Abstract
Background and purpose — According to previous Nordic Arthroplasty Register Association (NARA) data, the 10-year implant survival of cemented total hip arthroplasties (THAs) is 94% in patients aged 65–74 and 96% in patients aged 75 or more. Here we report a brand-level comparison of cemented THA based on the NARA database, which has not been done previously.
Patients and methods — We determined the rate of implant survival of the 9 most common cemented THAs in the NARA database. We used Kaplan-Meier analysis with 95% CI to study implant survival at 10 and 15 years, and Cox multiple regression to assess survival and hazard ratios (HRs), with revision for any reason as endpoint and with adjustment for age, sex, diagnosis, and femoral head material.
Results — Spectron EF THA (89.9% (CI: 89.3–90.5)) and Elite THA (89.8% (CI: 89.0–90.6)) had the lowest 10-year survivorship. Lubinus (95.7% survival, CI: 95.5–95.9), MS 30 (96.6%, CI: 95.8–97.4), and C-stem THA (95.8%, CI: 94.8–96.8) had a 10-year survivorship of at least 95%. Lubinus (revision risk (RR) = 0.77, CI: 0.73–0.81), Müller (RR =0.83, CI: 0.70–0.99), MS-30 (RR =0.73, CI: 0.63–0.86), C-stem (RR =0.70, CI: 0.55–0.90), and Exeter Duration THA (RR =0.84, CI: 0.77–0.90) had a lower risk of revision than Charnley THA, the reference implant.
Interpretation — The Spectron EF THA and the Elite THA had a lower implant survival than the Charnley, Exeter, and Lubinus THAs. Implant survival of the Müller, MS 30, CPT, and C-stem THAs was above the acceptable limit for 10-year survival.
Cemented low-friction arthroplasty, pioneered by Sir John Charnley, is the basis of the modern total hip arthroplasty (THA). Charnley THA (DePuy; Johnson and Johnson, New Brunswick, NJ) is still considered to be the gold standard against which all other devices are compared (Warth et al. Citation2014). The Lubinus THA (Waldemar Link, Hamburg, Germany) and Exeter THA (Stryker Howmedica, Mahwah, New Jersey, US) are well-documented devices with tens of thousands of implantations worldwide (SHAR Citation2014, NJR Citation2015). There are, however, several other less common cemented devices with limited data available on implant survival.
The Nordic Arthroplasty Register Association (NARA) was established in 2007 in Sweden, Norway, and Denmark with the overall aim of improving the quality of joint replacement surgery by registry-based research collaboration. Finland became a member of NARA in 2010. The total population of the 4 countries is currently 26 million.
It has been stated, based on NARA data, that the survival of cemented implants for total hip replacement is higher than that of uncemented implants in patients aged 65 years or more (Mäkelä et al. Citation2014a). In younger patients, uncemented implants do not perform better regarding overall revision rate, but they have a lower long-term risk of revision due to aseptic loosening (Pedersen et al. Citation2014). In countrywise analyses, the differences in THA survival rates in different Nordic countries turned out to be considerable, with inferior overall results for cemented THAs in Finland. Implant survival of cemented THAs was higher in Sweden than in other Nordic countries (Mäkelä et al. Citation2014b).
It is clear that brand-level implant survival data for cemented THA are required. We therefore determined the implant survival of the most common cemented THA brands in the Nordic countries based on the NARA database.
Patients and methods
Sources of data
The THA registries of Sweden, Denmark, Norway, and Finland participated. From 1995 through 2013, all 4 registries used individual-based registration of THAs and patients. A minimal NARA dataset was created, containing data that all the registries could deliver (Havelin et al. Citation2009). The degree of coverage and completeness in the Nordic registries is high (Pedersen et al. Citation2004, Arthursson et al. Citation2005, Espehaug et al. Citation2006, DHAR 2014, SHAR Citation2014, FAR Citation2015). Selection and transformation of the respective datasets and de-identification of the patients, including deletion of the national civil registration numbers, was performed within each national registry. Anonymous data were then merged into a common database.
