Abstract
Background and purpose Pharmacological modulation of skeletal muscle reperfusion injury after traumaassociated ischemia may improve limb salvage rates and prevent the associated systemic sequelae. Resuscitation with hypertonic saline restores the circulating volume and has favorable effects on tissue perfusion and blood pressure. We evaluated the effects of treatment with a bolus of hypertonic saline on skeletal muscle ischemia reperfusion (IR) injury and the associated end-organ injury.
Methods Adult male Sprague-Dawley rats (n = 27) were randomized into 3 groups: (1) a control group, (2) an IR group treated with normal saline, and (3) an IR group treated with hypertonic saline. Bilateral hindlimb ischemia was induced by application of a rubber band proximal to the level of the greater trochanters for 2.5 h. The treatment groups received either normal saline (4 mL/kg) or hypertonic saline (4 mL/kg) prior to tourniquet release. Following 12 h of reperfusion, the tibialis anterior muscle was dissected and muscle function was assessed electrophysiologically. The animals were then killed, and skeletal muscle and lung tissue were harvested for evaluation.
Results Hypertonic saline significantly attenuated skeletal muscle reperfusion injury, as shown by reduced myeloperoxidase content, wet-to-dry ratio, and electrical properties of skeletal muscle. There was a corresponding reduction in lung injury, as demonstrated by reduced myeloperoxidase content and reduced wet-to-dry ratio.
Interpretation Treatment with hypertonic saline attenuates skeletal muscle ischemia reperfusion injury and its associated systemic sequelae.
The local and systemic proinflammatory responses that accompany limb revascularization can adversely affect limb or patient survival (Yassin et al. Citation1996). Trauma is an important cause of an acutely ischemic limb, and the potential for limb salvage in this situation is greatly improved with modern methods of vascular surgery, fracture fixation, and soft tissue reconstruction. The muscle injury is characterized by permeability edema, impaired muscle function, and muscle necrosis (Ikebe et al. Citation2001). The associated systemic inflammatory response, of which lung is the most pertinent manifestation, represents the complex interplay between cytokine production and proinflammatory mediators—resulting in neutrophil recruitment and activation (Lechin and Varon Citation1994, Botha et al. Citation1995). Adhesion of neutrophils to the activated microvascular endothelium results in increased microvascular permeability and tissue injury from the release of reactive oxygen species and elastases (Welbourn et al. Citation1991, Weiser et al. Citation1996).
There has been renewed interest in hypertonic saline as a resuscitation fluid because of its favorable properties in the setting of trauma and hemorrhagic shock. It has been shown to be an effective means of restoring central hemodynamics, peripheral tissue perfusion, and oxygen delivery in states of hypovolemia (Younes et al. Citation1992). Furthermore, large randomized controlled human trials have established its safety and efficiency in this setting (Mattox et al. Citation1991, Wade et al. Citation1997). It also has beneficial immunomodulatory and anti-inflammatory properties, and has been shown to mediate dose-dependent inhibition of several neutrophil cytotoxic functions including expression of adhesion molecules, degranulation, and generation of reactive oxygen species (Junger et al. Citation1998, Ciesla et al. Citation2000). A treatment regime with a single bolus of hypertonic saline has not been thoroughly investigated in skeletal muscle ischemia reperfusion models. These observations led us to hypothesize that resuscitation with a bolus of hypertonic saline may protect against skeletal muscle ischemia reperfusion injury and its systemic sequelae.
Methods
Hindlimb ischemia and reperfusion model
We used adult male Sprague-Dawley rats (Biological Services Unit, University College Cork, Ireland) weighing 300–350 g in all experiments. A previously described rubber band model of tourniquet hindlimb ischemia and reperfusion was employed (Dillon et al. Citation2005). Briefly, under 60 mg/kg intraperitoneal (ip) thiopentone sodium anesthetic, bilateral rubber bands were applied above the greater trochanters to interrupt the arterial blood supply to the hindlimbs. Preliminary experiments employing several animals confirmed global ischemia and subsequent reperfusion with the aid of a laser Doppler blood flow monitor probe. The rubber bands were removed after 2.5 h, initiating hindlimb reperfusion. Animals used in this study were maintained in accordance with the guidelines of the Cruelty to Animals Act, 1876, of the Department of Health, Ireland, and those of the European Community Directive (86/609/EC).
