Abstract
Background High-volume local infiltration analgesia has been shown to be an effective pain treatment after knee replacement, but the role of bandaging to prolong analgesia has not been evaluated.
Methods 48 patients undergoing fast-track total knee replacement with high-volume (170 mL) 0.2% ropivacaine infiltration analgesia were randomized to receive a compression or a non-compression bandage, and pain was assessed at rest and with mobilization at regular intervals for 24 h postoperatively.
Results Pain at rest, during flexion, or on straight leg lift was lower for the first 8 h in patients with compression bandage than in those with non-compression bandage and with a similar low use of oxycodone. Mean hospital stay was similar (2.8 days and 3.3 days, respectively).
Interpretation A compression bandage is recommended to improve analgesia after high-volume local infiltration analgesia in total knee arthroplasty.
Postoperative pain after total knee arthroplasty (TKA) may be severe and may result in delayed rehabilitation. Various techniques of peripheral nerve blockade or continuous epidural analgesia can control postoperative pain, but they are associated with a number of side effects and require expertise and/or supervision (Choi et al. Citation2003, Davies et al. Citation2004, Boezaart Citation2006). Another approach is local infiltration analgesia (LIA), which has proven safe and efficient in reducing postoperative pain after TKA with no impairment of postoperative mobilization (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006, Andersen et al. Citation2007a, Citationb, Toft-dahl et al. 2007).
Briefly, LIA consists of systematic injection of a high volume (170 mL) of 0.2% ropivacaine with epinephrine in all tissues exposed, instrumented, or incised during surgery—including the joint capsule, ligaments, and other soft tissue as well as the subcutaneous layers. In order to prolong the effect of the local anesthetic, a compression bandage may be applied from the toes to the mid-thigh, which is assumed to prolong the analgesic effect by reducing absorption of local anesthetic as well as by reducing joint and wound swelling. For similar reasons, compression bandages have been used in the treatment of venomous snake bites. However, the role of bandaging has not been evaluated in any of the published LIA studies (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006, Toftdahl et al. Citation2007, Andersen et al. Citation2007a, Citationb,) or elsewhere. In this randomized study, we therefore evaluated the specific role of a compression bandage to improve the analgesic effect of LIA in TKA.
Patients and methods
The study was approved by the local ethics committee (reg. no. KF-01 327078) and the Danish data protection agency, and registered with Clini-calTrials.gov under the US National Library of Medicine (Code NCT00485212). 48 consecutive patients scheduled for total knee arthroplasty and local infiltration analgesia were randomized, by the use of a computer-generated random sequence and opaque sealed envelopes, to receive either a compression bandage or a non-compres-sion bandage (soft absorptive padding only) after surgery.
Inclusion criteria were: (1) being scheduled for unilateral total knee arthroplasty, (2) being able to understand and speak Danish, and (3) being able to give informed oral and written consent to participate. Exclusion criteria were: (1) being under treatment with opioids or steroids, (2) having rheumatoid arthritis or other immunological diseases, (3) having a history of stroke, or any neurological or psychiatric condition capable of influencing pain perception (e.g. depression, diabetic neuropathy etc.), (4) being allergic to any of the drugs administered, and (5) having a BMI > 40.
Procedures
Before surgery, all patients received 600 mg gabapentin, 1 g acetaminophen, and 400 mg celecoxib orally. All patients were operated by one of 2 surgeons, with insertion of a tricompartmental cemented AGC prosthesis (Biomet-Merck, Warsaw, IN) using a standard medial parapatellar approach. Surgery was done in a bloodless field by using a femoral tourniquet (100 mmHg above systolic blood pressure) from incision until cementation of the prosthesis was finished. Drains were not used. 15 min before incision, 500 mg of intravenous tranexamic acid was administered and another 500 mg was given before tourniquet release.
All patients received spinal anesthesia consisting of 2 mL 0.5% hyperbaric bupivacaine. Intraoperative sedation with propofol infusion at 0.5–5 mg/ kg/h was administered as required. A standardized intraoperative regimen for fluid administration was applied, consisting of 0.9% saline at 5 mL/kg/h and colloid (Voluven) at 7.5 mL/kg/h (Holte et al. Citation2007).
