Abstract
Background and purpose — Patient-specific instrumentation (PSI) for total knee arthroplasty (TKA) has been introduced to improve alignment and reduce outliers, increase efficiency, and reduce operation time. In order to improve our understanding of the outcomes of patient-specific instrumentation, we conducted a meta-analysis.
Patients and methods — We identified randomized and quasi-randomized controlled trials (RCTs) comparing patient-specific and conventional instrumentation in TKA. Weighted mean differences and risk ratios were determined for radiographic accuracy, operation time, hospital stay, blood loss, number of surgical trays required, and patient-reported outcome measures.
Results — 21 RCTs involving 1,587 TKAs were included. Patient-specific instrumentation resulted in slightly more accurate hip-knee-ankle axis (0.3°), coronal femoral alignment (0.3°, femoral flexion (0.9°), tibial slope (0.7°), and femoral component rotation (0.5°). The risk ratio of a coronal plane outlier (> 3° deviation of chosen target) for the tibial component was statistically significantly increased in the PSI group (RR =1.64). No significance was found for other radiographic measures. Operation time, blood loss, and transfusion rate were similar. Hospital stay was significantly shortened, by approximately 8 h, and the number of surgical trays used decreased by 4 in the PSI group. Knee Society scores and Oxford knee scores were similar.
Interpretation — Patient-specific instrumentation does not result in clinically meaningful improvement in alignment, fewer outliers, or better early patient-reported outcome measures. Efficiency is improved by reducing the number of trays used, but PSI does not reduce operation time.
Mechanical axis malalignment and component malalignment contribute to aseptic loosening, instability, and unexplained pain after total knee arthroplasty (TKA) (Sundfeldt et al. Citation2006, Del Gaizo and Della Valle Citation2011, Bell et al. Citation2014). Patient-specific instrumentation (PSI) has been introduced to improve alignment. Other postulated benefits are improved surgical efficacy and a possible reduction of complication risks due to avoidance of intramedullary canal violation. As for all new techniques, randomized controlled trials (RCTs) are needed to investigate whether patient-specific cutting guides do indeed perform as advantageously as advertised by the manufacturers.
A number of RCTs and meta-analyses have been published. Most of the meta-analyses included non-randomized studies and investigated radiographic outcome only. Furthermore, the maximum number of RCTs included so far is 10 (Thienpont et al. Citation2014, Voleti et al. Citation2014, Cavaignac et al. Citation2015, Fu et al. Citation2015).
In order to update our knowledge of the outcomes of patient-specific instrumentation, we conducted a meta-analysis of RCTs only. We investigated radiographic accuracy, operation time, hospital stay, blood loss, number of surgical trays required, and patient-reported outcome measures.
Patients and methods
We selected randomized controlled trials comparing patient-specific instrumentation with intramedullary or extramedullary cutting jigs in patients requiring TKA irrespective of underlying disease. Quasi-randomized trials and trials in which the treatment allocation was inadequately concealed were considered for inclusion.
Identified and selected outcome measures can be roughly categorized in 3 groups: radiographic measures, procedure-related details, and patient-reported outcome measures (PROMs). Both mean deviations from intended alignment and proportion of outliers, defined as >3 degrees deviation from intended alignment, were included. Papers were searched for operation time, hospital stay, blood loss, and the number of surgical trays used for the operation. Variations of these measures were assessed for pooling. Lastly, we included patient-reported outcome measures.
Identification and description of studies
We searched Medline and Embase via the Ovid platform up to May 28, 2015. From Embase, we selected only unique identifiers that were not found using Medline. PubMed clinical queries were searched for any missing RCTs. Key words were combinations of “knee arthroplasty” or “knee replacement” AND “patient match”, “patient specific”, or “custom” as truncated search terms. There were no language restrictions.
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al. Citation2009).
