Abstract
Background and purpose We studied whether osseointegration and fixation of plasma-sprayed titanium implants grafted with β-TCP granules (Ossaplast) can be improved by adding an osteogenic signal (Colloss E). The results were compared to implants grafted with fresh frozen morselized allograft with and without the Colloss E device.
Methods 4 porous-coated Ti implants were placed in the proximal humeri in each of 10 dogs. All implants were surrounded by a 2.5-mm defect, which was grafted with: (A) β-TCP, (B) β-TCP+20 mg Colloss E, (C) allograft, or (D) allograft+20 mg Colloss E. The observation time was 4 weeks.
Results Mechanical testing showed that the β-TCP group with Colloss E was twice as well fixed as the control group grafted with β-TCP granules alone, and comparable to both allograft groups. We found that every control implant in the β-TCP grafted group was covered by a dense fibrous membrane. No fibrous tissue was seen in the β-TCP group augmented with Colloss. These implants were well osseointegrated, with new bone covering 10–25% of the implant surface. Both treated groups had increased graft resorption compared to their respective control groups. Colloss E had no effect on new bone formation or fibrous tissue reduction around the allografted implants.
Interpretation The Colloss E device improved early osseointegration of implants grafted with β-TCP granules and increased their mechanical implant fixation to the level of allografted implants. The experiment indicates that ceramic bone substitutes may be a viable alternative to allograft when combined with an osteogenic signal such as Colloss E.
Cementless reconstructions of failed arthroplasties often require extensive bone grafting, but the use of autograft and allograft bone is associated with problems such as donor site morbidity, supply shortage, quality inconsistencies, and adverse immunological responses. Porous ceramics have long been considered as replacements for bone grafts, serving as osteoconductive scaffolds for new bone formation. The specific aim of this study was to investigate whether the fixation and osseointegration of implants surrounded by a ceramic bone graft substitute could be improved by adding an extract of equine bone matrix proteins.
Whereas bone consists of both organic and inorganic components, most commercially available ceramic bone substitutes are pure inorganic materials such as hydroxyapatite and tricalcium phosphate. These materials provide an osteoconductive scaffold on which new bone can be formed and incorporated. Theoretically, they can be designed as biphasic compounds to meet specific requirements in size, shape, strength, porosity, and resorption rate. However, ceramics are not osteoinductive, and to date they have been reported as promising bone graft extenders rather than actual bone graft substitutes (van Haaren et al. Citation2005, Jensen et al. Citation2007).
We used β-tricalcium phosphate granules (Ossaplast; Ossacur AG, Germany) as bone graft substitute, and an extract of equine extracellular matrix containing native osteogenic growth factors (Col-loss E; Ossacur AG, Germany) as augmentation. Implant fixation was evaluated by mechanical testing of the bone-implant interface and histomorphometrical quantification of the tissues on the implant surface and in the grafted defect surrounding the implant. We compared the results to fresh frozen allograft with and without Colloss E.
We hypothesized that Colloss E would improve the overall implant fixation and bioactivity of the graft substitute. For experimental purposes, we defined this as improved mechanical fixation, increased new bone formation in the grafted defect and on the implant surface, and reduction of fibrous tissue while at the same time maintaining a controlled graft resorption.
Material and methods
Design
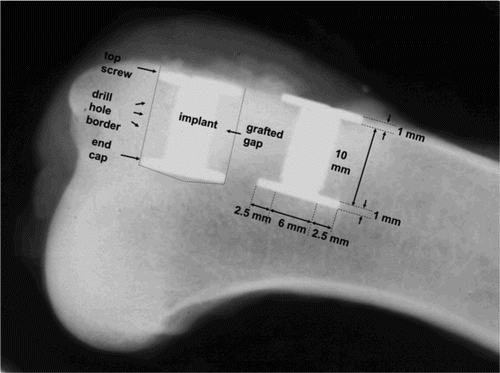
The experiment was designed as a paired animal study with 10 dogs. Each dog received 4 Ti implants in the proximal humeri ( and ). The controlled 2.5 mm defect around each implant was packed with bone graft material with or without Colloss E. The four implants in each dog were grafted in the following manner: (A) β-TCP, (B) β-TCP+Colloss E (20 mg), (C) allograft, and (D) allograft+Colloss E (20 mg per cm3 graft material) (). The implantation site of each group was alternated systematically with random start. The observation time was 4 weeks.
