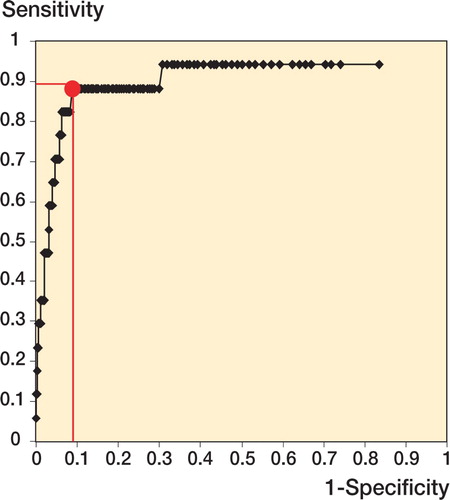
Results In the uneventful cases, a similar evolution in CRP concentrations was found: the peak level, which occurred on the second postoperative day, depended on the region (136 mg/L in femoral fractures and 45 mg/L in ankle fractures) and reflected the extent of surgical trauma. For deep wound infection, a cutoff level of 96 mg/L (sensitivity 92%, specificity 93%) after the fourth day of surgery was recorded.
Interpretation CRP kinetics permit establishment of a time‐dependent set of reference values of CRP after operative fracture treatment. Deviations of this course—especially CRP concentrations above 96 mg/L after the fourth day—may aid in early detection of surgical complications.
C‐reactive protein (CRP), an acute‐phase reactant, was discovered in the serum of patients with pneumonia by Tillett and Francis in Citation1930. At that early time, CRP was accepted as a parameter of severity for clinical diseases. CRP belongs to the humoral response of the immune system and functions by activating opsonization and the complement system, and in modulation of platelet activation. CRP is a marker of general tissue damage in addition to inflammation, which is also used in cardiology (Ledue and Rifai Citation2003). It is superior to the conventional parameters (leukocyte counts, eryth‐rocyte sedimentation rate) in highlighting surgical complications with bacterial infection (Ellitsgaad et al. Citation1991, Choudhry et al. Citation1992). However, CRP was not found to be predictive of organ failure and sepsis in a small number of patients (Giannoudis et al. Citation1998). CRP is also considered to reflect the extent of surgical trauma (Neumaier et al. Citation2006), whereas it appears to have no correlation with severity of injury or predicted survival in polytrau‐matized patients (Gosling and Dickson Citation1992). It is thought to be a basic parameter to enable the surgeon to monitor patients with infection (Foglar and Lindsey Citation1998). In orthopedic surgery, it may be difficult to differentiate between elevation of CRP caused by postoperative infection and elevation of CRP caused by surgery. To detect infections earlier, it is of importance to know the natural kinetics of CRP after operations. Specific data exist regarding CRP concentrations after abdominal surgery (Lindberg et al. Citation2002). Also, there are published CRP data for some specific orthopedic operations: total hip arthroplasty shows CRP levels on the second postoperative day ranging from 99 mg/L to 150 mg/L (Niskanen et al. Citation1996, White et al. Citation1998) and total knee arthroplasty has levels of about 160 mg/L (Kragsbjerg et al Citation1995, White et al. Citation1998). No reliable data exist for CRP concentrations after uneventful operative fracture treatment.
The current study was designed to establish a baseline for the use of CRP levels in operative fracture treatment and to define a set of reference values in uneventful cases and in cases with postoperative infection. Deviations from the normal course may indicate an infection or may help to detect surgical complications early and thus provide a better outcome for patients.
Patients and method
Data were collected prospectively. The study was approved by the Hospitals Study Committee, Munich. None of the patients had a blood test for the purposes of the study itself but based on the standard procedures and guidelines of the department. Exclusion criteria were clinical signs of infection on admission, neoplasia, a history of operative procedures within the previous 3 months, multiple injuries, and CRP levels greater than 50 mg/L preoperatively.
1,418 patients who had an operative fracture treatment were involved in the study. 631 patients had postoperative complications (large hematoma, urinary tract infection, thrombosis, dislocation, refracture, and (in 17) a deep infection) or missing values. In the remaining 787 patients, the postoperative course was uneventful and these patients were divided into 5 groups according to region of frac ture: group 1, femur (n = 362, mean age 73); group 2, tibia (n = 113, mean age 41); group 3, humerus (n = 88, mean age 64); group 4, elbow and forearm (n = 61, mean age 47); and group 5, ankle and miscellaneous small bones (n = 163, mean age 45).
