Abstract
Background and purpose Loosening of a total knee replacement may lead to loss of bone, requiring biological reconstruction at revision arthroplasty. Good results have been reported from revision arthroplasty of the hip using impaction bone grafting. We report our results of revision total knee arthroplasty using the same technique.
Patients and methods We retrospectively analyzed 30 patients (involving 34 knees) with a mean age of 63 (34–81) years who, between 1994 and 2002, underwent revision arthroplasty of the knee using hinge or rotational knee prostheses (Link) and impaction bone grafting. The average follow-up was 4 (2–9) years and included a questionnaire, a clinical examination, and standardized radiographs.
Results 25 patients were satisfied with their results. 10 patients reported no impairment in their activities of daily living attributed to their operation and did not need any walking support. In 5 patients, there were no clear radiographic signs of incorporation of the graft but that did not compromise the outcome. 5 other patients had complications due to aseptic loosening of their prostheses with radiographic failure of the graft, leading to a periprosthetic fracture in 2 cases.
Interpretation Our results with impaction bone grafting in knee revision arthroplasty appear to be similar to those obtained by the same technique in revision hip surgery.
Impaction bone grafting (IBG) was originally developed, and used with cement for acetabular reconstruction, by Sloof et al. (Citation1993). The technique was adapted by Gie et al. (Citation1993) for reconstruction of the femoral side in failed total hip arthroplasty. Loosening of total knee replacements may also lead to extensive bone defects. The cancellous bone of the distal femur and proximal tibia is usually severely damaged or lost completely, leaving a sclerotic tube that often has large peripheral deficiencies, whereas the diaphyseal cortical structure is usually intact above and below the knee. Reconstruction of bone defects is a challenge in revision total knee arthroplasty, and various techniques have been reported including IBG, and bulk allograft and metal augments (Rand Citation1991, Bradley Citation2000, Clatworthy et al. Citation2001, Dennis Citation2002).
For patients with small, contained defects (intact cortex), primary-type prostheses can be used. Larger defects require special implants with stems to engage the residual diaphyseal bone, and with augmented articular surfaces to rest on the rim of the deficient femoral and tibial metaphyses. Standard implants will not fit the size of larger defects without filling of the bone cavities by cement, which will lead to further peripheral defects of bone stock—worsening the situation if renewed loosening occurs.
The surgeon's choice of autogenous bone grafting is often complicated by the massive extent of the bone defect. In these cases, although autogenous bone might be the best choice, large quantities of autograft of sufficient quality are often difficult to harvest—especially in elderly patients. In view of the good long-term results of IBG in revision total hip arthroplasty (Schreurs et al. Citation1998), we used IBG for biological reconstruction in knee revisions, which has shown promising results (Wilde et al. Citation1990, Whiteside Citation1993, Ullmark and Hovelius Citation1996, Lotke et al. Citation2006). Here we report the outcome of exchange arthroplasty with a hinge or rotational knee prosthesis combined with IBG.
Patients and methods
36 patients underwent revision total knee replacement in the ENDO-Clinic, Hamburg, between 1994 and 2002. All patients had a revision total knee arthroplasty because of aseptic loosening and a type 1–3 bone defect (see below) that required allograft reconstruction in the femur, the tibia, or both. In all cases, infection was excluded by microbiological evaluation of preoperatively aspirated synovial fluid and intraoperative biopsies. 6 patients died of unrelated causes before the followup examination. They had had no pain or other substantial problems in the operated knee until they died. We performed a retrospective study of the remaining 30 patients (mean age 63 (34–81) years; 18 men, 34 reconstructions). The average follow-up time was 4 (2–9) years.
Femoral head allografts from the ENDO-Clinic bone bank were used in all cases. 8 grafts were placed on the femoral side and 8 were placed on the tibial side. 9 cases consisted of both femoral and tibial allograft augmentation. All patients had a bone reconstruction with IBG. In 3 cases, a rotational knee (Waldemar Link, Hamburg, Germany) was changed to a hinge prosthesis (Link) and the opposite was performed in 7 cases. Complete or partial exchange of a hinge prosthesis was performed in 20 cases. In 4 cases, the femoral component of a rotational knee (Link) was changed (). All prostheses were fixed with cement.
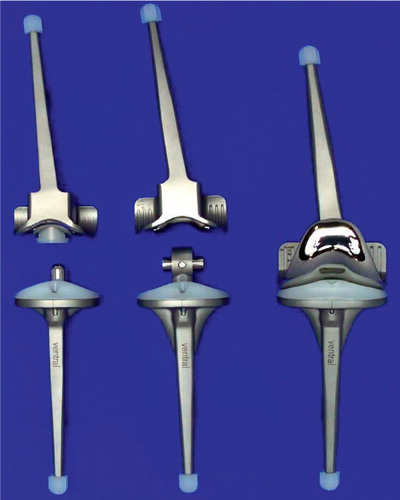
Figure 1. Both rotational (left) and hinge (center) knee prostheses, optionally furnished with patella flange (right). Flexion and rotation of the rotational knee prosthesis take place in a cross-joint. A connecting piece, which is fixed to the tibial component and links it to the femoral component of the hinge knee prosthesis, features a borehole for the joint axis.