Table 1. Experimental design. 9 dogs received 4 porouscoated Ti implants each grafted with ®-TCP or allograft with/without Colloss E in the proximal humeri
Table 2. Mechanical pushout test. Mean (SD)
Table 3. Histomorphometric data. Values are median (interquartile range)
Colloss E
Colloss E (Ossacur AG, Germany) is a lyophilized complex of the extracellular matrix proteins extracted from diaphyseal equine bone. The device has been proven to be osteoinductive in a rat model of ectopic bone formation (Li et al. Citation2006). It also induces ectopic bone formation in a subcutaneous titanium fiber mesh tube (Walboomers and Jansen Citation2005), and its bovine equivalent—Colloss—has been shown to improve early osseointegration and mechanical fixation of porous-coated Ti implants grafted with fresh frozen morselized allograft in a canine femur model (Baas et al. Citation2006). It is currently employed in clinical applications as bone void filler, for fracture healing and to increase bone volume in the vicinity of maxillofacial implants.
The Colloss E material has a cotton-like structure, takes up practically no volume when mixed with other substances, and has no mechanical strength. It cannot therefore be expected to be of benefit by itself in closing large defects around implants in need of mechanical support. Its effect on bone healing has not been investigated in combination with ceramic bone substitutes.
At surgery, 20 mg of Colloss E was mixed into 1 cm3 of graft material (morselized allograft or β-TCP granules) for the intervention groups. The dose was based on a previous dose-response study in augmentation of allograft bone (Baas et al. Citation2006).
Ossaplast
Ossaplast (Ossacur AG, Germany) is a commercially available, resorbable implant material for filling bone defects. It consists of sintered, β-phase tricalcium phosphate with a Ca/P ratio of 1.5. Its phase purity and biocompatibility comply with ASTM specification F1088-87. It was received from the manufacturer in granules of 1–1.6 mm diameter with an intragranula porosity of 50% and an overall porosity of 80% for this experiment.
Allograft
The bone graft was harvested immediately post mortem under sterile conditions from 2 dogs not included in the study, and stored at –80°C 14 months before the surgeries. The proximal humerus and the distal femurs were used. Before surgery, the bone graft was thawed and prepared: all soft tissue and cartilage was removed, and the bone was morselized with a standard bone mill on fine setting, creating bone chips of 1–4 mm. The chips from the different bones were mixed together, portioned in sterile double-containers, and stored at –80ºC. At surgery, bone chips longer than approximately 2 mm were sorted out, and the allograft divided into 2 portions of 1 cm3 in a standardized container.
Implants
We used 40 porous-coated titanium alloy (Ti-6A1-4V) implants for the experiment, which were manufactured by Biomet Inc. (Warsaw, IN) (). The implants were cylindrical with a height of 10 mm and a diameter 6 mm. The porous coating was plasma-sprayed, giving a mean pore diameter of 480 µm and a mean porosity of 44% as specified by the manufacturer. A footplate of 11 mm diameter was attached to one end of each implant. When inserted into an 11-mm drill hole, this centered the implant and provided a uniform 2.5-mm defect around it. After grafting of the defect, an 11-mm diameter top-washer was mounted on the outer end of the implant to ensure stability, concentricity, and containment of the graft material ().
Animals
10 American hound dogs with a mean weight of 22 (19–25) kg were used. Skeletal maturity was verified by radiography. As stated above, 2 additional dogs served as bone graft donors. The dogs were bred for scientific purposes, and the experiment was approved by the Institutional Animal Care and Use Committee. All animals were allowed unlimited activity. After 4 weeks observation time, the dogs were killed with an overdose of hypersaturated pentobarbital.