Devices
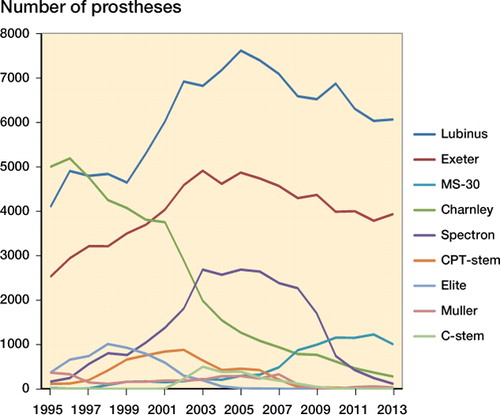
360,584 primary all-cemented THAs were registered in the NARA database from 1995 through 2013. The 9 most common cemented THAs were assessed: Lubinus, Exeter, Charnley, Spectron (Smith and Nephew, Memphis, TN), MS-30 (Zimmer, Winterthur, Switzerland), CPT (Zimmer, Warsaw, IN), Elite (DePuy; Johnson and Johnson, New Brunswick, NJ), Müller THA (Zimmer, Winterthur, Switzerland), and C-stem THA (DePuy, Johnson and Johnson, New Brunswick, NJ) ().
Table 1. Number and proportion of study implants, and demographic data
We assessed survivorship of implant families consisting of all versions of the device (see Table 2, Supplementary data), as several versions of the study implants were introduced during the study period. The different versions of the study implants were not necessarily the same in the 4 countries. Furthermore, the study devices were not necessarily coded similarly in the 4 registries. Only those cup/stem combinations with at least 100 implantations in a country were included. The cup/stem combinations assessed are listed in Table 2 (Supplementary data). Elite, MS 30, C-stem, CPT, and Müller THAs were created by combining the study stem with a cemented acetabular component by the same manufacturer.
Due to similar coding of the cup component in all 4 national registries, we had sufficient numbers to perform separate analyses of the Exeter X3 Rimfit, Exeter Contemporary, Exeter All-poly, and Exeter Duration ().
Table 3. Number and proportion of Exeter-subgroup devices, and demographic data
Statistics
We used Kaplan-Meier analysis with 95% confidence intervals (CIs) to assess implant survival at 10 and 15 years, until there were at least 100 THRs left at risk. Patients were censored at death or December 31, 2013, whichever came first. Outcome was revision for any reason, defined as removal or exchange of at least 1 of the components. Kaplan-Meier survivorship was also assessed separately for each device for 2 time periods, 1995–2004 and 2005–2013, using any reason for revision as endpoint. Furthermore, Kaplan-Meier survivorship for aseptic loosening of the cup, stem, or both components was assessed depending on the type of cement used (Palacos-type, Simplex-type, or other) (Espehaug et al. Citation2009). We used Cox multiple regression to determine survival rates and hazard ratios (HRs), with revision for any reason as endpoint, and with adjustment for age (< 60, 60–64, 65–69, 70–74, ≥ 75), sex, diagnosis (primary osteoarthritis, hip fracture, non-traumatic femoral head necrosis, inflammatory disease, childhood hip disease, or other/unknown), and femoral head material (metal, ceramics, or other/unknown). The assumption of proportional hazards was fulfilled, as evaluated by visual inspection of log-minus-log-plots.
Both Kaplan-Meier and Cox analysis are based on the assumption of non-informative censoring, an assumption that is not fulfilled when estimating revision risk and censoring for death. Thus, competing risk assessment was also performed using Stata 14 statistical software, and these data are available in Table 8 (see Supplementary data).
Patients with bilateral procedures were included, as earlier research has shown that this does not bias the results (Lie et al. Citation2004, Ranstam and Robertsson Citation2010). We considered any p-values less than 0.05 to be statistically significant. For most statistical analyses, we used SPSS version 22.0.
Ethics
Ethical approval of the study was obtained through each national registry.
Results
The Lubinus THA was the most common study device, being used in 41% of all implantations. Mean age at the time of surgery was highest for the CPT THA (73 years). The proportion of female patients was highest for Charnley THA (69%) ( and , and ).
