Abstract
Background High-volume local infiltration analgesia with additional intraarticular and wound administration of local anesthetic has been shown to be effective after knee replacement, but the optimum site of administration of the local anesthetic (i.e. intraarticular or extraarticular) has not been evaluated.
Patients and methods 32 patients undergoing total knee replacement with high-volume (170 mL) 0.2% ropivacaine infiltration analgesia were randomized to receive injection of 20 mL ropivacaine (0.2%) intraarticularly plus 30 mL saline in the extraarticular wound space 24 hours postoperatively or to receive 20 mL ropivacaine (0.2%) intraarticularly plus 30 mL ropivacaine (0.2%) in the extraarticular wound space 24 hours postoperatively. Pain intensity at rest and with mobilization was recorded for 4 hours after administration of additional local anesthetics.
Results Intensity of pain at rest, during flexion, or straight leg lift was not statistically significantly different between the two groups, but there was a tendency of improved analgesia with administration of additional local anesthetic in the extraarticular wound space.
Interpretation The optimal site of administration of local anesthetic in total knee arthroplasty cannot be determined from the present study. However, the insignificant analgesic effect from additional administration of extraarticular local anaesthetic may have been due to the relatively low pain scores observed 24 h postoperatively, confirming the efficiency of the high-volume infiltration analgesia technique. Further studies are required to define the optimal site of administration of local anesthetic following knee replacement surgery.
Pain after total knee arthroplasty (TKA) is usually severe, limiting early rehabilitation and prolonging hospital stay. Recently, several techniques to improve analgesia after TKA have been intro-duced—such as (1) intraarticular administration of local anesthetics (Badner et al. Citation1996, Mauerhan et al. Citation1997, Klasen et al. Citation1999, Ritter et al. Citation1999, Tanaka et al. Citation2001, Browne et al. Citation2004), (2) a high-volume combined infiltration and local intermittent administration of anesthetic with an intraarticular catheter (LIA) (Reilly et al. Citation2005, Vendittoli et al. Citation2006, Busch et al. Citation2006, Andersen et al. Citation2007a, Citationb, Toft-dahl et al. 2007) and (3) a combined technique with infiltration plus continuous infusion in the wound, including the subcutaneous tissues (Bianconi et al. Citation2003). These techniques have been successful, although intraarticular administration per se may be less effective (Ritter et al. Citation1999). Since continuous infusion of a wound with local anesthetic may have a significant role in future postoperative pain management in TKA (Liu et al. Citation2006), there is a need for detailed systematic studies to evaluate the optimal site of administration of local anesthetic. The aim of this randomized, double-blind and pla-cebo-controlled study was therefore to evaluate and compare the specific effects of injecting local anesthetic into the intraarticular or extraarticular tissues after TKA using the LIA technique (Rostlund and Kehlet Citation2007).
Demographic data from total knee arthroplasty and local infiltration analgesia patients (n=30) randomized to receive either injection of 30 mL 0.2% ropivacaine (group R) or injection of 30 mL 0.9% saline (group S) upon retraction of the intraarticular catheter. Values are expressed as mean (range) where relevant
Patients and methods
The study was approved by the local ethics committee (Copenhagen and Frederiksberg, Denmark, Reg.No. KF-01329190) and conducted in accordance with the Helsinki Declarations. It was also registered with ClinicalTrials.gov under the US National Library of Medicine (code NCT00518674) and the Danish Data Protection Agency (Copenhagen, Denmark).
32 patients who were operated with unilateral TKA between October 2006 and May 2007 were included in this randomized, double-blind, pla-cebo-controlled trial. Data collection took place from October 2006 until May 2007. Patient characteristics were similar in the two groups (Table).
All patients received local infiltration analgesia with placement of an intraarticular catheter, and the same standardized regimen was followed until 24 h postoperatively when they were randomly assigned to receive injection with either 30 mL saline or 30 mL ropivacaine (0.2%) and epinephrine (10 µg/mL) upon retraction and removal of the intraarticular catheter. Exclusion criteria were being under treatment with opioids or steroids, being unable to understand or speak Danish, or having rheumatoid arthritis, immunological disease, history of stroke, neurological or psychiatric disease influencing pain perception, allergy to any of the drugs administered, or a body mass index (BMI) of>40.