Surgical procedure
With the dogs under general anesthesia, and under sterile conditions, a skin incision was made with cautery on the lateral proximal humerus, approximately 7 cm in length. The proximal humerus was accessed by blunt dissection through the deltoid muscle fascia. A 2.5-mm guide wire was inserted anterolaterally in the exposed proximal humerus at the level of the greater tubercle, oriented perpendicular to the surface. With the aid of a wire guide instrument, another 2.5-mm guide wire was inserted 17 mm distally and parallel to the first. Over each guide wire, a cannulated drill (11.0 mm in diameter) was used to drill two 12-mm-deep cylindrical cavities at a maximum speed of 2 rotations per second. The edge of the holes was trimmed with a scalpel to remove excessive periosteum, and the cavities were irrigated with 10 mL saline for removal of loose bone chips. In each drill hole, the implants with footplates were inserted with a specially designed impaction tool to ensure uniform central placement. The same tool was then used to impact 1 cm3 graft material (corresponding to one of the 4 groups) into the 2.5-mm defect around each implant. Finally, the concentricity of each grafted defect was maintained by mounting an 11-mm top-washer, and the soft tissues were closed in layers. The procedure was repeated on the opposite side. All 40 implants were operated by the same surgeon (JB). The dogs were given Ceftriaxon (1 g) administered immediately before surgery and 3 days postoperatively. Buprenorphine hydrochloride (0.3 mg/mL, 0.0075 mg/kg/day intramuscularly) was given as postoperative analgesic treatment.
Specimen preparation
The proximal humeri were frozen and stored at –20ºC immediately after retrieval. The outermost 0.5 mm of the implant-bone specimen was cut off and discarded. The rest of the implant with surrounding bone was divided into two sections, perpendicular to the long axis of the implant, with a water-cooled diamond band saw.
The outermost section was cut to a thickness of 3.5 mm and stored at –20ºC until mechanical testing (Linde and Sorensen Citation1993).
The innermost section was cut to a thickness of 5.5 mm and prepared for histomorphometry. These specimens were dehydrated in graded ethanol (70–100%) containing basic fuchsin, and embedded in methylmethacrylate. Using vertical sectioning technique (Baddeley et al. Citation1986, Overgaard et al. Citation2000), each specimen was cut into four 30-µm-thick histological sections with a microtome (KDG-95; MeProTech, Heerhugowaard, Holland). Finally, these were surface counterstained with 2% light green for 2 min, rinsed and mounted on glass. This preparation provides red staining of uncalcified tissue and green staining of calcified tissue. The different types of calcified tissues, such as woven bone and lamellar bone, can be discriminated based on their morphological characteristics (Gotfredsen et al. Citation1989).
Mechanical testing
Thawed specimens were tested to failure by axial push-out test on an Instron Universal test machine (Instron Ltd., High Wycombe, UK). Testing was performed in blinded fashion and in one session. The specimens were placed with the cortical side facing up on a metal support jig with the implant centered over a 7.4-mm opening and under a cylindrical test probe of 5 mm diameter. A preload of 2 N defined the contact position for the start of the test. The implants were then pushed out of the surrounding tissue in the direction of the implant axis at a velocity of 5 mm/min. Load vs. implant displacement data was continuously recorded. From these data, the mechanical implant fixation parameters as described earlier (Soballe 1993) were calculated: ultimate shear strength, apparent shear stiffness, and total energy absorption.
Histological evaluation
Quantitative histomorphometry was performed using the stereological software C.A.S.T. Grid (Olympus Denmark AS, Ballerup, Denmark). With the aid of this software, 2 regions of interest were defined: zone 1 from the innermost parts of the implant surface and 500 µm into the grafted defect, and zone 2 in the 500–2,000 µm part of the grafted defect. In these zones, volume fractions of new bone, allograft, fibrous tissue, and marrow space were quantified by point-counting technique (Gundersen et al. Citation1988). On the implant surface, the area fractions of the same tissues were quantified by line-interception technique (Baddeley et al. Citation1986). Due to the histological differences evident between the ceramic granules and the allograft bone, blinding was only possible for whether or not the graft material was treated with Colloss E.
Statistics
The mechanical data followed a normal distribution, and fulfilled the assumptions for repeated measures ANOVA followed by paired t-test. The data are presented as mean with standard deviation. Differences between means were considered statistically significant for p-values<0.05.
The histological datasets were not normally distributed, as some parameters were close to zero for certain groups. For simplicity, all parameters were evaluated by Friedman's non-parametric analysis of paired data, followed by Wilcoxon signed rank test. The data are presented as median with inter-quartile ranges. Differences between medians were considered statistically significant for p-values < 0.05.