Table 4. Kaplan-Meier survivorship of the study devices at 10 and 15 years with revision for any reason as the endpoint, and adjusted revision rate (RR) (age, sex, diagnosis, femoral head material) for revision using Cox regression
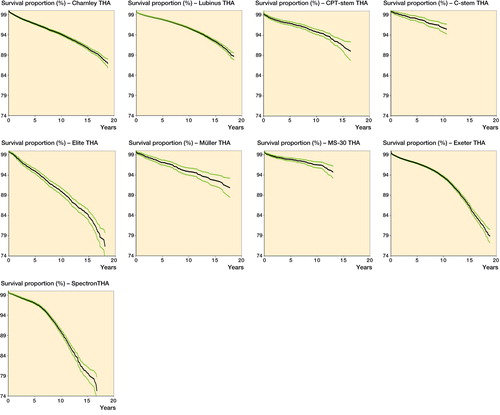
Several THAs had a 10-year survivorship of 95% or more, including Lubinus (95.7%, CI: 95.5–95.9), MS 30 (96.6%, CI: 95.8–97.4), and C-stem (95.8%, CI: 94.8–96.8). The lowest 10-year implant survival was observed in patients with Spectron EF THA (89.9%, CI: 89.3–90.5) and Elite THA (89.8%, CI: 89.0–90.6) ( and , and and ).
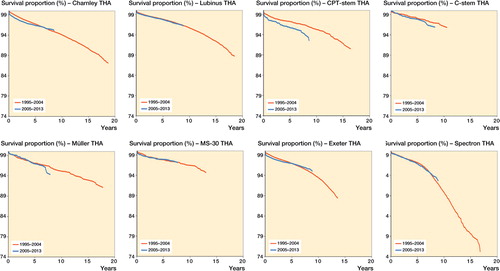
Table 5. Implant survival at 7 years for the time periods 1995–2004 and 2005–2013, with any reason for revision as the endpoint
Table 6. Kaplan-Meier survivorship of the study devices with either Palacos-type, Simplex-type, or other bone cement at 10 years with aseptic loosening as the endpoint
Implant survival of Charnley, Exeter, and Elite THAs with Palacos-type cement was higher than those of the same devices with other types of cement. Implant survival of Lubinus, Spectron, and Müller THAs with Palacos- and Simplex-type cement was higher than those of the same devices with other types of cement ().
Lubinus (revision risk (RR) = 0.77, CI: 0.73–0.81), Müller (RR =0.83, CI: 0.70–0.99), MS 30 (RR =0.73, CI: 0.63–0.86), and C-stem THAs (RR =0.70, CI: 0.55–0.90) had a lower revision risk than Charnley THA. Spectron EF (RR= 1.73, CI: 1.62–1.84), Exeter (RR =1.25, CI: 1.18–1.31), and Elite THAs (RR =1.65, CI: 1.51–1.80) had a higher revision risk than Charnley THA after adjusting for age, sex, and diagnosis ().
In subgroup analysis of the Exeter devices, the Exeter X3 Rimfit THA had a similar revision risk to that of the reference implant (Charnley) THA (RR =1.13, CI: 0.91–1.39). The Exeter Duration THA had a lower revision risk than the reference implant (RR =0.84, CI: 0.77–0.90) ().
Table 7. Adjusted revision risk (age, sex, diagnosis, femoral head material) for revision of the Exeter-subgroup devices
Results of the competing risk assessments are given in Table 8 (see Supplementary data). The results varied slightly, but they did not change the ranking of the implants.
Discussion
The Spectron EF and Elite THAs had a lower implant survival than the Charnley THA, the reference implant. Implant survival of Müller, MS 30, CPT, and C-stem THAs (94.9–96.6% at 10 years) was far above the acceptable limit for 10-year survival. However, the total amount of these devices was small compared to Charnley, Lubinus, and Exeter THAs, although all of them had been implanted in more than 2,000 hips. When an implant becomes more common and is used by an increasing number of surgeons, the results will be more representative since they can be assumed to reflect a wider range of differences in surgical technique.
A major strength of the present study was the unique collaboration of the 4 national registries to create a multinational database with large numbers of patients and a long follow-up time. The main weakness of the study was that we were unable to assess every updated version of each device separately. The study devices were implant families, consisting of several versions of the device. Another weakness was that we were not able to assess cup and stem survival separately with revision for any reason as the endpoint. These data are were not available from the Finnish Arthroplasty Register, and they were therefore not included in the 4-country minimal dataset either. Furthermore, our data did not include information on parameters such as surgeon volume, hospital volume, ASA grade, or preoperative patient-reported outcome measures (PROMs).