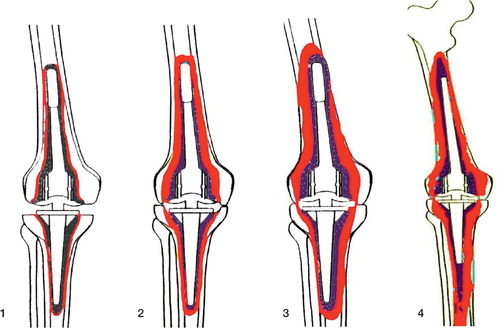
The amount and type of bone loss was rated according to the ENDO classification (Engelbrecht Citation1993), which is based on radiographic and intraoperative findings (). There were 23 type-1 defects, 10 in the femur and 13 in the tibia, 8 type- 2 defects, 6 in the femur and 2 in the tibia, and 3 type-3 defects, 1 in the femur and 2 in the tibia. Type-4 defects were not treated with IBG.
Figure 2. ENDO classification (Engelbrecht Citation1993) with regard to constrained knee prostheses: increasing loss of bone stock (colored red) from type 1 to type 4. Type-1 defects are minor bone defects with thin radiolucent lines but no subsidence of components. Type-2 defects involve moderate bone loss, evidenced by wider radiolucent lines around the entire implant and clear signs of subsidence. Depending on the model used, there may be thinning of the distal femoral or tibial proximal cortex or of the cancellous bone within the region of the femoral and tibial condyles. Type-3 defects are severe loss of bone with widening of the distal femur or proximal tibia, cortical thinning with perforations, and severe condylar defects. Type-4 defects are massive: there is total or severe loss of bone stock. More than half of the femur is defective, or the remains of the middle or proximal parts are weakened by severe cortical thinning or severe osteoporosis from lack of use.
Surgery
The loose prosthesis was removed together with cement, debris, and fibrous membrane. The caverns were reamed to obtain continuity with the femoral and tibial marrow canals, and to ensure exposure of viable bone at all accessible surfaces. The marrow canal was occluded by a firm-fitting acrylic plug or homologous bone plug placed at the end of the revision cavern. IBG was then performed with instruments for bone transplantation in knee revision surgery developed by Ullmark and Hovelius (Citation1996). A centralizing device screwed into the plug was left in place during the graft impaction procedure. Bone grafts were prepared from frozen femoral heads using a bone mill. The morselized grafts were partially defatted by washing in saline solution at 40°C. Then the grafts were packed firmly down on top of the plug in the distal end of the revision cavern with an impactor, until they reached some centimetres above the planned level of the tip of the stem. The entire cavern was then filled with morselized grafts that were gently pressed together. An impactor “dummy” was then inserted on the centralizer down in the grafted cavern to obtain firm graft impaction. Additional grafts were then impacted up to the end of the impactor. The centralizing device was then detached. After that, the impactor was extracted and antibiotic-loaded cement was filled into the remaining cavern with a cement gun. The cement was pressurized for a few seconds to force it into the grafted bone. The prosthesis was inserted at an early stage of cement polymerization.
The postoperative management varied according to the extent of the operation. Early movement was encouraged, and patients were allowed to bear weight (limited to 10 kg) for 2–10 weeks according to the surgeon's evaluation. This was followed by increasing weight bearing, ranging from 10–20 kg every week. Only 3 patients were permitted full weight bearing immediately after the operation. Full weight bearing was allowed when there was evidence of union at the allograft-host bone junction.
Follow-up
The Knee Society score (KSS) was used for clinical assessment (Insall et al. Citation1989). Anteroposterior and lateral radiographs were taken immediately in the recovery room after surgery, before starting full weight bearing, and at the followup examination. Radiographic allograft incorporation was evaluated according to de Waal Malefijt et al. (Citation1995) ().
Radiographic assessment of bone graft incorporation
Results
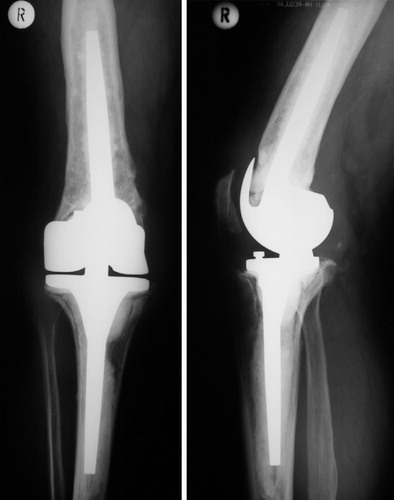
There were no substantial intraoperative complications and no deep infections. 5 knees (in 5 patients) were revised during the study period because of aseptic loosening. One patient (A) had failure of the femoral graft after 8 months (). The replacement was changed to a cemented hingetype prosthesis with restricted axial rotation mechanism. The cavity was reconstructed again using IBG, with a good result at latest follow-up (median KSS = 156 points).