Inclusion of patients and preparation of study medicine was done by an investigator who was not otherwise involved in data collection. Injection of the study medicine and registration of data was performed by one other investigator who was unaware of the randomization data.
Randomization was done using a computer-gen-erated random sequence concealed in 32 consecutively numbered opaque sealed envelopes, which were opened consecutively by the principal investigator after inclusion of each patient.
Procedures
All patients received lumbar spinal anesthesia with 12 mg hyperbaric marcaine (Bupivacaine) and optional sedation with propofol (0.5–5 mg/kg/h). In all patients, cefuroxim (1.5 g) was administered intravenously for infection prophylaxis and tranexamic acid (1 g) was given intravenously for control of hemostasis. Low-molecular-weight heparin (4,500 U) was administered subcutaneously 6–8 hours postoperatively for thromboprophylaxis, and then once daily until discharge. A standardized intraoperative regimen for fluid administration was applied, consisting of 0.9% saline at 5 mL/kg/h and colloid (Voluven) at 7.5 mL/kg/h. All patients received celecoxib (400 mg/day), acetaminophen (4 g/day), and gabapentin (900 mg/day) initiated preoperatively on the morning of surgery.
A tricompartmental cemented AGC prosthesis (Biomet-Merck, Warsaw, IN) inserted via a standard medial parapatellar approach was used in all patients. A femoral tourniquet (100 mmHg above systolic blood pressure) was used from incision until cementation of the prosthesis. No drain was used. Infiltration analgesia was performed with injection of 120 mL ropivacaine (0.2%) and epinephrine (10 µg/mL) and 50 mL ropivacaine (0.2%, without epinephrine), resulting in a total volume of 170 mL. The aim was to infiltrate all tissues incised or handled using a systematic technique, to ensure uniform delivery of the local anesthetic. The first 50 mL was injected into the posterior joint capsule and into both collateral ligaments after the bone cuts had been performed. 50 mL was then injected along the borders of and into the capsule, the cut quadriceps tendon, the infra-patellar ligament, possible remnants of the fat pad, the cruciate ligaments, and soft tissues surrounding the joint. Another 50 mL was infiltrated into the subcutaneous tissues before wound closure. To minimize the risk of cutaneous blister formation, the subcutaneous injections did not contain epinephrine. After insertion of the prosthesis and before joint closure, the surgeon placed an epidural 16-G catheter intraarticularly behind the medial posterior femoral condyle with the tip along the posterior aspect of the joint capsule. The catheter was inserted 10 cm proximal to the surgical incision in the lower anterior part of the thigh, through the subcutis and quadriceps tendon before penetrating the joint capsule. The remaining 20 mL of the drug mixture was injected through this catheter before wound closure.
6 and 12 h postoperatively, a bolus injection of 20 mL ropivacaine (0.2%) with epinephrine (10 µg/mL) was injected through the catheter as part of the postoperative analgesia regimen.
24 h postoperatively, both groups received an injection of 20 mL ropivacaine (0.2%) with epinephrine (10 µg/mL) intraarticularly through the catheter. In group R, 30 mL ropivacaine (0.2%) with epinephrine (10 µg/mL) was then injected upon retraction of the intraarticular catheter (1 mL/cm), ensuring uniform delivery of the local anesthetic to the extraarticular tissues from the joint capsule to the catheter insertion point, with delivery of local anesthetic primarily to the subcutaneous tissues. The distance from the insertion point of the catheter to the intraarticular space was estimated to be at least 15 cm, which would ensure delivery of at least 15 mL local anesthetic to the extraarticular and subcutaneous tissues. Group S received a similar injection with 30 mL 0.9% saline (1 mL/cm) upon retraction and removal of the catheter.
Study parameters
The primary endpoint was change in pain intensity over the 4-hour study period. All data were recorded by one investigator who was blinded as to the type of injection. Pain intensity was recorded from 24 h postoperatively, immediately before injection of medicine and every half hour for 4 h after injection. Pain was assessed using a numeric rank scale (NRS) from 0–10, where 0 indicated no pain and 10 indicated the worst imaginable pain. Pain was recorded at rest, upon 90° flexion of the knee, and with straight leg elevated 45°. In the 4-h study period, no additional analgesic was administered.