Implant survival of Charnley THAs was high (94.1% at 10 years), but slightly lower than that reported by the NJR (10-year survival of 95.1% for Charnley Ogee/Charnley and 97.0% for Charnley/Charnley) (NJR Citation2015). In Australia, 10-year survival for Charnley Ogee/Charnley was 91.6%, whereas that for Charnley/Charnley was 93.0% (AOANJRR Citation2015). The total amount of Charnley THAs in Australia was low (1,300), which might explain the slightly inferior results compared to ours. The Charnley THAs studied consisted of several versions of the Charnley stem, such as Charnley flanged, Charnley flanged heavy, and Charnley flat and round-backed stems. It has been stated previously that the implant survival of the Charnley THA after 1995 has been good, and differences in implant survival between Charnley stems are minor (Espehaug et al. Citation2009). Similarly, the cup designs of the Charnley THA that were assessed varied. The use of Charnley THAs decreased drastically towards the end of the study period.
The Elite Plus THA was introduced in 1994 as the second modular evolution of the original Charnley THA. Several changes were made to the shape and the dimensions of the femoral component, to improve proximal load transfer and reduce contact stresses. The design also incorporated an undercutting of the neck flange (DePuy Citation1993). Overall survivorship of Elite THAs in our study was inferior to that of the reference implant. The Elite Plus stem has been withdrawn from the market due to divergent clinical results (Hauptfleisch et al. Citation2006, Kim et al. Citation2007, von Schewelov et al. Citation2010). Our results support these earlier findings. A weakness of our Charnley vs. Elite THA analysis was that Elite cups were sometimes—although rarely—used with Charnley stems, and vice versa, which may have biased our results.
Implant survival of the third DePuy device assessed, the C-stem THA (95.8% at 10 years), was higher than that of the Charnley THA, and comparable to previous reports (a 10-year survival of 94.6% for C-stem/Elite Plus in the AOANJRR, and a10-year survival of 98% for C-stem/Elite Plus Ogee in the NJR). The triple-tapered, polished cemented C-stem introduced in 1993 was based on the original Charnley concept of the flat-back polished stem (Purbach et al. Citation2013). However, the total number of C-stem THAs that have been implanted to date in the Nordic countries—and also worldwide—is low compared to the original Charnley THA.
Long-term survival of the Lubinus THA in our study was higher than that of the reference implant. Most of the study stems were SP II models (see Appendix) with a high degree of documentation (Annaratone et al. Citation2000, Wierer et al. Citation2013, Prins et al. Citation2014, SHAR Citation2014). However, most of the Lubinus THAs (79%) in our study were performed in Sweden, which has twice the population of each of the 3 other countries. In general, the implant survival of cemented THAs in Sweden is substantially higher than in the other 3 countries (Mäkelä et al. 2014). The Swedish Hip Arthroplasty Register has provided feedback to the profession and also continued training in cementing techniques for more than 30 years. So the excellent implant survival of Lubinus THA in the present study may have been biased by the “Swedish factor”. The threshold for performing a revision operation is also culture-dependent, and may vary between the Nordic countries. X-linked Lubinus cups were coded separately in the NARA hip database only in Sweden, so we did not assess X-linked Lubinus THA separately. However, these devices will be able to be detected in the future using NARA data, when the catalog number-based register is ready.
