Abstract
Background and purpose It has been suggested that avascular osteonecrosis (AVN) of the femoral head develops early after renal transplantation. We evaluated the relationship between risk of AVN and dose of steroids administered in different time periods.
Methods Development of AVN was determined using MRI at 3–6 weeks, 9–12 weeks, 24 weeks, and 12 months after transplantation in 150 patients (96 males). We investigated possible associations between acute rejection reactions, the dose of cyclosporine, tacrolimus use, total steroid dose by the second, fourth, sixth, or eighth weeks after transplantation, and incidence of AVN.
Results There was no statistically significant difference between incidence of AVN and presence or absence of an acute rejection reaction. We found a statistically significant association between AVN incidence and the total dose of steroids administered during the first 2 months after transplantation, and there was a doseresponse relationship. No other statistically significant associations were found.
Interpretation Our findings confirm that the total dose of steroids given within the first 2 months after renal transplantation has a great influence on the incidence of AVN.
The pathogenesis of avascular osteonecrosis (AVN) has not been elucidated, but steroid administration, heavy consumption of alcohol, smoking, and abnormalities in lipid metabolism have been suggested to play a role (Heimann and Freiberger Citation1960, Felson and Anderson Citation1987, Hirota et al. Citation1993). Of these, steroids that are used for the treatment of underlying diseases—such as asthma or collagen disease, or in renal transplantation—appear to be strongly associated with the occurrence of AVN (Mont et al. Citation1997, Mok et al. Citation2000, Zonana-Nacach et al. Citation2000).
Renal transplantation requires administration of steroids for immune suppression. AVN is a major postoperative complication that develops within 12 weeks (Kubo et al. Citation1997, Fujioka et al. Citation2001) with a reported incidence of 3–40% (Harrington et al. Citation1971, Gustafsson et al. Citation1976, Ibels et al. Citation1978, Anderson and Nielsen Citation1981, Bradford et al. Citation1983, Julian et al. Citation1992, Tang et al. Citation2000). It has been shown that the total dose in a 1-year period or average daily dose of steroids is related to the risk of development of AVN (Harrington et al. Citation1971, Pierides et al. Citation1975, Chatterjee et al. Citation1976, Patton and Plaff Citation1988, Tang et al. Citation2000). However, no studies have been published that have evaluated the relationship between the risk of development of AVN and the total dose of steroids in the early period (). It may be that short-term, and also early, steroid administration may be a strong factor in the development of AVN.
Table 1. Previous reports on avascular necrosis (AVN) after renal transplantation
Since cyclosporine was introduced as an immunosuppressive agent to be used after renal transplantation, steroid dose and incidence of acute rejection has been reduced; as a result, the incidence of AVN has also been reduced (Canadian Multicentre Transplant Study Group Citation1983, Landmann et al. Citation1987). Today, the use of tacrolimus—a more potent immune suppressor—has reduced the steroid dosage further, and also the incidence of acute rejection. It has also been reported that tacrolimus itself may suppress the development of AVN (Sakai et al. Citation2003). To confirm these findings, we examined the difference in incidence of AVN after renal transplantation between a group that received cyclosporine and a group that received tacrolimus. We also studied the relationship between total dose of steroids over 2-, 4-, 6-, and 8-week periods after renal transplantation and the risk of AVN.
Patients and methods
Study design
We studied 150 patients (96 males) with a mean age of 34 (16–63) years. All had undergone renal transplantation at the Department of Transplantation and Endocrine Surgery, Kyoto Prefectural University of Medicine between March 1988 and June 1999. 108 patients received cyclosporine and 42 patients received tacrolimus. Cyclosporine was used in patients who underwent transplantation between March 1988 and December 1995. Either cyclosporine or tacrolimus was used in January 1996 (at the doctor's discretion), and tacrolimus was used in patients treated from February 1996 through June 1999. MRI of the hips was done made routinely in all patients preoperatively, at 3–6 weeks, at 9–12 weeks, at 24 weeks, and also 12 months after the transplantation. The presence or absence of AVN was determined based on the criteria of Sugano et al. (Citation1999). 37 patients (mean age 37 years) developed AVN and 113 patients (mean age 32 years) did not.
The incidence of osteonecrosis was compared with the total doses of cyclosporine and tacrolimus administered in the first 8 weeks. In the group that received cyclosporine alone, the administration started intravenously during surgery at a dose of 3 mg/kg, and continued orally from immediately after the surgery at a dose of 8 mg/kg. Then, depending on the result of the pharmacokinetic study conducted at that time, the dose was adjusted individually to the maintenance dose. The mean total dose of cyclosporine by 8 weeks after transplantation was 10,784 (SD 4,725, range 8,300–32,150) mg. In the group that received tacrolimus alone, the administration started intravenously during the surgery at a dose of 0.06 mg/kg, and continued orally from immediately after the surgery at a dose of 0.2 mg/kg. Then, depending on the result of the pharmacokinetic study, the dose was adjusted individually to the maintenance dose. The mean total dose of tacrolimus by 8 weeks after transplantation was 585 (SD 209, range 192–1,418) mg.