Animal groups
The animals were randomized into 3 groups, with 9 per group. Animals in group A underwent anesthesia alone as a control for the anesthesia. Group B animals were given an intravenous injection (4 mL/kg) of normal saline (0.9% NaCl) 15 min before tourniquet release, followed by 12 h of reperfusion. Group C underwent a similar volume of intravenous injection of hypertonic saline (7.5% NaCl, which has a Na load 8.3 times that of normal saline) at the same time point. Ischemia-reperfusion was induced by application of rubber bands above the greater trochanters bilaterally. Following 12 h of reperfusion, the animals were then killed for harvesting of tissue.
Functional assessment of tibialis anterior muscle
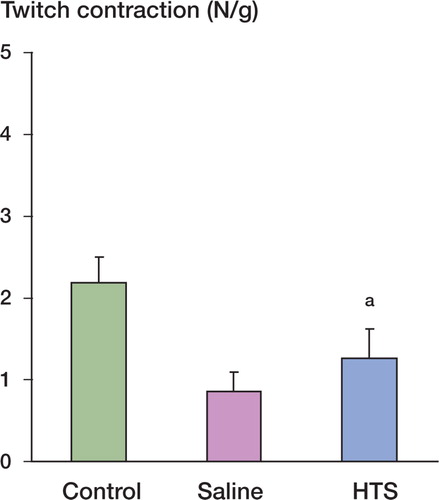
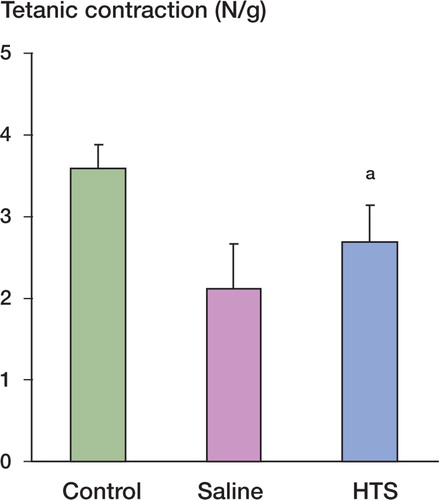
After 12 h of reperfusion, and while each animal was still under intraperitoneal anesthesia, the tibialis anterior muscle was exposed. The animals were fixed to an external frame in a supine position. A 2-0 silk suture was tied around the distal tendon, which was then sectioned and attached to a force transducer (AD Instruments) to measure the isometric contractile force. The muscle temperature was maintained at 33–34°C using an overhead heating lamp. The in situ muscle was stimulated directly (0.1 msec duration, 10 volts) via 2 electrodes connected to a stimulator (AD Instruments). The length of resting muscle was adjusted to produce maximum twitch tension. The isometric twitch contractile properties were determined. Tetanic tension in response to a tetanic electrical stimulus (50 Hz) was then recorded for each muscle. Twitch and tetanic contractions were reported as N per g of muscle weight.
Wet-to-dry ratio
Sections of gastrocnemius and lung were excised and weighed (wet weight). The muscle and lung tissue was then dried at 60°C in a convection oven for 72 h and reweighed (dry weight). Wet-to-dry ratios were calculated and used as an index of edema formation.
Myeloperoxidase assay
The right ventricle was cannulated and the right pulmonary hilum was clamped. The pulmonary vasculature of the left lung was flushed with 50 mL PBS to clear the lung of intravascular neutrophils. The lung was then weighed and homogenized in buffer A (0.021% K2HPO4, 0.66% KH2PO4 and 0.5% hexadecyltrimethyl ammonium bromide in distilled water). The homogenates were freeze-thawed 3 times and centrifuged at 2,000 rpm for 10 min. The supernatant was assayed spectrophotometrically for myeloperoxidase activity by adding 1 mL of supernatant to 2 mL of freshly prepared solution B. (Solution B was made by dissolving 0.0105 g K2HPO4 and 0.33 g KH2PO4 in 40 mL of distilled water and adding 5 mL of a 0.017% solution of dianisidine in methanol and 5 mL of 0.006% hydrogen peroxide in distilled water). The change in absorbance with time was then measured at 460 nm. 1 unit of myeloperoxidase was defined as that which degraded 1 micromole of hydrogen peroxide at 25°C, and was then calculated per gram of tissue. The procedure was repeated for fresh muscle samples.