Statistical analysis was performed using Intercooled STATA 8.0 software (StataCorp LP, College Station, TX).
Results
Methodological results
1 dog was excluded from the study due to a postoperative fracture of the right humerus. The remaining 9 dogs were fully weight bearing within 3 days of surgery and completed the 4-week observation period without complications. Sectioning and mechanical testing was uneventful. Double measurements on the quantitative histomorphometry were performed on random implants after a period of 1 year after the initial measurements, to determine the precision of the estimates. The intraobserver coefficients of variation (CVs) for “woven bone”, “bone graft”, “fibrous tissue”, and “marrow space” were 8%, 180%, 15%, and 15% for bone-to-implant contact, respectively, and 13%, 14%, 6%, and 20% for bone volume fractions, respectively. The mean surface intercept count for each implant was 537. The mean volume point count for zone 1 was 493, and for zone 2 it was 825.
Mechanical results (Table 2)
The mechanical fixation of the implants grafted with TCP granules was improved by more than 100% when Colloss E was added to the graft material. The treated TCP graft had a mechanical fixation similar to the allografted implants. There was no difference between the implants with allograft and those with allograft and Colloss E. The mechanical failure occurred in the immediate vicinity of the graft-implant interface for all implants.
Histological results (Table 3)
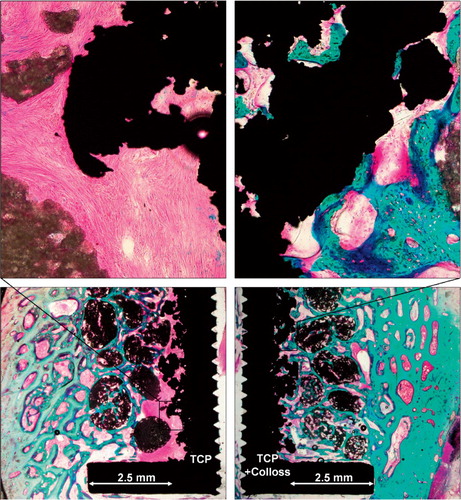
The control implants in the TCP group were covered by a dense fibrous membrane that extended up to half way into the grafted defect. Fibrous tissue was completely eliminated in the implants grafted with TCP granules with Colloss E, and these implants were well osseointegrated with newly formed bone covering 11–26% of the implant surface. The treated implants also had more new bone formation in the grafted defect, with a higher degree of osseointegration of the TCP granules and remodeling of the bone graft substitute ().
Figure 3. Representative histology from implant pair in the same dog. Left panels: engraftment with TCP granules. Encapsulation of implant with fibrous tissue. Right panels: engraftment with TCP granules with Colloss E added. Osseointegration of implant and ceramic granules.
The two allografted groups were similar with regard to the histomorphometrical features measured. The only statistically significant difference between the groups was a reduced volume fraction of allograft around the Colloss E-treated implants, indicating a higher rate of allograft remodeling around these implants.
Discussion
Our experimental model was designed to study the early fixation and osseointegration of an uncemented implant component inserted into a bed of impacted graft material (Lind et al. Citation2001, Jensen et al. Citation2002). We used dogs for the experiment because their epiphyseal cancellous bone closely resembles the composition, density, and quality of human bone (Aerssens et al. Citation1998). The implant model is not subject to clinically relevant influences such as joint fluid pressure and direct load transmission; nor does it provide the revision environment of compromised bone as replicated in the micromotion model (Bechtold et al. Citation2001). However, this basic experimental model is well controlled and has less statistical “noise” than is inherent in a weight-bearing model (Söballe Citation1993). The model allowed paired comparison of 4 treatment groups, by which the influence of biological differences between the animals was eliminated. Finally, it also permitted a defect around the implant that was large enough for simulation of impaction grafting.
We chose 50% microporous pure-phase β-TCP granules as a baseline representative of ceramic bone graft substitutes, since the purpose of the experiment was principally to show an effect of adding an osteogenic signal to augment implant fixation and osseointegration. These granules may not be able to withstand the dynamic compressive forces of a clinically loaded implant. The composition of bone graft substitutes is typically dual-phase, with both TCP and HA in specific relations—alternatively further strengthened with composite fibers.