Administration of steroids
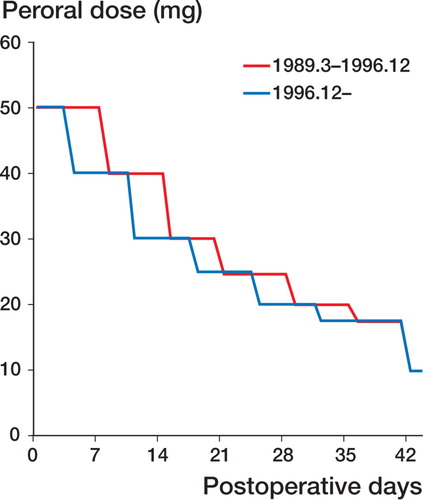
In all patients, methylprednisolone (MPSL; 500 mg) was administered intravenously during surgery, and prednisolone (PSL; 50 mg) was administered intravenously immediately after surgery. From the day after surgery, PSL at 50 mg, divided into 3 doses a day, was administered orally for 7 days (from March 1989 through November 1996) or for 3 days (after December 1996). After that, the oral dose was gradually reduced to 40, 30, 25, 20, and 17.5 mg every 7 days, and the maintenance dose of 10 mg was attained by the sixth week (Figure). In case of acute rejection (AR), bolus administration of MPSL was given. This bolus was used in 24 patients at an average dose of 3,111 (SD 1,748 mg, range 250–8,350) mg. The dose of MPSL depended on the severity of AR. The total doses of steroids given in the 2-, 4-, 6-, and 8-week periods after transplantation were calculated. Steroid administration as pulse therapy against AR was converted to PSL and added to the total dose in the 2-, 4-, 6-, and 8-week periods after transplantation. The dose of MPSL was added after conversion into PSL, based on its potency.
In order to examine the relationship between development of AVN and steroid dose, all cases (including those that developed AVN and those that did not) were divided into thirds (lower, middle, and upper thirds) based on the steroid dose in each period. Incidence of AVN was compared among each third at 2-, 4-, 6-, and 8-week periods after transplantation using univariate analysis and multivariate analysis adjusted for age, gender, AR status, and whether or not there was administration of tacrolimus. The steroid doses (lower, middle, and upper thirds) at the 2-week period were < 550 mg, 550–650 mg, and > 650 mg, respectively. Those of the 4-week period were < 895 mg, 895– 1,130 mg, and > 1,130 mg, respectively. Those of the 6-week period were < 1,165 mg, 1,165–1,488 mg, and > 1,488 mg, respectively. Those of the 8- week period were < 1,400 mg, 1,400–1,795 mg, and > 1,795 mg, respectively. Risk of AVN development in the middle- and upper-dose groups was evaluated against that of the lower-dose group.
Protocol for oral steroid administration. From the day after surgery, prednisolone (50 mg) was administered orally for 7 days (from March 1989 through November 1996), or for 3 days (after December 1996). After that, the oral dose was tapered down to 40, 30, 25, 20, and 17.5 mg every 7 days, and the maintenance dose of 10 mg was attained by the sixth week.
Statistics
The relationship between AVN and presence or absence of AR, and also sex, was analyzed using chi-squared tests. The incidences of AVN in the cyclosporine and the tacrolimus groups were compared by univariate analysis (crude odds ratio) and multivariate analysis (adjusted odds ratio), adjusting for age, sex, AR status, and steroid dose by 8 weeks after transplantation. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated using a conditional logistic regression model. These analyses were performed using SAS software version 6.12. We considered p-values less than 0.05 to be statistically significant.
Results
There was no statistically significant relationship between development of AVN and the presence or absence of AR, and sex (data not shown). The steroid dose in the tacrolimus group was lower than that in the cyclosporine group at all time periods after surgery ().
Table 2. Relationship between the type of immune suppressor and the dose of steroid administered
With the group that received cyclosporine taken as the reference, AVN in the group that received tacrolimus decreased to 0.52 and 0.67 by univariate and multivariate analyses, respectively. This difference was not statistically significant ().
