Abstract
Bone marrow transplantation can lead to osteogenic repair of intractable bone conditions. To achieve optimal clinical results, it is necessary to transplant as many cells with osteogenetic potential as possible. However, approaches involving special equipment and reagents for the extraction and purification of cells are expensive, and the complicated procedures involved are a hindrance to widespread acceptance of bone marrow transplantation for osteogenic repair. To standardize bone marrow transplantation for bone regeneration, a simple, safe, clean, and low‐cost system is required. We describe an easy‐to‐use method using a conventional manual blood bag centrifugation technique traditionally used for extracting buffy coats, for concentration of cells from bone marrow aspirates (BMAs) to obtain osteogenic progenitors.
Many groups have reported transplantation of autologous bone marrow with a minimally invasive approach for the osteonecrosis and nonunion of bone in which vascular networks and self‐repairing ability are compromised (Healey et al. Citation1990, Connolly et al. Citation1991). Bone regeneration can be expected after transplantation of bone marrow that contains multipotent cells (Barry Citation2003). Several recent reports have shown the efficacy of concen trated bone marrow aspirate (Gangji and Hauzeur Citation2005, Hernigou et al. Citation2005) and bone marrow mononuclear cells (Yasunaga et al. Citation2007) for osteogenic repair. To obtain optimal results, it is necessary to transplant as many cells with osteogenetic potential as possible. In previous studies, cell separator and density gradient centrifugation procedures have been used to obtain high cell concentrations. However, approaches involving special equipment and the reagents needed for extraction and purification of cells and growth factors are expensive. Also, complicated procedures prevent the widespread acceptance of bone marrow transplantation for osteogenic repair.
The “manual blood bag centrifugation” technique is traditionally used for extraction of buffy coat (BC), which contains most of the nucleated cells (Pietersz et al. Citation1990). We hypothesized that osteogenic progenitor cells can be efficiently extracted from bone marrow using a technique that is equivalent to buffy coat extraction. We introduced the simple method of using conventional manual blood bag centrifugation for concentration of osteogenic progenitor cells and growth factors from clinical bone marrow aspirates (BMAs).
Material and methods
Harvesting and concentration of bone marrow aspirates
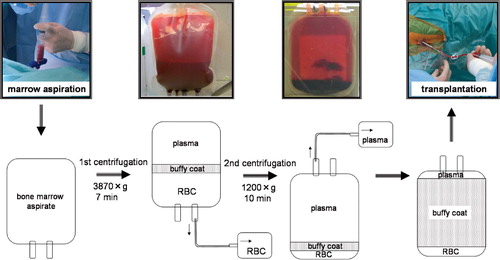
Under general anesthesia, bone marrow aspirates were obtained from the iliac crests of 10 patients diagnosed as having osteonecrosis of the femoral head, using an 11G bone marrow harvest needle (Medical Device Technologies, Inc., Gainesville, FL) with 20-mL syringes prefilled with 1.5 mL of the anticoagulant citrate dextrose to prevent blood clotting. The contents of each syringe were then transferred into a bag from a bone marrow collection kit (Baxter, Deerfield, IL). The bone marrow aspirate was then gravity‐filtered through a series of mesh filters with successively smaller diameters. The bone marrow aspirate was transferred to a quadruple blood bag (Terumo, Tokyo, Japan) for concentration of blood using the manual blood bag centrifugation technique. The quadrant blood bag containing the bone marrow aspirate was processed by a two‐step centrifugation method using a centrifuge (Kubota 9800, Kubota, Japan) at room temperature. After inverse centrifugation of the blood bag at 1,200 × g for 10 min, the red cells were transferred into the satellite bag until the interface between the plasma and the red blood cell layers reached a level 15 mm from the bottom of the bag. Next, using a high‐speed centrifugation at 3,870 × g for 7 min, plasma was transferred slowly into a satellite bag until about 4 cm of plasma was left on top of the buffy coat layer ().
All procedures were approved by an institutional ethical review committee of University of Tsukuba. Informed consent was obtained from all individuals who participated in this study prior to the operation.
Hematological analysis
10 series of samples of BMA and bone‐marrow blood cells (bm‐BCs) were analyzed. The numbers of nucleated cells were determined with an automated hematology analyzer (K‐4500; Sysmex, Kobe, Japan). The recovery rates of nucleated cells after concentration were calculated as follows. Recovery rate (%) = (total no. of nucleated cells in samples after concentration) / (total no. of nucleated cells in samples before concentration) x 100.