Analysis of CRP was done using an immunotur‐bidometric technique on a Hitachi 912 autoanaly ser (Analysesystem Roch/Hitachi Tina‐quant CRP Gen.3; Hitachi Europe Ltd, Frankfurt, Germany). Blood samples were obtained on admission, on the first preoperative day, and on at least 3 occasions between day 1 and day 12 postoperatively. An average of 6 measurements per patient were evaluated
Statistics
Analysis of variance (ANOVA, Scheffe post hoc test) was used for a comparison between groups. The Mann‐Whitney U test was used for compari son between two independent variables at the same time. The level of significance was set at p < 0.05 using a 2-tailed test. Statistical analysis was done using SPSS for Windows. A receiver operating characteristic (ROC) with analysis of the Youden index, defined as being equal to sensitivity + specificity - 1, was used to obtain a cutoff level for CRP in deep wound infection.
Results
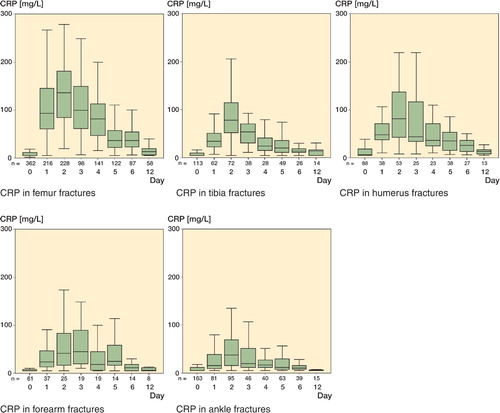
For uneventful cases, preoperative CRP levels were slightly increased (at most 10 mg/L) above normal reference values (< 5 mg/L). Postoperatively, the CRP rose to a maximum on the second day, and then concentrations returned to normal approximately 12 days after surgery. In each of the 5 groups, the postoperative maximum was reached on the second day after surgery ( and ). Femoral fractures showed the highest levels, differing statistically significantly from all other regions. Maximum levels showed the following order: femur fractures, humerus fractures, tibia fractures, forearm fractures, and finally ankle fractures.
Figure 1. CRP concentrations in the different fracture groups as shown by boxplots with median and interquartiles, and tenth and ninetieth percentiles.
Median CRP concentrations (mg/L) for each group on the second postoperative day, with 90th percentiles
There was an association between CRP levels and the amount of surgical trauma, comparing different surgical techniques within one group. Femoral neck fracture treatment with percutaneous screws gave a second‐day maximum of 72 mg/L compared to 159 mg/L after arthroplasty of femoral neck fractures (p < 0.001).
In 17 cases, deep wound infection occurred (as diagnosed by microbiological tests) and persistent elevation or a second rise in CRP concentrations was seen. No statistically significant differences in CRP concentrations were seen in the first 4 days after surgery. The first time a deviation became obvious was after the fourth day, depending on the onset of infection (). By ROC analysis (), a cutoff level of 96 mg/L after the fourth day of surgery was calculated for patients with a deep wound infection with a Youden index of 0.841 (sensitivity of 92% and a specificity of 93%).
Discussion
Preoperative CRP levels are important in order to rule out pre‐existing infection (Ng Citation1997). It is known that a distinctive pattern of natural CRP response occurs after an accident (Foglar and Lindsey Citation1998). The normal preoperative CRP level after fracture is slightly increased. Patients treated nonoperatively never reach the CRP levels seen in patients after operative treatment (Kallio et al. Citation1990). The CRP response does not appear to be correlated with age, sex of the patient, operation time, amount of bleeding, transfusion, drugs administered, or the type of anesthesia (Larsonn et al. 1992), but patients with autoimmune diseases such as rheumatoid arthritis may show elevated CRP levels (Ng Citation1997, Schwenger et al. Citation1998).
We have shown that CRP concentrations depend on the region of trauma, with the maximum levels occurring on the second postoperative day. Reliable and comparable data exist for arthroplasty of the hip or knee (140-160 mg/L) (Kragsbjerg et al. Citation1995, Okafor and Maclellan Citation1998, White et al. Citation1998) and spinal surgery (70-170 mg/L) (The lander and Larsson Citation1992).
Our findings confirm the results of previous studies (Kristiansson et al. Citation1999, Neumaier et al. Citation2006) that reported that CRP levels reflect the extent of surgical trauma. CRP concentrations on the second postoperative day were highest after femur fracture treatment and lowest after ankle fracture treatment.
For the 17 deep wound infections, a cutoff level of 96 mg/L was found after the fourth day of surgery. Although 17 patients do not provide good statistical power, all patients had similar CRP patterns that differed statistically significantly from those of healthy controls.