Statistics
Statistical analysis was performed by one-way analysis of variance (ANOVA) with post hoc Tukey test analysis. Differences were considered significant at p-values < 0.05.
Results
Functional assessment of tibialis anterior muscle
Muscle contractile function was measured after 12 h of reperfusion and expressed as the peak tension achieved for each muscle for both twitch and tetanic contractions. Ischemia-reperfusion injury (IRI) impaired muscle twitch contraction compared with the control group. Resuscitation with hypertonic saline preserved twitch contraction after IRI (). Muscle tetanic contraction was also significantly reduced by IRI when compared with control and again, this reduction was attenuated with hypertonic saline resuscitation ().
Tissue edema in gastrocnemius muscle
For assessment of tissue edema, tissue wet-to-dry ratios were measured. The muscle wet-to-dry ratios in the hypertonic saline-treated group were significantly lower than those in the saline-treated group .
Skeletal muscle and lung neutrophil infiltration (myeloperoxidase activity) and edema formation (wet-to-dry-ratio). Values are expressed as mean (SD)
Effect of IRI on muscle neutrophil infiltration
Myeloperoxidase activity was employed as a marker of skeletal muscle neutrophil infiltration. IRI treatment resulted in a significant increase in myeloperoxidase activity as compared to controls. This increase was attenuated with hypertonic saline resuscitation (Table).
Effect of ischemia-reperfusion on lung injury
IRI resulted in significant remote organ injury, as measured by lung wet-to-dry ratio and myeloperoxidase activity. These markers of lung injury were significantly better in the hypertonic saline-treated group than in the saline-treated group (Table).
Discussion
Restoration of blood flow to an acutely ischemic limb initiates a cascade of cellular and biochemical events that result in muscle edema, necrosis, and impaired muscle function (Kearns et al. Citation2001). The associated lung injury is mediated by the adherence of activated neutrophils to the endothelium of the pulmonary microvasculature, resulting in spillage of protein-rich transudate into the alveolar spaces—hallmarks of the acute respiratory distress syndrome (ARDS) (Barry et al. Citation1997). While many agents including adenosine infusion and NF-κB inhibitors have been shown to attenuate lower torso ischemia reperfusion injury, the pharmodynamics or toxicity of these agents has limited their introduction into the clinical setting (Schroeder et al. Citation1996, Lille et al. Citation2001).
Our study shows that resuscitation with hypertonic saline reduces the tissue damage associated with the reperfusion injury that occurs following acute lower torso ischemia. Skeletal muscle injury, measured from leukosequestration and edema formation, was significantly lower with hypertonic saline treatment than with saline treatment. The functional injury induced by IRI was reflected in the maximum twitch and tetanic contraction forces. The preservation of the contractile properties in the hypertonic saline-treated group further emphasizes the suppression of the inflammatory response provoked by hypertonic resuscitation.
The associated systemic inflammatory response is believed to promote overexuberant microcirculatory activation, the release of reactive oxygen species and elastases causing microvascular damage, and subsequent tissue and organ injury (Yassin et al. Citation2002). This systemic inflammatory response, as measured by pulmonary injury, was also dampened by hypertonic saline resuscitation. As already mentioned, an aberrant and unbridled neutrophil-endothelial interaction is believed to play a central role in ischemia reperfusion injury and the beneficial effects of administration of hypertonic saline can be partly explained by its ability to reduce neutrophil activation. Hypertonicity attenuates several receptor-mediated neutrophil cytotoxic responses including adhesion molecule expression, release of proteolytic enzymes, and production of reactive oxygen metabolites (Pascual et al. Citation2003).