Table 3. Relationship between immune suppressor and development of AVN. Adjusted for age, sex, acute rejection, and steroids at 8 weeks
When the group that received a middle-dose of steroids was compared with the low-dose group for the 2-week period, the ORs for development of AVN were not statistically significantly higher by univariate or multivariate analysis (crude OR = 2.4, p = 0.08). The OR for the upper-dose group was 2 (p = 0.2). There was no significant dose-response relationship between the steroid dose and the risk of AVN. For the 4-week period, ORs in the middledose group were higher, and the risk of developing AVN was higher in the group that received a higher dose of steroids (crude OR = 4.2, p = 0.008), but there was no statistically significant dose response in both the univariate and multivariate analyses (p = 0.2 and 0.3, respectively). The ORs for the upperdose group were not significantly higher than for the lower-dose group. In the 6-week period, differences were observed in the ORs of the middledose group by univariate and multivariate analyses (crude OR = 3.3, p = 0.02), but there was no statistical evidence of a dose-response relationship (p = 0.4 and 0.4, respectively) similar to that seen for the 4-week period. The OR for the upper-dose group was 1.8 (p = 0.277). In the 8-week period, the ORs for the middle-dose group were higher (crude OR = 4.3, p = 0.02; adjusted OR = 5.6, p = 0.02), and the ORs for the upper-dose group were also higher (crude OR = 4.0, p = 0.02; adjusted OR = 7.4, p = 0.01). In addition, there was a significant dose-response relationship in both groups (p = 0.04 and 0.02, respectively) ().
Table 4. The relationship between development of AVN and dose of steroids. Adjusted for age, sex, acute rejection, tacrolimus and cyclosporine
The odds ratio can overestimate the relative risk in cross-sectional studies with binary outcome analyzed by a logistic regression model. Thus, we performed additional analyses to estimate hazards ratio (HR) by Cox regression model, with equal follow-up times assigned to all individuals (Barros and Hirakata Citation2003). We found adjusted HRs (95% CIs) of 2.9 (0.9–9.1) at ≤1,795 mg and 3.2 (0.9–11) at > 1,795 mg, as compared to ≤1,400 mg of cumulative steroid dose over 8 weeks from renal transplantation (p = 0.07 and 0.07, respectively).
Discussion
Reported risk factors for AVN after renal transplantation include administration of steroids, the duration of dialysis before transplantation (Patton and Plaff Citation1988), the number of acute rejection episodes (Harrington et al. Citation1971), hyperparathyroidism (Chatterjee et al. Citation1976), and hypophosphatemia (Briggs et al. Citation1972). Numerous studies have been performed at multiple facilities regarding the dose and method of steroid administration in an attempt to reduce the incidence of AVN. Tacrolimus has gradually replaced cyclosporine. Tacrolimus is a more potent immune suppressor than cyclosporine, which has made it possible to reduce the dose of steroids after renal transplantation (Pirsch et al. Citation1997). In this study, we could confirm that a lower dose of steroid was indeed used in the tacrolimus group.
We found no relationship between the risk of developing AVN and the type of immune suppressor used (cyclosporine or tacrolimus): the steroid dose rather than the type of immune suppressor had the strongest influence on development of AVN.
Many studies have shown a connection between steroid administration and AVN development. Felson and Anderson (Citation1987) examined the total dose and oral dose of steroids administered in the 1-, 3-, 6-, and 12-month periods after transplantation and found a relationship between the oral dose in each time period and development of AVN. Harrington et al. (Citation1971) examined 150 patients who had undergone renal transplantation. Of 50 patients who had received an average total dose of steroids of 2,960 mg in the 3-week period after transplantation, 16 developed AVN. Of 101 patients who had received an average total dose of steroids of 1,180 mg in the 3-week period after transplantation, only 2 developed AVN. They therefore concluded that the total steroid dose in the 3-week period after transplantation was an etiological factor in the development of AVN. Tang et al. (Citation2000) confirmed development of AVN using MRI in 16 of 47 patients who had undergone renal transplantation, and they reported a relationship between the total dose of steroids in the 1-year period after transplantation and the development of AVN. However, since AVN develops early after the administration of steroids, there is a need to investigate the relationship between AVN development after transplantation and the short-term steroid dose.
We examined the relationship between steroid dose in the 2-, 4-, 6-, and 8-week periods after transplantation and the development of AVN, by both univariate and multivariate analysis. In the 2- week period after transplantation, odds ratios for AVN in the middle- and upper-dose steroid groups rose, though not statistically significantly. Thus, there is a possibility that steroid dose has some effect on the development of AVN even in the very early period of 2 weeks after transplantation. In the 4- and 6-week periods after transplantation, ORs for the middle-dose group rose significantly and those for the upper-dose group also rose, but not significantly. Although a significant dose-response relationship was not proven, this finding indicates that there is some relationship between increase in the steroid dose and development of AVN. Besides, in the 8-week period after transplantation, ORs for the middle- and upper-dose groups rose significantly, and a significant dose-response relationship was observed. This indicates that the risk of developing AVN increases depending on on the steroid dose as early as 8 weeks after transplantation, and reduction of the total dose administered in the 8- week period following transplantation is important in order to reduce the risk of AVN.