We found that long-term survival of Exeter THAs was satisfactory, although inferior to that of the reference device. The overall implant survival of Exeter THAs in our study (93.5%) was slightly lower than that reported by the NJR (10-year survival of 97.1% for Exeter V40/Contemporary, and 96.3% for Exeter V40/Exeter Duration), but similar to that reported by the AOANJRR (10-year survival for Exeter V40/Contemporary of 94.1%). In the current study, the Exeter THA family consisted almost exclusively of Exeter Universal stems. The Exeter V40 stem was separately coded only in Denmark in the current NARA database, so the number of definite Exeter V40 THAs in the current study was low. However, we were able to do subgroup analyses for Exeter THAs according to the acetabular component (). The Exeter X3 Rimfit THA (X3-stabilized UHMWPE, the latest version of high crosslinking) was coded separately in each of the 4 registries. Implant survival of this device was comparable to that of the reference implant. The total number of Exeter X3 Rimfit THAs was, however, small compared to Exeter Contemporary THAs and Exeter All-poly THAs, and the follow-up time was shorter. Contemporary cups are made of Duration-stabilized UHMWPE, whereas all-polyethylene cups are not Duration-stabilized. Duration-stabilized UHMWPE was the first annealed (heated below melting temperature) moderately crosslinked polyethylene with 3 Mrad of irradiation. The long-term implant survival of Exeter Contemporary THAs and Exeter All-poly THAs was good, although not quite as good as that of the best performers. Based on previous Swedish data, the implant survival of Lubinus THAs and Exeter THAs is similar in Sweden (SHAR Citation2014). The “Sweden factor” appears to have a major impact on the survival of the Exeter THA also. The most common Swedish device was the Exeter Duration THA, with excellent survivorship. The Exeter Duration THA was named specifically in Sweden and Denmark only and we did not ask for subclassification in the other countries for this particular study. In England and Wales, implant survival of Exeter/Contemporary THA was slightly higher than that of Exeter/Exeter Duration THA, which was contrary to our findings. In Australia, Exeter/Duration THA is not assessed separately. However, 15-year survivorship of the Exeter THA in our study was inferior to that of the best performers. 15-year survival data for the Exeter THA are not yet available from the NJR or the AOANJRR.
It should be taken into account that the Exeter stem is very easy to revise, and the revision method of cementing a new smaller stem without removing the old bone cement is well established (te Stroet et al. Citation2014). The ease of the Exeter stem revision could have biased survivorship of the Exeter THA in our study. However, the revision risk of the Exeter stem regarding periprosthetic fracture is higher than for the Lubinus SP II stem (Thien et al. Citation2014).
Implant survival of Spectron EF THAs in our study (89.9% at 10 years, and 79.8% at 15 years) was inferior to that of other THAs, and also inferior compared to previous reports (with a 10-year survival of 92.1% for Spectron EF/Reflection in the AOANJRR and in Norway (Espehaug et al. Citation2009)). Spectron THA in our study consisted mostly of Spectron EF stems and Reflection cups. It has been shown in RSA studies that cups with ethylene oxide-sterilized polyethylene (such as the Reflection cup) have higher wear rates than those with gamma irradiation-sterilized polyethylene (Digas Citation2005, Kadar et al. Citation2011a, Jonsson et al. Citation2015). The modular Spectron EF stem was introduced in 1988, and in 1989 the roughness of the proximal part of the stem was increased. 5 years later, further modifications to the stem were introduced and the name was altered to Spectron EF Primary. The collar became polished and smaller sizes were introduced. This design performed worse than its predecessor, especially the smallest sizes (, Thien and Kärrholm Citation2010, SHAR Citation2014). It has been suggested that the addition of a rough surface to the Spectron stem has been detrimental to the long-term success of the prosthesis (Gonzalez Della Valle et al. Citation2006, Grose et al. Citation2006, Espehaug et al. Citation2009, Kadar et al. Citation2011b). Our results support these findings.
Implant survival of Müller THAs (94.9% at 10 years, and 92.6% at 15 years) was higher than that of the reference implant, and comparable to that in previous reports (Mäkelä et al. Citation2008, Clauss et al. Citation2013, Nikolaou et al. Citation2013). The Müller THAs studied consisted mostly of Müller straight stems and of Müller all-polyethylene cups. Müller stems made of titanium alloy with a roughened surface finish were excluded due to previous reports of increased revision risk compared to polished cobalt-chrome Müller stems (Clauss et al. Citation2013, FAR Citation2015). Implant survival of another Zimmer device, the MS-30 THA (96.6% at 10 years) was also high, and comparable to those of previous reports (10-year survival of 97.5% for MS-30/Low Profile in the AOANJRR, and 10-year survival of 99% for MS-30/Low Profile in the NJR). However, stem survival as poor as 80% at 12 years has also been reported for the MS-30 (Witte et al. Citation2009). The original MS-30 (Morscher-Sportorno) stem was made of stainless steel, and was straight, three-dimensionally tapered, collarless, and matt-surfaced (Berli et al. Citation2005, Brigstocke et al. Citation2014). However, most MS-30 stems inserted in Sweden have been polished. The MS-30 stem was used in combination with Zimmer all-polyethylene cups such as the ZCA and highly crosslinked ZCA XLPE. A weakness of the Müller and MS-30 THA analysis was that all the Müller THAs studied were performed in Finland and in Sweden, and all the MS-30 THAs were performed in Sweden and Norway.