Fibroblastic colony‐forming‐unit (CFU‐F) assay
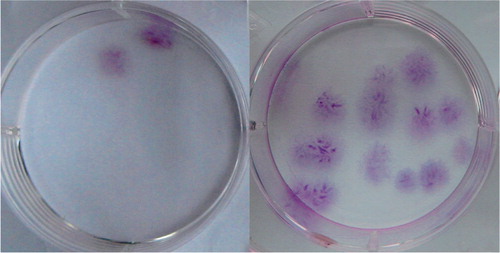
To determine the presence and proportion of progenitor cells, CFU‐F assays were performed with BMA and bm‐BC samples from all individuals. 100 |μL of the samples were washed with phosphate‐buffered saline twice and suspended in 3 mL growth medium. The growth medium consisted of Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (FBS) (Gibco) and antibiotics (anti‐biotic‐antimicotic solution; Gibco BRL). Samples were seeded onto 60‐cm2 dishes and cultured at 37°C in a humidified atmosphere of 5% CO2. The medium was replaced 2 days after plating and non‐adherent cells were removed. Thereafter, it was replaced twice a week. 2 weeks later, the medium was removed and the dishes were stained with 0.5% crystal violet (Sigma) in methanol for 5 min. The cells were washed twice with distilled water and the number of CFU‐Fs was counted. Colonies less than 2 mm in diameter and those colonies that were only faintly stained were ignored. The concentration (number of CFU‐Fs per mL of sample) and prevalence of the progenitor cells (number of CFU‐Fs per 106 nucleated cells) were calculated and plotted. The recovery rate of CFU‐Fs after concentration was calculated as follows.
Recovery rate (%) = (total no. of CFU‐Fs in samples after concentration) / (total no. of CFU‐Fs in samples before concentration) × 100.
Statistics
Differences in the nucleated cell counts, the concentrations of CFU‐Fs and the prevalence of CFU‐Fs between BMA samples and concentrated bone marrow samples were determined using the Mann‐Whitney U‐test. Error bars denote standard devia tion (SD). Differences with a p‐value of < 0.01 were considered statistically significant.
Results
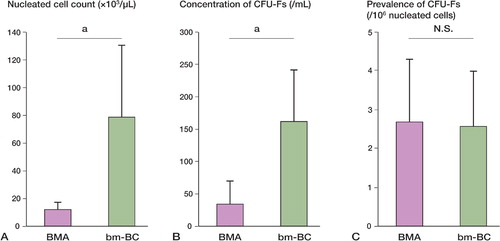
An average of 31 (SD 7.6) mL of bm‐BC extracts was obtained from an average of 336 (SD 88) mL of bone marrow aspirate with anticoagulant. 57% (SD 10) of the nucleated cells and 64 (SD 7)% of CFU‐Fs were obtained from the bm‐BC samples in an approximate volume of only 9% of the original bone marrow aspirate. In the bm‐BC samples, nucleated cells were concentrated 7‐fold (A). The concentrations of CFU‐Fs in the bm‐BC samples were approximately 5‐fold higher than in the preconcentrated bone‐marrow aspirates (B, ). The prevalence of CFU‐Fs was 2.65 cells per 106 nucleated cells in the preconcentrated bone marrow aspirates and 2.55 cells per 106 nucleated cells in bm‐BCs (C).
Figure 2. A. The nucleated cell counts were higher in the bm‐BC samples than in the BMA samples (p = 0.003). B. The concentrations of CFU‐Fs in concentrated bone marrow were higher than those in BMAs (p < 0.001). C. There were no statistically significant differences in the prevalence of CFU‐Fs in the 2 samples (p = 0.9).
Discussion
Hernigou and Beaujean (Citation2002) reported concentrated bone marrow transplantats for treatment of osteonecrosis and nonunion and recovered 84% of progenitor cells using blood cell separator. The efficiency of concentration of progenitor cells in our study was lower. However, the minimum number of transplanted osteoprogenitor cells that is necessary for the induction of bone formation has not yet been established. Additionally, we consider that effective bone regeneration can be achieved with the transplantation of concentrated bone marrow aspirate that contains a cocktail of various cell types.
For extracting an autologous tissue and using it as a material for clinical treatment, an easy‐to‐use method and reproducibility are required. Our procedure is simple and easy to operate; the time required for centrifugal separation is less than 1 h. Moreover, the system requires no specialized staff. The most important advantage of our system is that no special equipment other than the centrifuge and blood bag is required and can be carried out in most medical centers.
- Barry F P. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today 2003; 69(3)250–6
- Connolly J F, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop 1991, 266: 259–70
- Gangji V, Hauzeur J P. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg (Am) (Suppl 1 Pt 1) 2005; 87: 106–12
- Healey J H, Zimmerman P A, McDonnell J M, Lane J M. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop 1990, 256: 280–5
- Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop 2002, 405: 14–23
- Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg (Br) 2005; 87(7)896–902
- Pietersz R N, Dekker W J, Reesink H W. Comparison of a conventional quadruple-bag system with a ‘top-and-bottom’ system for blood processing. Vox Sang 1990; 59(4)205–8
- Yasunaga Y, Terayama H, Yamasaki T, Ishikawa M, Ochi M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow mononuclear cells. Clin Calcium 2007; 17(6)910–5