Data collection
2 researchers (HJTAMH and ES) independently selected eligible papers and performed risk-of-bias assessments. Differences were resolved by discussion. Data were extracted from the papers by HJTAMH using Cochrane Collaboration Review Manager 5.3 software (the Nordic Cochrane Centre, the Cochrane Collaboration, 2014) and an Excel spreadsheet was used for authors, year of publication, patient-specific system, sample size, age, and body mass index (BMI). When not available in the paper, additional information regarding means, standard deviations, and intention-to-treat data were requested from the authors. If the authors did not respond to 2 consecutive e-mails (which was the case for 3 of 10 authors), the study data were excluded from further analysis. Intention-to-treat data were used from all studies.
Analysis
Assessment of risk of bias was done according to the Cochrane handbook. Bias was categorized as selection bias, performance bias, detection bias, attrition bias, or reporting bias. A “risk-of-bias summary” was generated using Review Manager 5.3 software.
Weighted mean differences (e.g. the absolute difference between the mean value in PSI and standard groups) and 95% confidence intervals (CI) were calculated for continuous outcomes. For dichotomous outcomes, risk ratios (RRs) and CIs were calculated. The risk ratio was the ratio of the risk of an outlier in the PSI group and the risk of an outlier in the standard group. Results from individual trials were pooled wherever possible and appropriate, using the fixed-effects model. Heterogeneity between comparable trials was assessed by visual inspection of the forest plot (analysis) along with consideration of the standard chi-squared test and the I2 statistic (Higgins et al. Citation2003). The I2 statistic describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins et al. Citation2003). Where there was statistical or graphical evidence of heterogeneity, the results were checked using the random-effects model. Generally, the results for the random-effects model are presented when there was substantial heterogeneity (p < 0.1; I2 = 50% or more) in the results of individual trials. Intervention effects of fixed- and random-effects estimates were compared to assess the influence of small-study effects. Whenever the random-effects estimate was more beneficial, effect size was assessed again, excluding the smallest study.
Results
Description of studies
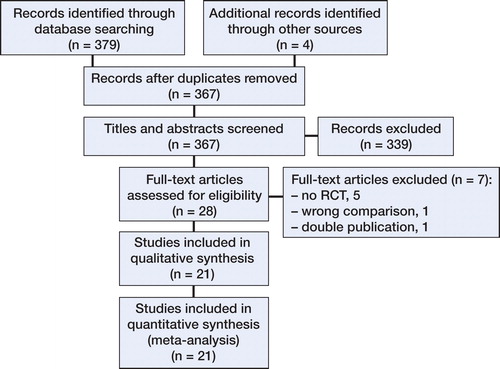
Eventually, 20 published studies and 1 “in press” paper—representing 1,587 TKAs—were included ( and ) (Noble et al. Citation2012, Boonen et al. Citation2013, Chareancholvanich et al. Citation2013, Hamilton et al. Citation2013, Parratte et al. Citation2013, Roh et al. Citation2013, Vundelinckx et al. Citation2013, Pietsch et al. Citation2013b, Abdel et al. Citation2014, Chen et al. Citation2014, Chotanaphuti et al. Citation2014, Kotela and Kotela Citation2014, Ng et al. Citation2014, Silva et al. Citation2014, Victor et al. Citation2014, Woolson et al. Citation2014, Abane et al. Citation2015, Ferrara et al. Citation2015, Molicnik et al. Citation2015, Yan et al. Citation2015, Huijbregts et al. Citation2016). 3 authors did not respond to e-mails requesting additional data. Data from those studies were partially excluded (Parratte et al. Citation2013, Ng et al. Citation2014, Abane et al. Citation2015).
Table 1. Baseline characteristics of the studies included
Radiographic outcome
Hip-knee-ankle axis and component accuracy were determined by recording the deviation from intended alignment. Intended alignment is usually perpendicular to the mechanical axis in the coronal plane, for both components. In the sagittal plane, posterior slope varied from neutral to 7 degrees and femoral flexion varied from neutral to 3 degrees. Femoral rotation was usually set parallel to the epicondylar axis. There was no uniform method of referencing and measuring tibial rotation.
Outliers were included when defined as more than 3 degrees of deviation from intended alignment. 2 studies used different definitions, and these were excluded from further radiographic analysis (Ng et al. Citation2014, Ferrara et al. Citation2015).