LIA was performed with 120 mL 0.2% ropivacaine with epinephrine (10 µg/mL) (RE mixture) and 50 mL 0.2% ropivacaine (without epinephrine), resulting in a total volume of 170 mL. The aim was to infiltrate all tissues that were incised, handled, or otherwise instrumented. After the bone cuts had been made, before insertion of the prosthesis, 50 mL was injected in the posterior capsule and collateral ligaments. After insertion of the prosthesis and deflation of the tourniquet, another 50 mL was injected into the joint capsule and into the incised quadriceps tendon, infra-patellar ligament, possible remnants of the fat pad, cruciate ligaments, and soft tissue surrounding the joint. A 16G epidural catheter was then inserted 10 cm proximal to the incision and tunneled into the knee joint (behind the medial posterior femoral condyle), with placement of the tip along the posterior aspect of the joint capsule. 20 mL of the RE mixture was injected through the catheter to ensure that it was not kinked. Finally, the subcutaneous layers were systematically injected with 50 mL 0.2% ropivacaine (without epinephrine) immediately before wound closure.
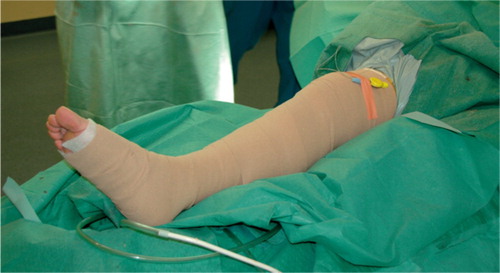
The compression bandage was then applied firmly from the toes to the mid-thigh and consisted of an inner double layer of soft padding (Soffban; BSN Medical Ltd., Brierfield, UK) surrounded by an overlapping layer of elastic adhesive bandage (Acrylastic; BSN Medical SAS, Vibraye, France)(). The non-compression bandage was applied from the mid-calf to the mid-thigh and consisted of an inner double layer of soft padding and an outer layer of standard wrapping. Bandages were kept in place during the entire 24-hour postoperative period.
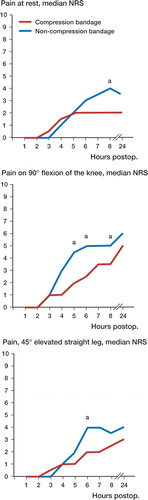
Figure 1. Pain assessment on a numeric rank scale for 24 h following total knee arthroplasty and local infiltration analgesia (n=48), at rest and upon mobilization, comparing the efficacy of a compression bandage with that of a non-compression bandage. a p<0.05. NRS: numeric rank scale (0–10), with 0=no pain and 10=worst imaginable pain.
Postoperative management
Following surgery and bandaging, all patients were admitted to the postoperative care unit for about 2 h, after which they were transferred to the orthopedics department. The postoperative analgesic regimen consisted of oral gabapentin (900 mg/day), acetaminophen (4 g/day), and celecoxib (400 mg/ day) until the seventh postoperative day.
To “top up” the LIA, an additional 20 mL of 0.2% ropivacaine with epinephrine (10 µg/mL) was injected through the intraarticular catheter at 6 and 12 h, and 50 mL 24 h postoperatively while removing the catheter, in an attempt to infiltrate both intra- and periarticular tissues.
Study parameters
All data were recorded and collected by two investigators. To assess pain, a numeric rank scale (NRS) ranging from 0 mm (indicating no pain) to 100 mm (indicating extreme pain) in 10-mm increments was used. Pain was assessed at rest, with 90° flexion of the knee, and with elevated straight leg at 45°. Pain assessment was carried out every hour for the first 8 postoperative hours, and 24 h postoperatively before injection of additional local anesthetic.
Supplementary opioid requirements (oxycodone) were recorded throughout the 24-h postoperative period. As a secondary outcome, we recorded the postoperative hospitalization period.
All patients were discharged directly to their homes and the discharge criteria were of a functional nature: ability to get in and out of bed, to get dressed, and to get into and up from a chair; also, the ability to walk independently for 50 meters with appropriate walking aids, and acceptance of discharge.