Implant survival of the third Zimmer device studied, the CPT THA (94.9% at 10 years, and 91.6% at 15 years), was comparable to that of the reference THA, and to those in previous reports (a 10-year survival of 95.4% for CPT/ZCA in the AOANJRR, and a 10-year survival of 96.4% for CPT/ZCA in the NJR). The CPT (collarless polished tapered) stem was originally developed as a collarless, highly polished, double-tapered prosthesis for distribution in the USA. Like the Exeter stem, from which the principles of its design were taken, the CPT also uses the taper slip concept. The CPT differs from the Exeter Universal stem in its broad lateral shoulder, more complete lateral taper, and more rectangular proximal geometry (Burston et al. 2012). CPT stems made of stainless steel and made of chromium cobalt were included in the current study, which may have caused bias. Although performed in all 4 countries, the total number of CPT THAs was small.
The long-term performance of cemented THAs depends on many factors in addition to the implant, such as the characteristics of the patient, surgical and cementing technique, and the properties of the bone cement used. Although all bone cements used today are based on methylmethacrylate, their performance may vary. Poor results have been found for some low-viscosity cements (Havelin et al. Citation1995, Furnes et al. Citation1997, Espehaug et al. Citation2002). We therefore also determined implant survival according to the type of cement used (Palacos-type, Simplex-type, or other) (Espehaug 2009). Implant survival of Charnley, Elite, and Exeter THAs was higher when used with high-viscosity, Palacos-type cement. Our results support previous findings (Havelin et al. Citation1995, Espehaug et al. Citation2002). Implant survival of the study devices was similar in the period 1995–2004 and the period 2005–2013. Cementing techniques appear to have become standardized in the Nordic countries over the last 2 decades.
In summary, several cemented THA brands perform well in the long term. However, there are substantial differences in implant survival between high and low performers.
Supplementary data
Tables 2 and 8 are available on the Acta Orthopaedica website att www.orthop.org, number 10004.
This paper is the result of close team work, all the authors participated in planning and design of the study and interpretion of the results. PP performed statistical analyses. MJ was responsible for writing the manuscript.
No competing interests declared.
- AOANJRR. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2015. https://aoanjrr.sahmri.com/
- Annaratone G, Surace F M, Salerno P, Regis G F. Survival analysis of the cemented SP II stem. J Orthopaed Traumatol 2000; 1: 41–5.
- Arthursson A J, Furnes O, Espehaug B, Havelin L I, Söreide J A. Validation of data in the Norwegian Arthroplasty Register and the Norwegian Patient Register: 5,134 primary total hip arthroplasties and revisions operated at a single hospital between 1987 and 2003. Acta Orthop 2005; 76: 823–8.
- Berli B J, Schäfer D, Morscher E W. Ten-year survival of the MS-30 matt-surfaced cemented stem. J Bone Joint Surg Br 2005; 87(7): 928–33.
- Brigstocke G H, Mitchell P A, Rosson J W. Total hip arthroplasty with the MS-30 polished surface cemented stem: a single surgeon consecutive series study at 10 year follow-up. Eur J Orthop Surg Traumatol 2014; 24(1): 63–6.
- Clauss M, Gersbach S, Butscher A, Ilchmann T. Risk factors for aseptic loosening of Müller-type straight stems. A registry-based analysis of 828 consecutive cases with a minimum follow-up of 16 years. Acta Orthop 2013; 84 (4): 353–359.
- DePuy. Elite Plus Total Hip Replacement System. Product Rationale. Leeds: DePuy International Ltd, 1993.
- DHAR. Danish Hip Arthroplasty Register. www.dhr.dk 2013.
- Digas G. New polymer materials in total hip arthroplasty. Evaluation with radiostereometry, bone densitometry, radiography and clinical parameters. Acta Orthop (Suppl 315) 2005; 76: 3–82.