Hip-knee-ankle axis
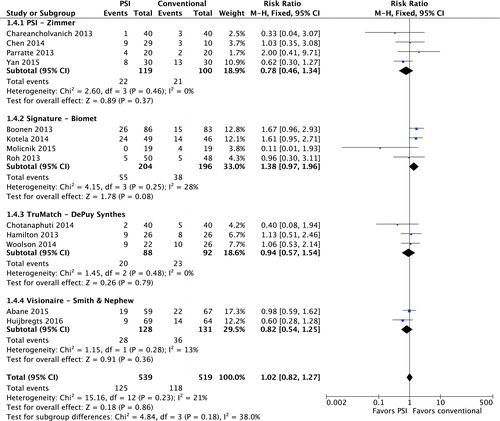
Deviation from intended hip-knee-ankle (HKA) axis was reported in 15 studies involving 1,157 patients (studies 3–11, 13, 16, and 18–21) () Heterogeneity was low (I2 = 5%). HKA axis was 0.3 degrees more accurate in the PSI group (95% CI: 0.07–0.57). Pooled data from 14 studies (1,182 TKAs, I2 = 15%; studies 1, 3–6, 8–11, 14, 16, 18, 20, and 21) () resulted in an HKA-axis outlier risk ratio of 1.00 (95% CI: 0.82–1.22). For 13 studies, the proportion of outliers could be distributed in subgroups according to the patient-specific system. The risk ratio was 1.38 (95% CI: 0.97–1.96) for the Signature system (Biomet, Warsaw, IN) (). When the study with the lowest number of participants was excluded, the risk ratio was 1.54 (95% CI: 1.07–2.22), indicating a 54% increased risk of HKA-axis outlier when using the Signature cutting guide system.
Coronal alignment
Coronal femoral component positioning was 0.3 degrees (95% CI: 0.06–0.50) more accurate in the PSI group (1,026 TKAs, I2 = 22%; studies 3–5, 7–10, 12, 16, 18, 20, and 21) (). The femoral coronal outlier risk ratio was not decreased in the PSI group: RR =0.81 (95% CI: 0.60–1.09) (1,044 TKAs, I2 = 16%; studies 1, 3–5, 8–10, 16, 18, 20, and 21) ().
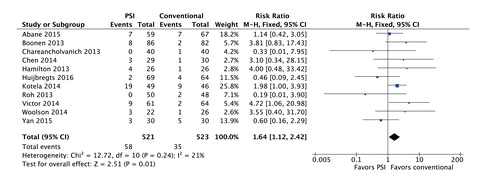
Tibial coronal alignment was similar to that in the conventional group: 0.04 degrees (95% CI: −0.17 to 0.25) (1,026 TKAs, I2 = 0%; studies 3–5, 7–10, 12, 16, 18, 20, and 21) (). The tibial coronal outlier risk ratio was 1.64 (95% CI: 1.12–2.42) in the PSI group ().
Sagittal alignment
Using a random-effects model for 980 TKAs, femoral flexion was 0.9 degrees (95% CI: 0.08–1.74) more accurate in the PSI group (studies 3, 5–10, 12, 16, 18, and 21) (). Significance was lost when the smallest study was excluded: 0.8 degrees (95% CI: −0.05 to 1.73). The sagittal femoral outlier risk ratio was 0.97 (95% CI: 0.82–1.15) (859 TKAs, I2 = 35%; studies 1, 3, 8–10, 16, 18, and 21) ().
Tibial slope was 0.7 degrees (95% CI: 0.40–0.94) more accurate in the PSI group (1,090 TKAs, I2 = 40%; studies 3, 5–10, 12, 16, 18–21) (). However, using a random-effects model for 8 studies (781 TKAs, I2 = 53%), the tibial slope outlier risk ratio was borderline-significantly increased in the PSI group: RR =1.47 (95% CI: 0.97–2.23) (studies 3, 8–10, 16, 18, 20, and 21) ().