Beta-TCP granules combined with Colloss E gave a twofold increase in mechanical implant fixation, to a level comparable to that in the allografted implants. The addition of Colloss E eliminated the presence of fibrous tissue and increased the fraction of new bone directly supporting the implant surface and within the β-TCP-granules around the implants. The combination of Colloss E and allograft offered no advantage in this study. We consider the mechanical fixation of the implants to be the main parameter in evaluating therapies with this model. The histomorphometric data complements this and provides information about the tissue response behind the implant fixation.
The poorly fixed β-TCP-grafted control implants were covered by fibrous tissue. The well-fixed Colloss E-treated implants had no fibrous tissue, and the osseointegration of both β-TCP granules and implant was excellent. From human cadaver studies, we know that allografted implants can be anchored in an inert composite of graft chips and fibrous tissue (Linder Citation2000). Some experiments have suggested that such a composite of fibrous tissue and allograft may be a mechanism that is sufficient for anchorage of an implant (Tägil and Aspenberg Citation2001). Our results indicate that bone ingrowth further strengthens the β-TCP granules by forming a bridge between the existing bone and the graft material, as well as between the graft granules. More importantly, this seems to be a prerequisite for the osseous bridging of the graft-implant interface, which was the critical interface in this study during mechanical testing. This corresponds well with the existing literature on cemented impaction allografting, where the formation of a fibrous membrane on the graft-cement interface was found to be associated with mechanical failure (Schimmel et al. Citation1998).
The Colloss E-treated β-TCP granules were largely invaded by new bone and were partially disintegrated. This was also reflected by the increased rate of resorption in this group compared to the controls. A study that evaluated β-TCP blocks with different pore sizes found that fast resorption was associated with lower bone content and more soft tissue within the blocks (von Doernberg et al. Citation2006). Another study of porous β-TCP struts implanted into an osteochondral defect in rabbits showed signs of rapid resorption, and β-TCP degradation products were found to provoke an inflammatory response that impaired and reversed bone apposition (Hing et al. Citation2007). Our histological preparation of the implant-tissue interface did not allow an analysis of inflammatory response, but there was no sign of inhibition of bone growth in implants with advanced resorption. The aforementioned studies did not involve the use of osteogenic growth factors. If there was, in fact, a relative inhibition of bone growth caused by an unidentified resorption-related inflammatory response in our study, then it was by far outweighed by the benefit of the osteogenic stimulus. Any fixation strength lost to the increased resorption of the β-TCP granules seems to have been compensated by the increased formation of new bone. Our results indicate that osseous integration and gradual resorption of the graft is preferential, and that the direct contact between living bone and implant provides superior fixation.
Based on previous experiments with bovine Col-loss, we know that doses that are too high can give adversely increased allograft remodeling, causing a decline in implant fixation (Baas et al. Citation2006). Extreme resorption has been observed in other experimental studies using OP-1/rhBMP7 (Jensen et al. Citation2002, Jeppsson et al. Citation2003) and rhBMP2 (Baas et al. Citation2008) with allograft, and could explain the early failures of revision THR where allograft and OP-1 were used in combination (Kärrholm et al. 2006). This has raised the question of whether adjuvant therapies with BMPs mainly raise the overall bone turnover at its application site, rather than favoring an anabolic increase in new bone formation (Little et al. Citation2007). Colloss E contains trace amounts of bone growth factors embedded within the lyophilized collagen (van der Zande et al. Citation2006, El-Sabban et al. Citation2007). No conclusions on optimal dose, release rate, or growth factor combinations can be made based on this study. The balance between bone formation and resorption is still not fully understood, which further warrants the need for dose-response studies of BMP-based adjuvant therapies before human trials.
Resorption of the β-TCP granules was increased by the use of Colloss E in the present study. However, relatively speaking, this increase in resorption was smaller than in the corresponding allograft groups. The same relationship was found using an equivalent canine model for revision arthroplasty, where an HA-based bone graft substitute (ProOsteon) and also allograft were augmented with OP-1 (Jensen et al. Citation2002). This directs attention to another possible advantage of bone graft substitutes: in contrast to biological bone grafts, they can be composed to better resist resorption. The BMP-related problem of a transient mechanical weakening of the implant fixation due to accelerated allograft resorption (Jeppsson et al. Citation2003) may therefore be controlled by correct composition of the bone graft substitute.