Statistics
A formal power analysis was not performed due to lack of data on the topic in the literature. 48 patients were therefore chosen arbitrarily. For statistical analysis, the Mann-Whitney U test for non-parametric data was used. Values of p less than 0.05 were considered statistically significant.
Results
The data collection took place from September 2006 through May 2007. A total of 48 consecutive patients fulfilling the inclusion criteria participated. Patient demographics were similar in the two groups, apart from higher age in the compression bandage group (Table).
Demographic data for total knee arthroplasty patients randomized to compression bandage or non-compres-sion bandage
At 8 h postoperatively, pain at rest was lower in patients who received a compression bandage (median 2 and interquartile range (IQR) 1–4)) than in those who received a non-compression bandage (median 4 and IQR 2–6) (p=0.03) (). Pain upon 90° flexion of the knee was lower in patients who received a compression bandage than in those who received a non-compression bandage—at 5 h postoperatively (median 2 and IQR 0–3.75 as opposed to median 4.5 and IQR 1.25–7; p<0.02), 6 h postoperatively (median 2.5 and IQR 1–5 as opposed to median 5 and IQR 3.25–7; p<0.01), and 8 h postoperatively (median 3.5 and IQR 1–5.75 as opposed to median 5 and IQR 3.25– 7.75; p<0.02) (Figure). Pain with a 45°-elevated straight leg was lower in patients who received a compression bandage than in those who received a non-compression bandage at 6 h postoperatively (median 2 and IQR 0.25–3 as opposed to median 4 and IQR 2–6; p<0.02) ().
There was no difference in supplementary administration of oxycodone between the compression bandage group (mean 11 mg, SD 10) and the non-compression bandage group (mean 12 mg, SD 10) (p=0.6).
Mean length of hospital stay (LOS) was 2.8 days in the compression bandage group and 3.3 days in the non-compression bandage group (p=0.7) For both groups, the combined mean LOS was 3.0 days.
Discussion
The technique of peri- and intraarticular infiltration with ropivacaine and epinephrine has been evaluated in 4 randomized studies in knee arthroplasty surgery (Reilly et al. Citation2005, Busch et al. Citation2006, Vendittoli et al. Citation2006, Toftdahl et al. Citation2007). These studies have shown that there is reduced postoperative pain and opioid requirements, and also a reduction in length of hospital stay (LOS). These studies were not, however, performed in a fast-track setting; thus, hospital stay was about 5 days except for the Oxford study (Reilly et al. Citation2005), which was however not a total knee replacement but a unicompartmental joint replacement. The specific role of the bandaging technique was not described or evaluated in any of these studies, despite being recommended by Kerr and Kohan (Citation2008) who invented the technique (personal communication). So far, no serious adverse events have been reported, and the technique is considered safe. Two studies (Busch et al. Citation2006, Vendittoli et al. Citation2006) have reported plasma ropivacaine levels below the toxic level of 1.5 µg/mL (Scott et al. Citation1989, Knud-sen et al. 1997).
The LIA technique has 4 central components: (1) the drug mixture, in terms of choice of local anesthetic, and addition of epinephrine and/or NSAID; (2) the injection technique, which should be systematic and include injection into all tissues involved during surgery; (3) an intraarticular catheter, put in place by the surgeon, preferably near the posterior aspect of the joint capsule; and (4) the application of a compression bandage, in order to reduce degradation and diffusion of the local anesthetic into the bloodstream and away from the site of injection. An additional advantage in applying a compression bandage may be the prevention of swelling of the joint and periarticular tissues.
Only a few studies have investigated the effect of compression bandages after TKA—and as far as we know, none with respect to LIA. One study concluded that a compression bandage reduces swelling and postoperative pain when compared to a non-compression bandage (Charalambides et al. Citation2005). Our study was therefore carried out to investigate one of the central components in the LIA technique—the compression bandage. We found that there was reduced pain when a compression bandage was used from 5 to 8 h postoperatively. At 24 h postoperatively, the compression bandage group had less pain than the control group, although this was not statistically significant.