Statistics
The number of participants was arbitrarily set at 32, since no valid power calculation could be performed from previously published studies. For statistical analysis, the Mann-Whitney U test for non-parametric data was used for continuous numeric data that were not normally distributed. We considered p-values of<0.05 to be statistically significant. All data analysis was done with SPSS for Windows version 12.0.
Results
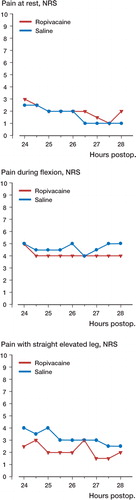
The two groups were similar when comparing the difference in median NRS pain scores reported before injection until 1.5 hours after injection, either at rest (p=0.07), upon 90° flexion of the knee (p=0.4), or with straight leg elevated 45° (p=0.1) (). Similarly, comparisons at all other time points were not statistically significant.
Figure 1. Pain scores for all 32 patients randomized to receive either 30 mL 0.2% ropivacaine (n=16) or 30 mL 0.9% saline (n=16) in the extraarticular wound space following total knee arthroplasty. p>0.05 when comparing groups in all assessments.
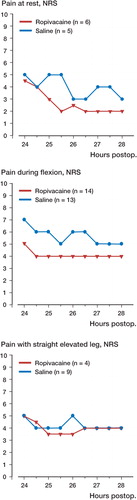
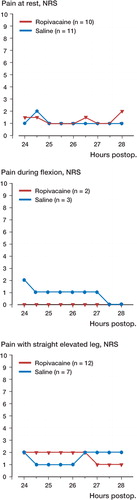
A subgroup analysis of patients who reported an NRS pain score of≥4 before injection showed a decrease at rest (mean difference 0.75, 95% confidence interval -0.25–1.75) in the combined group compared to intraarticular administration alone 1.5 hours after injection (), but this decrease was not statistically significant. There was no significant difference between groups regarding changes in NRS pain score in patients who reported NRS pain scores of<4 before injection (p>0.5) ().
Discussion
Our findings confirm that the LIA technique (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006, Toftdahl et al. Citation2007) is generally quite effective 24 hours after TKA, although many patients still have an NRS of≥4 during mobilization. Furthermore, our results show that in a well-defined group of patients there is on average no clinically relevant difference in analgesic effect of administration of 20 mL 0.2% ropivacaine intraarticularly or 20–35 mL 0.2% ropivacaine plus 15–30 mL 0.2% ropivacaine in the extraarticular wound space. This may be due to the fact that many patients had relatively low pain scores. In patients with a pain score of≥4 before administration of local anesthetic, there was a tendency (albeit not statistically significant) of improved analgesia when the additional 30 mL 0.2% ropivacaine was given in the extraarticular wound spaces while 20 mL 0.2% ropivacaine intraarticularly alone did not improve analgesia. These results may therefore have important clinical implications for future continuous local anesthetic administration studies in major joint replacement by suggesting that a significant amount, if not all, of the local anesthetic should be administered at the extraarticular wound spaces.
Our results may not be discordant with those reported previously, but with a different design. Thus, most randomized controlled trials on single-dose, local administration of anesthetic intraarticularly after TKA have shown no effect on pain (Badner et al. Citation1996, Klasen et al. Citation1999, Ritter et al. Citation1999, Browne et al. Citation2004) and the 6 randomized studies on LIA technique with a combined infiltration and local administration of anesthetic intraarticularly have shown relatively low pain scores, but no information is available on the analgesic effect of injection through the intraarticular catheter per se (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006, Andersen et al. Citation2007a, Citationb, Toftdahl et al. Citation2007). In the only randomized study with a combination of injection technique and continuous wound infusion at extraarticular sites, reduced pain scores were obtained (Bianconi et al Citation2003). Based on the results in the literature and those in the present study, future studies to improve analgesia with continuous wound administration techniques should therefore specifically focus on administration of all the local anesthetic in extraarticular wound spaces as opposed to in the intraarticular space per se. Such studies will be important to define the optimal site of administration of local anesthetic, since there is a limit to the amount of doses that can be given. In this context, there is also a need for volume vs. concentration studies since variable regimens have been used in the literature, which does not permit any conclusions to be drawn regarding the optimal dose and volume. Since the overall wound space after TKA is relatively small, it may be important to use a higher concentration of local anesthetic than those reported in the literature (about 0.2–0.25% ropivacaine or bupivacaine) and with a smaller volume. Such studies will undoubtedly have clinical implications for improvement of analgesia after TKA since the wound infusion techniques from all types of surgery have been reported to be safe and simple, with acceptable patient compliance (Liu et al Citation2006).