The marked difference in implant fixation and osseointegration between the β-TCP control group on the one hand and the β-TCP with Colloss E and both allograft groups on the other, indicates that such calcium phosphate materials alone are not sufficient substitutes for bone grafts. The ability of ceramic materials to scaffold new bone formation depends on an appropriate scaffold structure—as well as the correct chemistry to support or stimulate an appropriate host response (Hing Citation2004). In this experiment, the Colloss E device was able to serve as an adequate adjuvant signal for bone formation and remodeling. It seems imperative not only to replace the osteoconductive inorganic component of bone, but also to replace parts of the osteoinductive organic components that are embedded in the matrix of natural bone.
We found no difference in mechanical fixation between the allografted implant groups with and without Colloss E. This was an unexpected finding, as we have previously observed a doubling of mechanical fixation of allografted implants when bovine Colloss was added to the allograft. The materials used in the two experiments were not the same, as the Colloss E device is produced from equine diaphyseal bone, not bovine. Another important difference between the two experiments was the allograft itself. The bones used in the previous experiment were collected and frozen at –80°C 2 weeks before surgery, whereas the graft bone for the current experiment was harvested and frozen more than a year before surgery. We believe that the long storage of the allograft in the current experiment may have reduced the its immunogenicity by unintended lyophilization. Given the restrictions of comparing data from different experiments, we saw that the fixation in the allografted control group in the current study was nearly twice as good as in the control group in the previous study, and there was comparable fixation to that in the Colloss-treated groups in the previous study (Baas et al. Citation2006). Furthermore, neither the allografted controls nor the treated implants in the current study were covered by fibrous tissue. In the previous study, the control implants had 39% coverage by fibrous tissue (median) and the Col-loss-treated implants had 0% fibrous tissue coverage (median). This suggests that the allograft in the current study may have been more biocompatible and more easily integrated into new bone, giving little room for improvement with the addition of adjuvant therapy. It also indicates that reduction of allograft immunogenicity may improve allograft biocompatibility, and thereby implant fixation.
In summary, we found that the use of Colloss E with a β-TCP bone graft substitute resulted in a two-fold increase in implant fixation—to the level of morselized allograft, with reduced presence of fibrous tissue and increased new bone formation. The osseointegration of ceramic bone graft substitutes seems to improve when both osteoconductive and osteoinductive components of bone are substituted. In the ongoing development of these materials, bioactivity should be considered as well as biomechanics and biocompatibility.
The authors wish to thank laboratory technician Jane Pauli for excellent lab work with the histological sections, and Niels Trolle Andersen for statistical guidance. The Colloss E device and the Ossaplast granules were donated unconditionally by Ossacur AG, Germany. Ossacur AG also provided unconditional institutional research support to co-fund the study. The implants were provided unconditionally by Biomet Inc., Warsaw, IN. Unconditional support for the work was also provided by the Augustinus Fund, Denmark, and the Interdisciplinary Research Group Nanoscience and Biocompatibility funded by the Danish Research Council (2052-01-006). The sponsors did not participate in the design of the study, in the evaluation of the results, or in writing of the article.
No competing interests declared.
Contributions of authors
JBa: design, surgery, specimen preparation, analysis, and writing of manuscript. BE: design, surgery, analysis, and revision of the manuscript. JBe: design, surgery, and revision of the manuscript. XC: surgery and revision of the manuscript. KS: design, surgery, and revision of the manuscript.
- Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology 1998; 139: 663–70
- Baas J, Lamberg A, Jensen T B, Elmengaard B, Soballe K. The bovine bone protein lyophilisate Colloss improves fixation of allografted implants - an experimental study in dogs. Acta Orthop 2006; 77: 791–8
- Baas J, Elmengaard B, Jensen T B, Jakobsen T, Andersen N T, Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials 2008; 29: 2915–22
- Baddeley A J, Gundersen H J, Cruz-Orive L M. Estimation of surface area from vertical sections. J Microsc 1986; 142(Pt 3)259–76
- Bechtold J E, Kubic V, Soballe K. A controlled experimental model of revision implants: Part I. Development. Acta Orthop Scand 2001; 72: 642–9
- El-Sabban M E, El-Khoury H, Hamdan-Khalil R, Sindet-Pedersen S, Bazarbachi A. Xenogenic bone matrix extracts induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Regen Med 2007; 2: 383–90
- Gotfredsen K, Budtz-Jorgensen E, Jensen L N. A method for preparing and staining histological sections containing titanium implants for light microscopy. Stain Technol 1989; 64: 121–7
- Gundersen H J, Bendtsen T F, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, Sorensen F B, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96: 379–94
- Hing K A. Bone repair in the twenty-first century: biology, chemistry or engineering?. Philos Transact A Math Phys Eng Sci 2004; 362: 2821–50
- Hing K A, Wilson L F, Buckland T. Comparative performance of three ceramic bone graft substitutes. Spine J 2007; 7: 475–90
- Jensen T B, Overgaard S, Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein 1 device increases bone formation and bone graft resorption around cementless implants. Acta Orthop Scand 2002; 73: 31–9
- Jensen T B, Overgaard S, Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein-1 increases the fixation of implants grafted with morcellised bone allograft and ProOsteon bone substitute: An experimental study in dogs. J Bone Joint Surg (Br) 2007; 89: 121–6
- Jeppsson C, Astrand J, Tagil M, Aspenberg P. A combination of bisphosphonate and BMP additives in impacted bone allografts. Acta Orthop Scand 2003; 74: 483–9
- Karrholm J, Hourigan P, Timperley J, Razaznejad R. Mixing bone graft with OP-1 does not improve cup or stem fixation in revision surgery of the hip: 5-year follow-up of 10 acetabular and 11 femoral study cases and 40 control cases. Acta Orthop 2006; 77: 39–48
- Li H, Springer M, Zou X, Briest A, Bunger C. Ectopic bone induction by equine bone protein extract. Adv Exp Med Biol 2006; 585: 393–402
- Lind M, Overgaard S, Jensen T B, Song Y, Goodman S B, Bunger C, Soballe K. Effect of osteogenic protein 1/colla-gen composite combined with impacted allograft around hydroxyapatite-coated titanium alloy implants is moderate. J Biomed Mater Res 2001; 55: 89–95
- Linde F, Sorensen H C. The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech 1993; 26: 1249–52
- Linder L. Cancellous impaction grafting in the human femur: histological and radiographic observations in 6 autopsy femurs and 8 biopsies. Acta Orthop Scand 2000; 71: 543–52
- Little D G, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg (Br) 2007; 89: 425–33
- Overgaard S, Soballe K, Jorgen H, Gundersen G. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res 2000; 18: 313–21
- Schimmel J W, Buma P, Versleyen D, Huiskes R, Slooff T J. Acetabular reconstruction with impacted morselized cancellous allografts in cemented hip arthroplasty: a histological and biomechanical study on the goat. J Arthroplasty 1998; 13: 438–48
- Söballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand (Suppl 255) 1993; 1–58
- Tägil M, Aspenberg P. Fibrous tissue armoring increases the mechanical strength of an impacted bone graft. Acta Orthop Scand 2001; 72: 78–82
- van der Zande M, Springer M, Krause A, Briest A, Poulsen K, Jensen O N, Wallboomers X F, Jansen J A (2006) Analysis of the Osteoinductive Materials Colloss and Col-loss E. Paper presented at European Conference on Biomaterials, NantesFrance, September, 2006, Ref Type: Abstract
- van Haaren E H, Smit T H, Phipps K, Wuisman P I, Blunn G, Heyligers I C. Tricalcium-phosphate and hydroxyapatite bone-graft extender for use in impaction grafting revision surgery. An in vitro study on human femora. J Bone Joint Surg (Br) 2005; 87: 267–71
- von Doernberg M C, von R B, Bohner M, Grunenfelder S, van Lenthe G H, Muller R, Gasser B, Mathys R, Baroud G, Auer J. In vivo behavior of calcium phosphate scaffolds with four different pore sizes. Biomaterials. Biomaterials 2006; 27: 5186–98
- Walboomers X F, Jansen J A. Bone tissue induction, using a Colloss-filled titanium fibre mesh-scaffolding material. Biomaterials 2005; 26: 4779–85