Contributions of authors
This study was supported by the fund of the Japanese Investigation Committee for Osteonecrosis of the Femoral Head, under the auspices of the Ministry of Health and Welfare of Japan.
- Andersonm J, Nielsen H E. Osteonecrosis in renal transplant recipients. Early radiological detection and course. Acta Orthop Scand 1981; 52(5)475–9
- Barros A J, Hirakata V N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3: 21
- Bradford D S, James P C, Simmons R S, Najarian J S. Total hip arthroplasty in renal transplant recipients. Clin Orthop 1983, 181: 107–14
- Briggs W A, Hampers C L, Merrill J P, Hager E B, Wilson R E, Birtch A G, Murray J E. Aseptic necrosis in the femur after renal transplantation. Ann Surg 1972; 175: 282–9
- Canadian Multicentre Transplant Study Group. A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med 1983; 309: 809–15
- Chatterjee S N, Massry S G, Friedler R M, Singer F R, Berne T V. The high incidence of persistent secondary hyperparathyroidism after renal transplantation. Surg Gynecol Obstet 1976; 143: 440–2
- Felson D T, Anderson J J. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet 1987; 18: 902–5
- Fujioka M, Kubo T, Nakamura F, Shibatani M, Ueshima K, Hamaguchi H, et al. Initial changes of non-traumatic osteonecrosis of femoral head in fat suppression images: bone marrow edema was not found before the appearance of band patterns. Magentic Resonance Imaging 2001; 19: 985–91
- Gustafsson L A, Meyers M H, Berne T V. Total hip replacement in renal transplant recipients with aseptic necrosis of the femoral head. Lancet 1976; 2: 606–8
- Harrington K D, Murray W R, Kountz S L, Belzer F O. Avasucular necrosis of bone after renal transplantation. J Bone Joint Surg (Am) 1971; 53(2)203–15
- Heimann W G, Freiberger R H. Avascular necrosis of the femoral and humeral head after high dosage corticosteroid therapy. N Engl J Med 1960; 263: 672–5
- Hirota Y, Hirohata T, Fukuda K, Mori M, Yanagawa H, Ohno Y, Sugioka Y. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol 1993; 137: 530–8
- Ibels L S, Alfrey A C, Huffer W E, et al. Aseptic necrosis of bone following renal transplantation: Experience in 194 transplant recipients and review of literature. Medicine (Baltimore) 1978; 57: 25–45
- Julian B A, Quarles D, Niemann K M W. In-depth review. Musculoskeletal complications after renal transplantation : Pathogenesis and treatment. Am L Kidney Diseases 1992; XIX: 99–120
- Kubo T, Yamazoe S, Sugano N, Fujioka M, Naruse S, Yoshimura N, et al. Initial MRI. ndings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging 1997; 15: 1017–23
- Landmann J, Renner N, Gachter A, Thiel G. Cyclospolin A and osteonecrosis of femoral head. J Bone Joint Surg (Am) 1987; 69: 1226–8
- Mok M Y, Farewell V T, Isenberg D A. Risk factors for avascular necrosis of bone in patients with systemic lupus erythematosus: Is there a role for antiphospholipid antibodies?. Ann Rheum Dis 2000; 59: 462–7
- Mont M A, Glueck C J, Pacheco I H, Wang P, Hungerford D S, Petri M. Risk factors for osteonecrosis in systemic lupus erythematosus. J Rheumatol 1997; 24: 654–62
- Patton P R, Plaff W W. Aseptic bone necrosis after renal transplantation. Surgery 1988; 103: 63–8
- Pierides A M, Simpson W, Stainsby D, et al. Avascular necrosis of bone following renal transplantation. Q J Med 1975; 44: 459–80
- Pirsch J D, Miller J, Deierhoi M H, Vincenti F, Filo R S. A comparison of tacrolimus(FK506) and cyclosporine in vivo. Transplantation 1997; 63: 977–83
- Sakai T, Sugano N, Kokado Y, Takahara S, Ohzono K, Yoshikawa H. Tacrolimus may be associated with lowet osteonecrosis rates after renal transplantation. Clin Orthop 2003, 415: 163–70
- Sugano N, Kubo T, Takaoka K, Ohzono K, Hotokebuchi T, Matsumoto T, et al. Multicenter study of diagnostic criteria for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg (Br) 1999; 81: 590–5
- Tang S, Chan T M, Lui S L, Li F K. Risk factors for avascular bone necrosis in renal transplantation. Transplantation Proc 2000; 32: 1873–5
- Zonana-Nacach A, Barr S G, Magder L S, Petri M. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum 2000; 43: 1801–8