This study was supported by the IMK Almene Fund, Copenhagen, Denmark, and Astra-Zeneca, Södertälje, Sweden. The authors are grateful to Dr Kerr and Dr Kohan, St. Luke's Hospital, Sydney, Australia, for teaching us the technique of high-volume infiltration analgesia.
The sponsors had no role in the conception and design of the study, the collection, analysis, and interpretation of the data and the writing of the manuscript.
LØA, HH, KSO, BBK: none. HK has received a lecture fee from Astra-Zeneca, Södertälje, Sweden.
All authors took part in the planning and design of the study and all revised and approved the final manuscript. LA: data collection and interpretation, and writing of the manuscript. HH, KO, and BK: performed the operations (HH, KO) and anesthesia (BK) and contributed to planning, adjustment, and interpretation of data. HK: planning, design, and interpretation of results.
- Andersen K V, Pfeiffer-Jensen M, Haraldsted V, Soballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: A randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop 2007a; 78: 180–6
- Andersen L J, Poulsen T, Krogh B, Nielsen T. Postoperative analgesia in total hip arthroplasty: A randomized double-blinded, placebo-controlled study on peroperative and postoperative ropivacaine, ketorolac, and adrenaline wound infiltration. Acta Orthop 2007b; 78: 187–92
- Badner N H, Bourne R B, Rorabeck C H, MacDonald S J, Doyle J A. Intra-articular injection of bupivacaine in knee-replacement operations. Results of use for analgesia and for preemptive blockade. J Bone Joint Surg (Am) 1996; 78: 734–8
- Bianconi M, Ferraro L, Traina G C, Zanoli G, Antonelli T, Guberti A, Ricci R, Massari L. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 2003; 91: 830–5
- Browne C, Copp S, Reden L, Pulido P, Colwell C, Jr. Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty 2004; 19: 377–80
- Busch C A, Shore B J, Bhandari R, Ganapathy S, MacDonald S J, Bourne R B, Rorabeck C H, McCalden R W. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg (Am) 2006; 88: 959–63
- Klasen J A, Opitz S A, Melzer C, Thiel A, Hempelmann G. Intraarticular, epidural, and intravenous analgesia after total knee arthroplasty. Acta Anaesthesiol Scand 1999; 43: 1021–6
- Liu S S, Richman J M, Thirlby R C, Wu C L. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006; 203: 914–32
- Mauerhan D R, Campbell M, Miller J S, Mokris J G, Gregory A, Kiebzak G M. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty 1997; 12: 546–52
- Reilly K A, Beard D J, Barker K L, Dodd C A, Price A J, Murray D W. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty–a ran domised controlled trial. Knee 2005; 12: 351–7
- Ritter M A, Koehler M, Keating E M, Faris P M, Meding J B. Intra-articular morphine and/or bupivacaine after total knee replacement. J Bone Joint Surg (Br) 1999; 81: 301–3
- Rostlund T, Kehlet H. High-dose local infiltration analgesia after hip and knee replacement -what is it, why does it work, and what are the future challenges?. Acta Orthop 2007; 78: 159–61
- Tanaka N, Sakahashi H, Sato E, Hirose K, Ishii S. The efficacy of intra-articular analgesia after total knee arthroplasty in patients with rheumatoid arthritis and in patients with osteoarthritis. J Arthroplasty 2001; 16: 306–11
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen E K, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: A randomized clinical trial. Acta Orthop 2007; 78: 172–9
- Vendittoli P A, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M C, Varin F. A multimodal analgesia proto col for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg (Am) 2006; 88: 282–9