Axial alignment
Femoral rotation was 0.45 degrees (95% CI: 0.18–0.72) more accurate in the PSI group (). The rotational outlier risk ratio was not significant in 6 studies, involving 524 TKAs: RR =0.80 (95% CI: 0.52–1.23) (studies 6, 9, 14, 16, 18, and 20) ().
Tibial rotation was determined in 5 studies (studies 7, 9, 12, 14, and 17) (). However, CT protocols varied among the studies or were not clearly described, and rotational intentions were different for the study groups, so the data could not be pooled. 1 study found more accurately rotated components in the PSI group (Ng et al. Citation2014), but the other studies found no differences.
Operation time
Various parts of the operation were timed in the studies selected. Data for total operation time and tourniquet time could be pooled. Using a random-effects model, total operation time was not shorter in the PSI group (, see Supplementary data). Tourniquet time (168 TKAs, I2 = 96%) was 6 min (95% CI: −10.1 to 21.2) shorter for PSI (Ng et al. Citation2014, Yan et al. Citation2015, Ferrara et al. Citation2015). When the study with the lowest number of participants was excluded, heterogeneity dropped significantly, allowing the use of a fixed-effects model. Tourniquet time was now reduced by 4.5 min (95% CI: 2.1–6.8) in the conventional instrumentation group (I2 = 43%).
Hospital stay
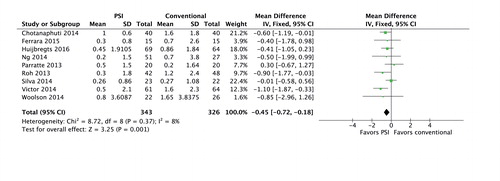
Hospital stay was approximately 8 hours (95% CI: 3.1–12.5) shorter in the PSI group (507 TKAs, I2 = 47%) (studies 3, 4, 7, 12, 13, 19, and 20) ().
Blood loss
Data from 2 studies could be pooled for total blood loss (Boonen et al. Citation2013, Chareancholvanich et al. Citation2013), intraoperative blood loss (Noble et al. Citation2012, Ferrara et al. Citation2015), blood loss by 48-hour drain (Pietsch et al. Citation2013b, Ferrara et al. Citation2015), and hemoglobin loss on day 3 (Vundelinckx et al. Citation2013, Pietsch et al. Citation2013b). Using random-effects models, total blood loss was reduced by 44 mL (95% CI: −91 to 178) and intraoperative blood loss by 68 mL (95% CI: −87 to 223) for PSI. 48-hour drain production was reduced by 194 mL (95% CI: 110–279) in the PSI group (110 TKAs, I2 = 0%). Hemoglobin loss was similar in the PSI group: 0.30 g/dL (95% CI: −0.10 to 0.70) (142 TKAs, I2 = 22%).
Blood transfusion risk ratio was 0.71 (95% CI: 0.40–1.25) for PSI (, see Supplementary data).
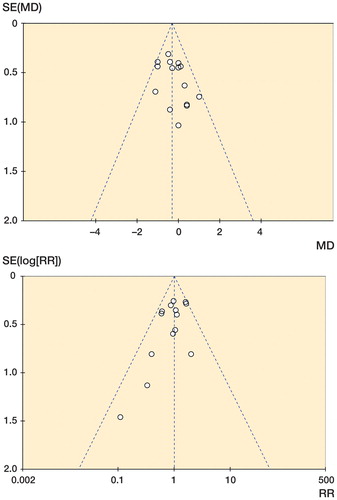
Figure 8. Funnel plots. A. Funnel plot of publication bias for deviation of intended hip-knee-ankle axis. B. Funnel plot of publication bias for hip-knee-ankle axis outliers.
2 studies (Molicnik et al. Citation2015, Ferrara et al. Citation2015) found reduced hemoglobin loss at 1 day postoperatively in the PSI group, but Molicnik et al. did not give their reference values. Abane et al. (Citation2015) used a formula to calculate blood loss and found no difference between PSI and conventional instrumentation.
Number of surgical trays used for the operation
2 pooled studies involving 81 TKAs (I2 = 89%) had a reduction of 4 surgical trays (95% CI: 2.48–5.61) in the PSI group (Noble et al. Citation2012, Hamilton et al. Citation2013).
