Abstract
Background and purpose — Porous tantalum cups have been introduced as an alternative to various reinforcement rings in revision hip surgery. We hypothesized that porous tantalum cups would be superior to Müller acetabular roof reinforcement rings (MARRs) in revision hip surgery with re-revision for aseptic loosening as the primary outcome measure.
Patients and methods — 207 hips operated with either a porous tantalum cup (TM cup, n = 111) or a MARR (n = 96) at index procedure were identified in our local arthroplasty register. Acetabular defects were classified according to Paprosky. There were 96 men and 111 women with a median age of 71 (35–95) years, presenting acetabular defect size type I in 39 cases, IIA in 22, IIB in 27, IIC in 43, IIIA in 32, and IIIB in 37 cases. Analysis of medical records identified all patients with subsequent re-revision and reasons for re-revisions. Kaplan-Meier survival functions were used to estimate implant survival.
Results — With re-revision for aseptic loosening as the endpoint, the 6-year unadjusted cumulative survival was 97% (95% CI: 94–100) for TM cups and 96% (CI: 92–100) for MARR (p = 0.6). Using re-revision for any reason as the endpoint, 6-year survival was 87% (CI: 81–94) for TM cups and 95% (CI: 90–99) for MARR (p = 0.06). The main reason for re-revision in the TM group was dislocation (n = 10), followed by loosening (n = 3), whereas the main reason for re-revision in the MARR group was aseptic loosening (n = 8). Duration of the index procedure and perioperative blood loss were lower in the TM group.
Interpretation — Both TM and MARR lead to good 6-year results in acetabular revision surgery. The methods differ in their respective failure mechanisms. We conclude that TM cups are a valuable treatment option in acetabular revision surgery, but the reasons underlying dislocations after the use of TM cups must be analyzed further.
13% of all patients undergoing total hip arthroplasty (THA) require revision surgery within 10 years postoperatively (Labek et al. Citation2011). Most revision procedures involve the acetabular component, either in combination with a stem revision or without (Swedish Hip Arthroplasty Register Citation2014). The main cause of revision surgery is aseptic loosening of one or both components, followed by infection, dislocation, or periprosthetic fracture (Swedish Hip Arthroplasty Register Citation2013). Projections predict a 2-fold increase in revision hip surgery by the year 2026 (Kurtz et al. Citation2007), and thereby increase in overall costs and impairment of quality of life for patients (Burns and Bourne Citation2006).
Historically, devices such as the Müller acetabular roof reinforcement ring (MARR) proved to be fairly successful in revision surgery, but the technique has its challenges and pitfalls (Kremers et al. Citation2012). Furthermore, the outcome is dependent on bone contact, and in situations where osteolysis compromises the acetabular rim—especially superiorly—MARR proved to be an unsatisfactory concept (Starker et al. Citation1998). Burch-Schneider reinforcement cages and other devices are used in situations with more extensive bone loss (Berry and Müller Citation1992).
Porous tantalum cups were introduced as an alternative strategy for acetabular revision surgery. At the microscopic level, its porous structure (resembling cancellous bone) offers a surface that facilitates bone ingrowth (Balla et al. Citation2010). Elasticity and high friction of porous tantalum allow good primary and secondary stability. Given these properties, porous tantalum cups appear to offer theoretical advantages in revision hip surgery (Bobyn et al. Citation1999, Kremers et al. Citation2012, Batuyong et al. Citation2014). Published studies on porous tantalum cups used in revision surgery mostly have good or satisfactory results: Unger et al. (Citation2005) reported only 1 re-revision due to aseptic loosening in their study population of 60 hips. In a study of 805 patients with TM cups, Mohaddes et al. (Citation2015) found an unadjusted 5-year survival rate of 89% for porous tantalum cups used in revision surgery.
Only a few papers on acetabular revision surgery using porous tantalum cups have reported comparisons between this type of implant and concepts such as the MARR. We therefore investigated a consecutive local cohort of patients who underwent acetabular revision surgery either using porous tantalum cups or MARR, taking acetabular defect size into account. We hypothesized that porous tantalum cups would be superior to MARR with re-revision due to aseptic loosening as the primary endpoint and re-revision for any reason as a secondary endpoint.