It may be argued that the reduction in pain demonstrated here is related to reduced swelling of the joint in the postoperative period, or by a slower absorption of the local anesthetic, thereby improving and possibly prolonging analgesia. With the present study design, we cannot answer these ques-tions—which would require analysis of absorption kinetics of ropivacaine. Whatever the mechanism, our results clearly demonstrate the importance of a compression bandage to improve analgesia with the LIA technique.
The application of a compression bandage does not restrict patient mobilization, although mild discomfort may be reported during flexion of the knee above 45°, due to the non-elastic properties of the dressing. In our opinion, the reduction in pain demonstrated in this study outweighs the mild disadvantages the compression bandage may have.
Hospital stay was short (about 3 days) before discharge to home with our fast-track regimen (Husted and Holm Citation2006), and there was no difference between groups. However, the study was not powered to detect potential differences in hospital stay as a result of improved analgesia.
The study was supported by grants from the IMK Almene Fond, Copenhagen, Denmark, and Astra-Zeneca, Södertälje, Sweden. The sponsor did not participate in the design of the study, in the evaluation of results, or in the writing of the manuscript.
We are grateful to Drs Kerr and Kohan, St. Luke's Hospital, Sydney, Australia for their help in teaching us the high-volume infiltration analgesia technique.
No competing interests declared.
HK received a lecture fee from Astra-Zeneca, Södertälje, Sweden.
All authors took part in the planning and design of the study and all revised and approved the final manuscript. LA: data collection and interpretation, and writing of the manuscript. HH, KO, and BK: performed the operations (HH, KO) and anesthesia (BK) and contributed to planning, adjustments, and interpretation of data. HK: planning, design, interpretation, and intellectual contributions.
- Andersen K V, Pfeiffer-Jensen M, Haraldsted V, Soballe K. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: A randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop 2007a; 78: 180–6
- Andersen L J, Poulsen T, Krogh B, Nielsen T. Postoperative analgesia in total hip arthroplasty: A randomized double-blinded, placebo-controlled study on peroperative and postoperative ropivacaine, ketorolac, and adrenaline wound infiltration. Acta Orthop 2007b; 78: 187–92
- Boezaart A P. Perineural infusion of local anesthetics. Anesthesiology 2006; 104: 872–80
- Busch C A, Shore B J, Bhandari R, Ganapathy S, MacDonald S J, Bourne R B, Rorabeck C H, McCalden R W. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg (Am) 2006; 88: 959–63
- Charalambides C, Beer M, Melhuish J, Williams R J, Cobb A G. Bandaging technique after knee replacement. Acta Orthop 2005; 76: 89–94
- Choi P T, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev 2003; CD003071
- Davies A F, Segar E P, Murdoch J, Wright D E, Wilson I H. Epidural infusion or combined femoral and sciatic nerve blocks as perioperative analgesia for knee arthroplasty. Br J Anaesth 2004; 93: 368–74
- Holte K, Kristensen B B, Valentiner L, Foss N B, Husted H, Kehlet H. Liberal versus restrictive fluid management in knee arthroplasty: a randomized, double-blind study. Anesth Analg 2007; 105: 465–74
- Husted H, Holm G. Fast Track in total hip and knee arthroplasty -experiences from Hvidovre University Hospital, Denmark. Injury, Int J Care Injured 2006; 37: S31–S35
- Kerr D R, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery. A case study of 325 patients. Acta Orthop 2008; 79(2)174–83
- Knudsen K, Beckman S M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth 1997; 78: 507–14
- Reilly K A, Beard D J, Barker K L, Dodd C A, Price A J, Murray D W. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty—a randomised controlled trial. Knee 2005; 12: 351–7
- Scott D B, Lee A, Fagan D, Bowler G M, Bloomfireld P, Lundh R. Acute toxicity of ropivacaine compared with that of bupivacaine. Anesth Analg 1989; 69: 563–9
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen E K, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: A randomized clinical trial. Acta Orthop 2007; 78: 172–9
- Vendittoli P A, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin M C, Varin F. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg (Am) 2006; 88: 282–9