Patient-reported outcome measures
3-month Knee Society scores from 2 studies involving 140 TKAs could be pooled (Pietsch et al. Citation2013b, Yan et al. Citation2015). Weighed mean difference for the Knee and Function scores (I2 = 7% and I2 = 0%, respectively) were negligible at 0.2 (95% CI: –2.21 to 2.61) points and 0.3 (CI: −3.75 to 4.38) points in favor of PSI. 3-month Oxford knee scores were reported in 2 studies involving 189 TKAs (I2 = 0%) (Yan et al. Citation2015, Huijbregts et al. Citation2016). The difference was 0.7 (95% CI: −1.27 to 2.65) points in favor of PSI.
No statistically significant differences were observed regarding new Knee Society scores, KOOS, or SF-12 scores at 3 months postoperatively (Abdel et al. Citation2014, Yan et al. Citation2015, Huijbregts et al. Citation2016). Vundelinckx et al. (Citation2013) collected KOOS scores at unscheduled moments and data had to be excluded. Woolson et al. (Citation2014) found no differences in Knee Society scores at 6-month follow-up. Huijbregts et al. (Citation2016) found that 1-year Oxford knee scores were marginally improved in the PSI group.
Risk of bias in the studies included
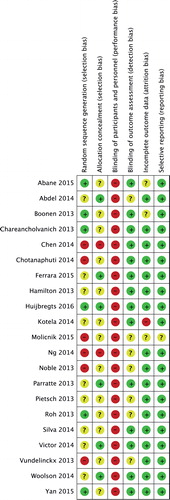
As surgeons cannot be blinded regarding the treatment allocated, the risk of performance bias was regarded as being high ().
Reporting bias
Risk of publication bias in the studies that reported on hip-knee-ankle axis was assessed by graphical assessment of funnel plots (). Both plots show minimal evidence of publication bias.
Discussion
Patient-specific instrumentation (PSI) has been introduced to improve alignment and reduce outliers after TKA. Other suggested benefits are increased efficiency, reduced operation time, and a possible reduction of complication risks due to avoidance of intramedullary canal violation.
Compared to conventional instrumentation, the mean HKA axis was 0.3 degrees more accurate in the PSI group. HKA-axis outlier risk ratio was not significantly different between the groups. Pfitzner et al. (Citation2014) recently demonstrated that HKA axis is slightly more accurate after use of MRI-based patient-specific instrumentation compared to CT-based patient-specific instrumentation. In the Signature group, 2 out of 4 studies were CT-based and the HKA outlier risk ratio was 1.38. However, the 3 studies using TruMatch (DePuy, Warsaw, IN) were all CT-based, resulting in a risk ratio of 0.94. Thus, the increased HKA-axis outlier risk appears to be related to the patient-specific system.
PSI resulted in more accurate femoral component positioning. However, mean differences were less than 0.5 degrees, which is not clinically meaningful. Alteration of intraoperative technique (moving of the pivot point for femoral rotation from the intramedullary canal to the surface of the posterior condyle) may improve femoral rotation further (Fitz et al. Citation2015).
Mean tibial slope was closer to intended in the PSI group, but coronal and sagittal plane outlier risks had increased. Likely explanations are inadequate design and suboptimal fitting technique. Although the tibial tubercle was usually used for referencing, its position varies more than any other point in the mediolateral plane (Cobb et al. Citation2008). Any axis defined by the tubercle may substantially affect rotational alignment of the tibial component (Howell et al. Citation2013). Improvement of available CT protocols is mandatory, to allow more accurate and reproducible tibial rotational measurements.
Abandonment of PSI technique has been reported to be as high as 16–32% (Roh et al. Citation2013, Victor et al. Citation2014, Woolson et al. Citation2014). Intraoperative changes to the preoperative plan often occur also (Stronach et al. Citation2013, Pietsch et al. Citation2013a). Assuming that these adjustments prevented malalignment, the difference in alignment would in fact be smaller compared to the use of conventional cutting jigs.