Patients and methods
We identified all patients in our local arthroplasty register who underwent acetabular revision surgery using either a porous tantalum cup during the period 2008–2012 or a MARR (Zimmer) from 1998 to 2012. Whenever an individual had bilateral revision surgery, only the first procedure was included as the index procedure in order to avoid dependency issues created by bilateral observations. The acetabular revision procedures identified were termed "index procedures". Since all re-revisions were also recorded in the register, we gathered information on whether the patients included underwent subsequent re-revision, at what time point the re-revision was performed, and for what reasons. In order to investigate whether some of our patients underwent re-revision surgery at other departments, we cross-validated our dataset with the Swedish hip arthroplasty register (SHAR) and found 1 additional re-revision registered in the SHAR that was done at another hospital (and had therefore not been recorded in our local register). Dates of death were collected for all deceased patients.
Analysis of data from the medical charts of all the patients included was performed in order to obtain additional information on primary diagnoses, i.e. the diagnosis underlying the primary THA that was later revised during the index procedure, the diagnosis at index procedure (e.g. aseptic loosening, infection, dislocation, or other), and reasons for re-revision where applicable. Acetabular defect size prior to the index procedure was assessed by analyzing radiographs obtained preoperatively using the Paprosky classification (Paprosky et al. Citation1994). 2 observers (AB and EF) independently assessed standard anteroposterior pelvic radiographs in order to classify bone loss according to the categories suggested by Paprosky, and whenever the 2 observers failed to reach agreement a consensus classification was achieved under the guidance of the senior author. Since not all the surgeons involved had given sufficiently detailed descriptions of acetabular defect size in their operative notes, we were unable to systematically compare the defect size found at surgery with the classification according to the radiographic categories defined by Paprosky.
Surgical technique and implants
At our unit, the direct lateral approach is most commonly used in revision surgery (171 out of 207; 15 transfemoral, 20 unknown, and 1 posterior). In 91 of the 207 cases, stem revision was necessary at the index procedure; the remaining 116 revisions addressed only the cup. When MARRs (Zimmer, Warsaw, IN) were inserted, frozen morselized bone allograft was incubated in physiological saline solution at 37 °C and used whenever needed in order to fill bone defects behind the MARR (67 out of 96 patients; missing data in 8 cases). MARRs were then fixed to the pelvic bone using multiple cancellous screws. Subsequently, standard polyethylene liners (n = 90) or a dual-mobility cup (Avantage; Biomet; n = 6) were cemented into the MARR using Palacos, allowing restoration of the center of rotation.
In this study, the term "TM cups" refers to both Continuum cups and TM revision shells by the same manufacturer (Zimmer). Lower-degree defects (mostly Paprosky type I) received Continuum cups; larger-size defects (Paprosky type II and above) received TM revision shells. Both constructs were introduced press-fit into reamed acetabular defects. Additional screw fixation to the pelvis using cancellous screws was performed when necessary (n = 71, median 3 (1–9) screws). In the case of small to medium-sized TM cups, standard liners were inserted into the cups, either snap-fit (in the case of Continuum cups) or cemented (whenever larger TM revision shells were used). In cases of doubtful joint stability (i.e. after a history of previous dislocations or when there was loss of gluteus medius integrity) and if the size of the TM revision shell permitted this, a dual-mobility cup (Avantage; Biomet) was cemented into the TM revision shell (n = 26).
Different head sizes ranging from 22 to 32 mm were combined with the chosen liners as required. We mainly used 28-mm heads (in 128 of the 207 patients). Only 7 patients received a 32-mm head. Data on head size were missing for 54 patients, mainly from the early period of the observation time.
As a rule, our patients were advised to bear weight as tolerated, both after complex acetabular revision surgery and after transfemoral approaches to the femur. Adequate positioning of the implants was evaluated with postoperative radiography, and both clinical and radiographic follow-up was performed at 3–4 months postoperatively. Thereafter, further follow-up visits were only scheduled when necessary.