Figure 3. Postoperative control after IBG reconstruction of the femoral cavity in patient A, who had a type-3 defect.
3 patients (B, C, and D) had failure of the tibial graft after 19, 32, and 34 months, respectively. In 2 of these cases (B and C) the loose prosthesis was removed, the tibial marrow canal was prepared, and the tibial components were cemented—with good results by the time of follow-up (median KSS: 149 points and 161 points). Failure of the tibial graft in patient D required implantation of a cemented custom-made long-stemmed prosthesis, with moderate result at the time of follow-up (KSS = 121 points).
One patient (E) had failure of both the femoral and the tibial graft after 42 months, leading to a periprosthetic femoral fracture. A cemented custom-made implant had to be inserted, with moderate result at the follow-up examination (KSS = 101 points).
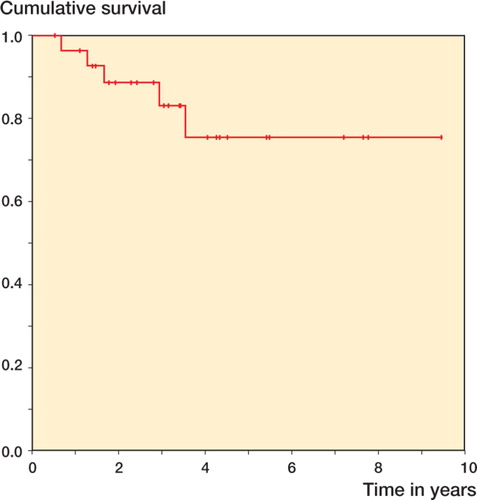
Prosthesis survival at 5 years was 76% (95% CI: 56–96). No knees were revised later than 5 years ().
Figure 4. Kaplan-Meier survivorship. The endpoint used was: any part of the prosthesis removed or revised for any reason.
In the 29 knees where our primary reconstruction had not been revised, there was no apparent radiographic bone loss. Bone graft incorporation with formation of trabecular pattern was found in 24 cases, with no signs of graft demarcation or graft resorption. The incorporation was unclear in 5 cases, with no organized trabecular formations and no well-defined graft demarcations. None of these patients had developed progressive widening of small circumscribed lytic lesions indicating graft resorption. Disintegration of the graft occurred in 5 other cases, with no trabecular pattern but osteolysis of the graft and adjacent host bone. Mild cortical hypertrophy was occasionally seen.
The median Knee Society score was 125 (93– 162) in the 21 patients with remaining prostheses and radiographic graft incorporation (24 with IBG). The score was median 130 (101–142) in the 5 patients with unclear incorporation (all 5 with IBG).
Discussion
The outcomes of revision arthroplasties are determined by a number of factors, the degree and management of bone stock loss being one of them. Cement filling or IBG is good for small, contained defects (Dorr et al. Citation1986, Lucey et al. Citation2000, Ritter and Harty Citation2004). However, there is no concensus on the best method to fill massive bone defects. Several treatment options have been discussed— including structural allograft, porous metal augments, or custom implants (Harrison et al. Citation2006, Radnay and Scuderi Citation2006, Engh and Ammeen Citation2007).
IBG, successfully used for hip revisions, was reported by Samuelson (Citation1988) in revision total knee arthroplasty of 22 patients using cementless stemmed components. The clinical results were similar to those in primary arthroplasty. Most grafts showed radiographic evidence of remodeling. Various bone reactions, not fitting any particular pattern, occurred around the stems. Early migration of the implants occurred in over a third of the grafts, but stabilized later on.
Whiteside (Citation1993) described morselized reconstruction of bony defects in 56 knees using longstemmed, uncemented prostheses augmented by screws through the tibial baseplate. All grafts had radiographic evidence of remodeling and all implants except 2 achieved stable fixation to bone. De Waal Malefijt et al. (Citation1995) performed IBG on the tibial side in 17, and on the femoral side in 6, of 36 cemented knee replacements. There was unquestionable radiographic incorporation in all except 2 in the tibia. Lotke et al. (Citation2006) reported favorable medium-term results in 48 consecutive cemented revision total knee arthroplasties with uncontained defects, and treated with IBG and additional steel mesh. He described no mechanical failures; there was incorporation and remodeling of all bone grafts, but there were 2 periprosthetic fractures related to poor host bone quality, 2 infections, and 2 patella clunk syndromes.