Total operation time varied from 12 min in favor of PSI to almost 13 min in favor of conventional instrumentation. The random-effects model did not result in any difference between the study groups. Hamilton et al. (Citation2013) powered their study on operation time and found that distal femur and AP/chamfer cuts took more time in the PSI group. They attributed their findings to the inability to adjust during surgery when PSI is used. Although surgeon training and previous case-load of high volume might bias operation time in favor of conventional techniques, it is questionable whether more experience with the PSI technique would shift time efficiency towards PSI (Huijbregts et al. Citation2016). Another purported efficiency variable was length of stay, which was minimally reduced in the PSI group. As postoperative recovery is multifactorial, the alignment technique alone would be unlikely to affect duration of admission substantially.
Increased operational efficiency was observed from a reduction in the number of surgical trays used for the procedure. However, minimal effect of radiographic alignment and operation time warrant questions about cost-effectiveness. Contradictory results have been reported in this regard (Nunley et al. Citation2012, Barrack et al. Citation2012, Tibesku et al. Citation2013). Barrack et al. (Citation2012) found lower total operative time and instrument processing time, but increased additional costs of the MRI and the cutting guide, resulting in lower overall costs for standard instrumentation. 2 studies found a reduction in preoperative preparation time when PSI was used (Watters et al. Citation2011, Tibesku et al. Citation2013). Operation room efficiency might therefore be increased by using PSI. Nevertheless, its cost-effectiveness should be questioned.
Overall blood loss and transfusion rates were similar between the study groups. Only 48-hour drain production was statistically significantly reduced in the PSI group. Perhaps diminished dissection has a role in reduction of blood loss. As postoperative intra-articular bleeding can be prevented by introduction of a bony plug or cement into the distal end of the intramedullary canal, drain production can be reduced. In addition, we did not come across any papers describing a decrease in embolic events due to avoidance of intramedullary canal violation. Thus, there is a lack of evidence suggesting a reduction in complications.
The risk-of-bias summary revealed that 6 studies had a high risk of randomization bias, and for 9 others, random sequence generation was unclear. Allocation concealment was clearly described in only 6 studies. The first limitation of our meta-analysis was a lack of methodological rigor in some part of the available data.
A limited number of studies included short-term patient-reported outcome measures. Knee Society scores and Oxford knee scores could be pooled, but were similar for PSI and conventional instrumentation. Similar results were described when new Knee Society score or KOOS was used. 1 study found marginal differences in 1-year Oxford knee score (Huijbregts et al. Citation2016). Confirmation of these results will be required to conclude the PSI discussion.
Conclusions
Our meta-analysis did not show clinically meaningful improvements in radiographic alignment, surgical efficiency, or patient-reported outcome measures. Therefore, our results do not support the routine use of patient-specific cutting guides.
- Abane L, Anract P, Boisgard S, Descamps S, Courpied J P, Hamadouche M. A comparison of patient-specific and conventional instrumentation for total knee arthroplasty: A multicentre randomised controlled trial. Bone Joint J 2015; 97-B (1): 56–63.
- Abdel M P, Parratte S, Blanc G, Ollivier M, Pomero V, Viehweger E, Argenson J N. No benefit of patient-specific instrumentation in TKA on functional and gait outcomes: A randomized clinical trial. Clin Orthop Relat Res 2014; 472 (8): 2468–76.
- Barrack R L, Ruh E L, Williams B M, Ford A D, Foreman K, Nunley R M. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br 2012; 94 (11 Suppl A): 95–9.
- Bell S W, Young P, Drury C, Smith J, Anthony I, Jones B, Blyth M, McLean A. Component rotational alignment in unexplained painful primary total knee arthroplasty. Knee 2014; 21 (1): 272–7.
- Boonen B, Schotanus M G, Kerens B, van der Weegen W, van Drumpt R A, Kort N P. Intra-operative results and radiological outcome of conventional and patient-specific surgery in total knee arthroplasty: A multicentre, randomised controlled trial. Knee Surg Sports Traumatol Arthrosc 2013; 21 (10): 2206–12.