- Espehaug B, Furnes O, Havelin L I, Engesaeter L B, Vollset SE. The type of cement and failure of total hip replacements. J Bone Joint Surg Br 2002; 84-B: 832–8.
- Espehaug B, Furnes O, Havelin L I, Engesaeter L B, Vollset S E, Kindseth O. Registration completeness in the Norwegian Arthroplasty Register. Acta Orthop 2006; 77: 49–56.
- Espehaug B, Furnes O, Engesaeter L B, Havelin L I. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register: concerns about some newer implants. Acta Orthop 2009; 80(4): 402–12.
- FAR. Finnish Arthroplasty Register. www.thl.fi/far 2015.
- Furnes O, Lie S A, Havelin L I, Vollset S E, Engsaeter L B. Exeter and Charnley arthroplasties with Boneloc or high viscosity cement: comparison of 1,127 arthroplasties followed for 5 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 1997; 68: 515–20.
- Gonzalez Della Valle A, Rana A, Nestor B, Bostrom M, Westrich G, Salvati E A. Metallic shedding, surface finish changes, and extensive femoral osteolysis in the loose Spectron EF stem. Clin Orthop 2006; (442): 165–70.
- Grose A, Gonzalez Della Valle A, Bullough P, Lyman S, Tomek I, Pellicci P. High failure rate of a modern, proximally roughened, cemented stem for total hip arthroplasty. Int Orthop 2006; 30 (4): 243–7.
- Hauptfleisch J, Glyn-Jones S, Beard D J, Gill H S, Murray D W. The premature failure of the Charnley Elite-Plus stem: a confirmation of RSA predictions. J Bone Joint Surg Br 2006; 88 (2): 179–83.
- Havelin L I, Espehaug B, Vollset S E, Engesaeter L B. The effect of the type of cement on early revision of Charnley total hip prostheses: a review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg Am 1995; 77-A: 1543–50.
- Havelin L I, Fenstad A M, Salomonsson R, Mehnert F, Furnes O, Overgaard S, Pedersen A B, Herberts P, Kärrholm J, Garellick G. The Nordic Arthroplasty Register Association. A unique collaboration between 3 national hiparthroplasty registries with 280,201 THRs. Acta Orthop 2009; 80 (4): 393–401.
- Jonsson B A, Kadar T, Havelin L I, Haugan K, Espehaug B, Indrekvam K, Furnes O, Hallan G. Oxinium modular femoral heads do not reduce polyethylene wear in cemented total hip arthroplasty at five years. A randomized trial of 120 hips using radiostereometric analysis. Bone Joint J 2015; 97-B: 1463–9.
- Kadar T, Hallan G, Aamodt A, Indrekvam K, Badawy M, Skredderstuen A, Havelin L I, Stokke T, Haugan K, Espehaug B, Furnes O. Wear and migration of highly cross-linked and conventional cemented polyethylene cups with cobalt chrome or oxinium femoral heads. A randomized radiostereometric study of 150 patients. J Orthop Res 2011a; 29(8): 1222–9.
- Kadar T, Hallan G, Aamodt A, Indrekvam K, Badawy M, Havelin LI, Stokke T, Haugan K, Espehaug B, Furnes O. A randomized study on migration of the Spectron EF and the Charnley flanged 40 cemented femoral components using radiostereometric analysis at 2 years. Acta Orthop. 2011b; 82(5): 538–44.
- Kim Y H, Kim J S, Yoon S H. Long-term survivorship of the Charnley Elite Plus femoral component in young patients. J Bone Joint Surg Br 2007; 89(4): 449–54.
- Lie S A, Engesaeter L B, Havelin L I, Gjessing H K, Vollset S E. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients. Stat Med 2004; 23(20): 3227–40.
- Mäkelä K, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V. Cemented total hip replacement for primary osteoarthritis in patients aged 55 years or older: results of the 12 most common cemented implants followed for 25 years in the Finnish Arthroplasty Register. J Bone Joint Surg Br 2008; 90(12): 1562–9
- Mäkelä KT, Matilainen M, Pulkkinen P, Fenstad AM, Havelin L, Engesaeter L, Furnes O, Pedersen AB, Overgaard S, Kärrholm J, Malchau H, Garellick G, Ranstam J, Eskelinen A. Failure rate of cemented and uncemented total hip replacements: register study of combined Nordic database of four nations. BMJ 2014a; 348: f7592.