Just using a single CRP value (after the fourth day) is, however, unreliable for detection of deep infection, and a minimum of a preoperative and a second‐day CRP are needed. Since some patients might be discharged before the fifth postoperative day, monitoring of CRP levels on an outpatient basis would be helpful. CRP concentrations must be correlated to the clinical situation because every bacterial inflammation can cause high levels of CRP (e.g. pulmonary infection). No laboratory value alone can account for clinical decision making.
CRP has previously been shown to be of value for detection of postoperative complications, especially deep wound infection (Sanzen and Carls son Citation1989, Larsson et al. Citation1992, Foglar and Lindsey Citation1998). A second rise in and/or a persistent elevation of CRP is indicative of complications. It is thus necessary to know the natural CRP response and the baseline for different fracture treatments and trauma regions. A patient with a CRP level above 96 mg/L after the fourth day of surgery is very likely to have a deep wound infection if no other reason (e.g. pneumonia) can be found. CRP concentrations above the 90% percentile in the time‐dependent reference limit diagram () after operative fracture treatment of the extremities are suspicious and warrant further diagnostic measures.
Contributions of authors
MN: collection of data, processing of data, statistics, writing of manuscript. MAS: adviser, supervisor, writing of manuscript.
No competing interests declared.
- Choudhry R R, Rice R P, Triffitt P D, Harper W M, Gregg P J. Plasma viscosity and C-reactive protein after total hip and knee arthroplasty. J Bone Joint Surg (Br) 1992; 74: 523–4
- Ellitsgaad N, Andersson A P, Jensen K V, Jorgensen M. Changes in C-reactive protein and erythrocyte sedimentation rate after hip fractures. Int Orthop 1991; 15: 311–4
- Foglar C, Lindsey R W. C-reactive protein in orthopedics. Orthopedics 1998; 21: 687–91
- Gaine W J, Ramamohan N A, Hussein N A, Hullin M G, McCreath S W. Wound infection in hip and knee arthroplasty. J Bone Joint Surg (Br) 2000; 82: 561–5
- Giannoudis P V, Smith M R, Evans R T, Bellamy M C, Guillou P J. Serum CRP and IL-6 levels after trauma. Acta Orthop Scand 1998; 69: 184–8
- Gosling P, Dickson G R. Serum C-reactive protein in patients with serious trauma. Injury 1992; 23: 483–6
- Kallio P, Michelsson J, Lalla M, Holm T. C-reactive protein in tibial Fractures. J Bone Joint Surg (Br) 1990; 72: 615–7
- Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrationes of interleukin-6, tumour necrosis factor-α, and Creactive protein in patients undergoing major operations. EurJSurg 1995; 161: 17–22
- Kristiansson M, Saraste L, Soop M, Sundqvist K G, Thörne A. Diminished interleukin-6 and C-reactive protein responses to laparoscopic versus open cholecystectomy. Acta Anaesthesiol Scand 1999; 43: 146–52
- Larsson S, Thelander U, Friberg S. C-reactive protein levels after elective orthopedic surgery. Clin Orthop 1992; 275: 237–42
- Ledue T B, Rifai N. Preanalytic and analytic sources of variations in C-reactive protein measurement: implications for cardiovascular disease risk assessment. Clin Chem 2003; 49: 1258–71
- Lindberg M, Hole A, Johnsen H, et al. Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand J Clin Lab Invest 2002; 62: 189–94
- Neumaier M, Metak G, Scherer M A. C-reactive Protein as a parameter of surgical trauma. Acta Orthop 2006; 77: 788–90
- Ng T. Erythrocyte sedimentation rate, plasma viscosity and C-reactive protein in clinical practice. Br J Hosp Med 1997; 58: 521–3
- Niskanen R O, Korkala O, Pammo H. Serum C-reactive protein levels after total hip and knee arthroplasty. J Bone Joint Surg (Br) 1996; 78: 431–3
- Okafor B, Maclellan G. Postoperative changes of erythrocyte sedimentation rate, plasma viscosity and C-reactive protein levels after hip surgery. Acta Orthop Belg 1998; 64: 52–6
- Sanzen L, Carlsson A S. The diagnostic value of C-reactive protein in infected total hip arthroplasies. J Bone Joint Surg (Br) 1989; 71: 638–41
- Schwenger V, Sis J, Breitbart A, Andrassy K. CRP levels in autoimmune disease can be specified by measurement of procalcitonin. Infection 1998; 26: 274–6
- Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine 1992; 17: 400–4
- Tillett W S, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med 1930; 52: 561–71
- White J, Kelly M, Dunsmuir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg (Br) 1998; 80: 909–11