In our study, 5 of 34 allograft reconstructions failed. We had 2 periprosthetic fractures (in 3 ENDO-classification type-3 defects), which indicates that type-3 defects may represent a borderline situation with regard to IBG.
For radiographic evaluation, we used the division into 4 groups according to a previous study in which 3 radiographic aspects of the graft were assessed (de Waal Malefijt et al. Citation1995). An unclear (n = 5) or failed (n = 5) integration of the graft was found in 10 of the 34 reconstructions. Increased stability (reduction of micromovements between graft and implant) may be obtained by the use of larger-sized bone chips than those we used (Ullmark Citation2000).
Contributions of authors
WS: concept and design, acquisition of data, analysis and interpretation of data, drafting of article, and approval of the final version of the manuscript. JF: concept and design, analysis and interpretation of data, and writing of manuscript. JW: acquisition of data, interpretation, and analysis. AK: concept and design, and approval of the final version of the manuscript.
No competing interests declared.
- Bradley G W. Revision total knee arthroplasty by impaction bone grafting. Clin Orthop 2000; 371: 113–8
- Clatworthy M G, Balance J, Brick G W, Chandler H P, Gross A E. The use of structural allograft for uncontained defects in revision total knee arthroplasty. A minimum five-year review. J Bone Joint Surg (Am) 2001; 83(3)404–11
- Dennis D A. The structural allograft composite in revision total knee arthroplast. J Arthroplasty 2002; 17(Suppl 4)90–3
- de Waal Malefijt, van Kampen A, Sloof T J. Bone grafting in cemented knee replacement. Acta Orthop Scand 1995; 66(4)325–8
- Dorr L D, Ranawat C S, Sculco T A, McKaskill B, Orisek B S. Bone graft for tibial defects in total knee arthroplasty. Clin Orthop 1986; 205(4)153–65
- Engelbrecht E. Errors and pitfalls in total knee replacement. Eur Instr Course Lect 1993; 1: 10–8
- Engh G A, Ammeen D J. Use of structural allograft in revision total knee arthroplasty in knees with severe bone loss. J Bone Joint Surg (Am) 2007; 89(12)2640–7
- Gie G A, Linder L, Ling R S, Simon J P, Sloof T J, Timperley A J. Impacted cancellous allograft and cement for revision total hip arthroplasty. J Bone Joint Surg (Br) 1993; 75(1)14–21
- Harrison R J, Jr, Thacker M M, Pitcher J D, Temple H T, Scully S P. Distal femur replacement is useful in complex total knee arthroplasty revisions. Clin Orthop 2006; 446(5)113–20
- Insall J N, Dorr L D, Scott R, Scott W N. Rationale of the knee society clinical rating system. Clin Orthop 1989; 248(11)13–4
- Lotke P A, Carolan G F, Puri N. Impaction grafting for bone defects in revision total knee arthroplasty. Clin Orthop 2006; 446(5)99–103
- Lucey S D, Scuderi G R, Kelly M A, Insall J N. A practical approach to dealing with bone loss in revision total knee arthroplasty. Orthopedics 2000; 23(10)1036–41
- Radnay C S, Scuderi G R. Managment of bone loss: augments, cones, offset stems. Clin Orthop 2006; 446(5)83–92
- Rand J A. Bone deficiency in total knee arthroplasty. Use of metal wedge augmentation. Clin Orthop 1991; 271(10)63–71
- Ritter M A, Harty L D. Medial screws and cement: a possible mechanical augmentation in total knee arthroplasty. J Arthroplasty 2004; 19(5)587–9
- Samuelson K M. Bone grafting and non cemented revision arthroplasty of the knee. Clin Orthop 1988; 226(1)93–101
- Schreurs B W, Sloof T J, Burna P, Gardeniers J W, Huiskes R. Acetabular reconstruction with impacted morsellized cancellous bone graft and cement. J Bone Joint Surg (Br) 1998; 80(3)391–5
- Sloof T J, Schimmel J, Buma P. Cemented fixation with bone grafts. Orthop Clin North Am 1993; 24(4)667–72
- Ullmark G. Bigger size and defatting of bone chips will increase cup stability. Arch Orthop Trauma Surg 2000; 120(7–8)445–7
- Ullmark G, Hovelius L. Impacted morsellized allografts and cement for revision arthroplasty: a preliminary report of 3 cases. Acta Orthop Scand 1996; 67(1)10–2
- Whiteside L A. Cementless revision total knee arthroplasty. Clin Orthop 1993; 286(1)160–7
- Wilde A H, Schickendantz M S, Stulberg B N, Go R T. The incorporation of tibial allografts in total knee arthroplasty. J Bone Joint Surg (Am) 1990; 72(6)815–24