- Mäkelä KT, Matilainen M, Pulkkinen P, Fenstad AM, Havelin LI, Engesaeter L, Furnes O, Overgaard S, Pedersen AB, Kärrholm J, Malchau H, Garellick G, Ranstam J, Eskelinen A. Countrywise results of total hip replacement. An analysis of 438,733 hips based on the Nordic Arthroplasty Register Association database. Acta Orthop 2014b; 85(2): 107–16.
- Nikolaou V S, Korres D, Lallos S, Mavrogenis A, Lazarettos I, Sourlas I, Efstathopoulos N. Cemented Müller straight stem total hip replacement: 18 year survival, clinical and radiological outcomes. World J Orthop 2013 Oct 18; 4(4): 303–8.
- NJR. National Joint Registry. NJR 12th Annual Report. http://www.njrcentre.org.uk 2015.
- Pedersen A, Johnsen S, Overgaard S, Søballe K, Sørensen H T, Lucht U. Registration in the danish hip arthroplasty registry: completeness of total hip arthroplasties and positive predictive value of registered diagnosis and postoperative complications. Acta Orthop Scand 2004; 75(4): 434–41.
- Pedersen A B, Mehnert F, Havelin LI, Furnes O, Herberts P, Kärrholm J, Garellick G, Mäkela K, Eskelinen A, Overgaard S. Association between fixation technique and revision risk in total hip arthroplasty patients younger than 55 years of age. Results from the Nordic Arthroplasty Register Association. Osteoarthritis Cartilage 2014; 22(5): 659–67.
- Prins W, Meijer R, Kollen B J, Verheyen C C, Ettema H B. Excellent results with the cemented Lubinus SP II 130-mm femoral stem at 10 years of follow-up: 932 hips followed for 5-15 years. Acta Orthop 2014; 85(3): 276–9.
- Purbach B, Kay P R, Siney P D, Fleming P A, Wroblewski B M. The C-stem in clinical practice: fifteen-year follow-up of a triple tapered polished cemented stem. J Arthroplasty 2013; 28(8): 1367–71.
- Ranstam J, Robertsson O. Statistical analysis of arthroplasty register data. Acta Orthop 2010; 81(1): 10–4.
- SHAR. Swedish Hip Arthroplasty Register. Annual Yearbook 2014. www.shpr.se/
- te Stroet M A, Moret-Wever S G, de Kam D C, Gardeniers J W, Schreurs B W. Cement-in-cement femoral revisions using a specially designed polished short revision stem; 24 consecutive stems followed for five to seven years. Hip Int 2014; 24(5): 428–33.
- Thien T M, Kärrholm J. Design-related risk factors for revision of primary cemented stems. Acta Orthop 2010; 81(4): 407–12.
- Thien T M, Chatziagorou G, Garellick G, Furnes O, Havelin L I, Mäkelä K, Overgaard S, Pedersen A, Eskelinen A, Pulkkinen P, Kärrholm J. Periprosthetic femoral fracture within two years after total hip replacement: analysis of 437,629 operations in the nordic arthroplasty register association database. J Bone Joint Surg Am 2014; 96(19): e167.
- Warth L C, Callaghan J J, Liu S S, Klaassen A L, Goetz D D, Johnston R C. Thirty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old. A concise follow-up of previous reports. J Bone Joint Surg Am 2014; 96(21): 1814–9.
- Wierer T, Forst R, Mueller L A, Sesselmann S. Radiostereometric migration analysis of the lubinus SP II hip stem: 59 hips followed for 2 years. Biomed Tech (Berl) 2013; 58 (4): 333–41.
- Witte D, Klimm M, Parsch D, Clarius M, Breusch S, Aldinger PR. Ten-year survival of the cemented MS-30 femoral stem: increased revision rate in male patients. Acta Orthop Belg 2009; 75(6): 767–75.
- von Schewelov T, Sanzén L, Besjakov J, Carlsson A. The Elite-Plus stem migrates more than the flanged Charnley stem. Acta Orthop 2010; 81(3): 280–5.