Much of the previous work on skeletal muscle ischemia reperfusion injury has focused on the effects of pretreatment with various agents (Kearns et al. Citation2001). While this is important to further our understanding of this complex process and to elucidate the underlying mechanisms involved, this does not always correlate with the clinical setting where ischemia is often a sudden unpredictable event. In our experiments, hypertonic saline was given after the ischemic insult and a significant reduction in injury was observed, thereby suggesting that it could be used as a therapeutic intervention.
In conclusion, our study demonstrates that resuscitation with hypertonic saline attenuates skeletal muscle ischemia reperfusion injury and its associated systemic sequelae in a rodent model. We therefore feel that resuscitation with hypertonic saline may be a potential therapeutic intervention for skeletal muscle reperfusion in the clinical setting.
Contributions of authors
JPD, AJL, and CJS: formulation of idea, experimental design, experiments, and writing of manuscript. JRSC: experimental design, experiments, and processing of samples. JHW: formulation of research idea, experimental design, and experiments. AMcG and HPR: formulation of research idea, experimental design, and research supervisors.
No competing interests declared
- Barry M C, Kelly C, Burke P, et al. Immunological and physiological responses to aortic surgery: effect of reperfusion on neutrophil and monocyte activation and pulmonary function. Br J Surg 1997; 84: 513–9
- Botha A J, Moore F A, Moore E E, et al. Early neutrophil sequestration after injury: A pathogenic mechanism for multiple organ failure. J Trauma 1995; 39: 411–7
- Ciesla D J, Moore E E, Gonzalez R J, et al. Hypertonic saline inhibits PMN priming via attenuation of p38 MAPK signaling. Shock 2000; 14: 265–70
- Dillon J P, Laing A J, Cahill R A, et al. Activated protein C attenuates acute ischaemia reperfusion injury in skeletal muscle. J Orthop Res 2005; 23: 1454–9
- Ikebe K, Kato T, Yamaga M, et al. Increased ischaemiareperfusion blood flow impairs the skeletal muscle contractile function. J Surg Res 2001; 99(1)1–6
- Junger W G, Hoyt D B, Davis R, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest 1998; 101: 2768–79
- Kearns S R, Moneley D, Murray P, et al. Oral vitamin C attenuates acute ischaemia-reperfusion injury in skeletal muscle. J Bone Joint Surg (Br) 2001; 83: 1202–6
- Lechin A E, Varon J. Adult respiratory distress syndrome (ARDS): the basics. J Emerg Med 1994; 12: 63–8
- Lille S T, Lefler S R, Mowlavi A, et al. Inhibition of the initial wave of NF-κB activity in rat muscle reduces ischaemia/ reperfusion injury. Muscle Nerve 2001; 24(4)534–41
- Mattox K L, Maningas P A, Moore E E, et al. Prehospital hypertonic saline/dextraninfusion for post-traumatic hypotension: the U.S.A. Multicenter Trial. Ann Surg 1991; 213: 482–91
- Pascual J L, Khwaja K A, Chaudhury P, Christou N V. Hypertonic saline and the microcirculation. J Trauma 2003; 54(5)S133–40
- Schroeder C A, Jr, Lee H T, Shah P M, et al. Preconditioning with ischaemia or adenosine protects skeletal muscle from ischaemic tissue reperfusion injury. J Surg Res 1996; 63: 29–34
- Wade C E, Kramer G C, Grady J J, et al. Efficacy of hypertonic 7.5% saline and 6% dextran-70 in treating trauma: a meta-analysis of controlled clinical studies. Surgery 1997; 122: 609–16
- Weiser M R, Gibbs S A, Valeri C R, et al. Anti-selectin therapy modifies skeletal muscle ischaemia and reperfusion injury. Shock 1996; 5: 402–7
- Welbourn C R, Goldman G, Paterson I S. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg 1991; 78: 651–5
- Yassin M, Barros D'Sa A, Parks G, et al. Mortality following lower limb ischaemia-reperfusion: A systemic inflammatory response?. World J Surg 1996; 20: 961–7
- Yassin M M, Harkin D W, Barros D'Sa A A, et al. Lower limb ischaemia reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg 2002; 26: 115–21
- Younes R N, Aun F, Accioly C Q, et al. Hypertonic solutions in the treatment of hypovolemic shock: a prospective, randomized study in patients admitted to the emergency room. Surgery 1992; 111: 70–2