- Cavaignac E, Pailhe R, Laumond G, Murgier J, Reina N, Laffosse J M, Berard E, Chiron P. Evaluation of the accuracy of patient-specific cutting blocks for total knee arthroplasty: A meta-analysis. Int Orthop 2015; 39 (8): 1541–52.
- Chareancholvanich K, Narkbunnam R, Pornrattanamaneewong C. A prospective randomised controlled study of patient-specific cutting guides compared with conventional instrumentation in total knee replacement. Bone Joint J 2013; 95-B (3): 354–9.
- Chen J Y, Yeo S J, Yew A K, Tay D K, Chia S L, Lo N N, Chin P L. The radiological outcomes of patient-specific instrumentation versus conventional total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2014; 22 (3): 630–5.
- Chotanaphuti T, Wangwittayakul V, Khuangsirikul S, Foojareonyos T. The accuracy of component alignment in custom cutting blocks compared with conventional total knee arthroplasty instrumentation: Prospective control trial. Knee 2014; 21 (1): 185–8.
- Cobb J P, Dixon H, Dandachli W, Iranpour F. The anatomical tibial axis: Reliable rotational orientation in knee replacement. J Bone Joint Surg Br 2008; 90 (8): 1032–8.
- Del Gaizo D J, Della Valle C J. Instability in primary total knee arthroplasty. Orthopedics 2011; 34 (9): e519–21.
- Ferrara F, Cipriani A, Magarelli N, Rapisarda S, De Santis V, Burrofato A, Leone A, Bonomo L. Implant positioning in TKA: Comparison between conventional and patient-specific instrumentation. Orthopedics 2015; 38 (4): e271–80.
- Fitz W, Jager S, Rieger J S, Seebach E, Bitsch R G. Femoral rotation in total knee arthroplasty: A comparison of patient individualized jigs with gap balancing in relation to anatomic landmarks. Knee Surg Sports Traumatol Arthrosc 2015; [Epub ahead of print]
- Fu H, Wang J, Zhou S, Cheng T, Zhang W, Wang Q, Zhang X. No difference in mechanical alignment and femoral component placement between patient-specific instrumentation and conventional instrumentation in TKA. Knee Surg Sports Traumatol Arthrosc 2015; 23 (11): 3288–95.
- Hamilton W G, Parks N L, Saxena A. Patient-specific instrumentation does not shorten surgical time: A prospective, randomized trial. J Arthroplasty 2013; 28 (8 Suppl): 96–100.
- Higgins J P, Thompson S G, Deeks J J, Altman D G. Measuring inconsistency in meta-analyses. BMJ 2003; 327 (7414): 557–60.
- Howell S M, Chen J, Hull M L. Variability of the location of the tibial tubercle affects the rotational alignment of the tibial component in kinematically aligned total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2013; 21 (10): 2288–95.
- Huijbregts H J, Khan R J, Fick,D.P., Hall,M., Punwar S A, Sorensen E, Reid M, Dalle Vedove S, Haebich S. Component alignment and clinical outcome following total knee replacement: A randomised controlled trial comparing an intramedullary alignment system with patient-matched instrumentation. Bone Joint J 2016; In press
- Kotela A, Kotela I. Patient-specific computed tomography based instrumentation in total knee arthroplasty: A prospective randomized controlled study. Int Orthop 2014; 38 (10): 2099–107.
- Moher D, Liberati A, Tetzlaff J, Altman D G, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6 (7): e1000097.
- Molicnik A, Naranda J, Dolinar D. Patient-matched instruments versus standard instrumentation in total knee arthroplasty: A prospective randomized study. Wien Klin Wochenschr 2015; 127 (Suppl 5): 235–40.
- Ng V Y, Arnott L, Li J, Hopkins R, Lewis J, Sutphen S, Nicholson L, Reader D, McShane M A. Comparison of custom to standard TKA instrumentation with computed tomography. Knee Surg Sports Traumatol Arthrosc 2014; 22 (8): 1833–42.
- Noble J W, Jr, Moore C A, Liu N. The value of patient-matched instrumentation in total knee arthroplasty. J Arthroplasty 2012; 27 (1): 153–5.
- Nunley R M, Ellison B S, Ruh E L, Williams B M, Foreman K, Ford A D, Barrack R L. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res 2012; 470 (3): 889–94.
- Parratte S, Blanc G, Boussemart T, Ollivier M, Le Corroller T, Argenson J N. Rotation in total knee arthroplasty: No difference between patient-specific and conventional instrumentation. Knee Surg Sports Traumatol Arthrosc 2013; 21 (10): 2213–9.
- Pfitzner T, Abdel M P, von Roth P, Perka C, Hommel H. Small improvements in mechanical axis alignment achieved with MRI versus CT-based patient-specific instruments in TKA: A randomized clinical trial. Clin Orthop Relat Res 2014; 472 (10): 2913–22.
- Pietsch M, Djahani O, Hochegger M, Plattner F, Hofmann S. Patient-specific total knee arthroplasty: The importance of planning by the surgeon. Knee Surg Sports Traumatol Arthrosc 2013a; 21 (10): 2220–6.
- Pietsch M, Djahani O, Zweiger C, Plattner F, Radl R, Tschauner C, Hofmann S. Custom-fit minimally invasive total knee arthroplasty: Effect on blood loss and early clinical outcomes. Knee Surg Sports Traumatol Arthrosc 2013b; 21 (10): 2234–40.
- Roh Y W, Kim T W, Lee S, Seong S C, Lee M C. Is TKA using patient-specific instruments comparable to conventional TKA? A randomized controlled study of one system. Clin Orthop Relat Res 2013; 471 (12): 3988–95.
- Silva A, Sampaio R, Pinto E. Patient-specific instrumentation improves tibial component rotation in TKA. Knee Surg Sports Traumatol Arthrosc 2014; 22 (3): 636–42.
- Stronach B M, Pelt C E, Erickson J, Peters C L. Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res 2013; 471 (1): 169–74.
- Sundfeldt M, Carlsson L V, Johansson C B, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop 2006; 77 (2): 177–97.
- Thienpont E, Schwab P E, Fennema P. A systematic review and meta-analysis of patient-specific instrumentation for improving alignment of the components in total knee replacement. Bone Joint J 2014; 96-B (8): 1052–61.
- Tibesku C O, Hofer P, Portegies W, Ruys C J, Fennema P. Benefits of using customized instrumentation in total knee arthroplasty: Results from an activity-based costing model. Arch Orthop Trauma Surg 2013; 133 (3): 405–11.
- Victor J, Dujardin J, Vandenneucker H, Arnout N, Bellemans J. Patient-specific guides do not improve accuracy in total knee arthroplasty: A prospective randomized controlled trial. Clin Orthop Relat Res 2014; 472 (1): 263–71.
- Voleti P B, Hamula M J, Baldwin K D, Lee G C. Current data do not support routine use of patient-specific instrumentation in total knee arthroplasty. J Arthroplasty 2014; 29 (9): 1709–12.
- Vundelinckx B J, Bruckers L, De Mulder K, De Schepper J, Van Esbroeck G. Functional and radiographic short-term outcome evaluation of the visionaire system, a patient-matched instrumentation system for total knee arthroplasty. J Arthroplasty 2013; 28 (6): 964–70.
- Watters T S, Mather R C,3rd, Browne J A, Berend K R, Lombardi A V,Jr, Bolognesi M P. Analysis of procedure-related costs and proposed benefits of using patient-specific approach in total knee arthroplasty. J Surg Orthop Adv 2011; 20 (2): 112–6.
- Woolson S T, Harris A H, Wagner D W, Giori N J. Component alignment during total knee arthroplasty with use of standard or custom instrumentation: A randomized clinical trial using computed tomography for postoperative alignment measurement. J Bone Joint Surg Am 2014; 96 (5): 366–72.
- Yan C H, Chiu K Y, Ng F Y, Chan P K, Fang C X. Comparison between patient-specific instruments and conventional instruments and computer navigation in total knee arthroplasty: A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2015; 23 (12): 3637–45.