Characteristics of the study population
Using the inclusion criteria described above, we identified and included 111 patients (111 hips) who had received a TM cup and 96 patients (96 hips) who had received a MARR during acetabular revision surgery. MARRs were used from 1998 to 2012, but were used less frequently from 2008 onwards, since the TM cup was introduced in our department during that year. Due to the stepwise substitution of the MARR with the TM cup, the mean follow-up time was 4.9 (2.1–6.9) years for the group of patients who had received a TM cup and 12 (2.3–17) years for those who had received a MARR. The 2 groups investigated were similar with respect to age, sex, and diagnosis at the index procedure. Analysis of radiographs obtained preoperatively according to the Paprosky classification indicated that there was no difference in the distribution of acetabular defect sizes between the 2 groups investigated ( and ).
Table 1. Description of the entire study populationTable Footnotea
Table 2. Age, blood loss, and duration of surgery
Statistics
Continuous data are described using means, medians, and ranges. 95% confidence intervals (CIs) are used to describe estimation uncertainty. Categorical data were summarized in cross-tables and the Chi-square test was used to investigate differences between groups. We compared TM cups with MARR devices with re-revision for aseptic loosening as the primary endpoint, and re-revision of these devices for any reason as the secondary endpoint. The Kaplan-Meier method was used to calculate cumulative unadjusted component survival, and the Mantel-Haenszel log-rank test was used to assess whether survival differed between groups. Cox multivariable regression models were fitted in order to calculate hazard ratios for the risk of experiencing the primary or secondary endpoints, adjusted for relevant confounders such as sex, age, and acetabular defect size at the index procedure. The potential confounders were selected based on their assumed relevance to implant survival by using the method of constructing directed acyclical graphs. Scaled Schönfeld residuals were plotted and calculated to verify that the assumption of proportional hazards was fulfilled.
Due to concerns related to competing risks that may bias Kaplan-Meier survival analyses, additional analyses to calculate cumulative incidence functions were performed. Since follow-up times differed between the 2 groups investigated, we thus addressed the issue of non-informative censoring. We performed a sensitivity analysis with all patients within the MARR group being censored at the maximum follow-up time of the TM group (7 years). Any p-values <0.05 were considered statistically significant. All data were analyzed using R software version 3.1.3 together with the "rms", "Gmisc", and "cmprsk" packages (R Core Team Citation2015).
Ethics
The study was conducted in accordance with the Helsinki Declaration. The local ethics board (Regionala Etikprövningsnämnden Uppsala) approved the study (entry no. 2014/108, date of issue April 16, 2014).
Results
3 of 111 hips in the TM group had been re-revised due to aseptic loosening, as compared to 8 of 96 hips in the MARR group (). For the endpoint aseptic loosening, we thus found a 6-year unadjusted cumulative implant survival of 97% (CI: 94–100) in the TM group and of 96% (CI: 92–100) in the MARR group (p = 0.6). In the TM group, 14 hips had been re-revised for any reason, as compared to 9 hips in the MARR group (see for details on the subgroup of re-revised hips). In the TM group, the cumulative unadjusted implant survival with the endpoint re-revision for any reason was 87% (CI: 81–94) after 6 years and it was 95% (CI: 90–99) for MARR (p = 0.06) (). We calculated cumulative incidence functions and performed a sensitivity analysis censoring all observations after 7 years to validate the above findings (see Tables 4 and 5 in Supplementary data for details).
Figure 1. Kaplan-Meier survival curves with re-revision for aseptic loosening as the endpoint. Whiskers show 95% confidence intervals; p = 0.6 (derived from Mantel-Haenszel log-rank test). Numbers at risk, for TM and MARR, are given above the x-axis.
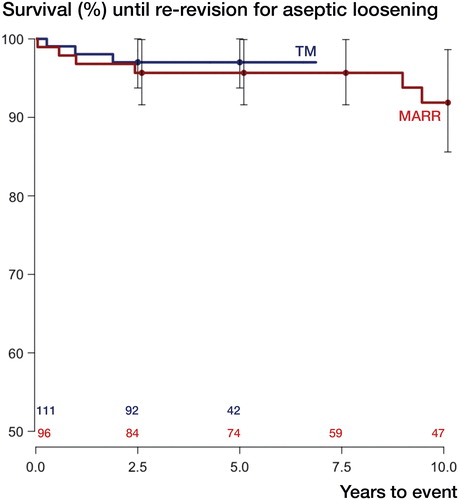
Figure 2. Kaplan-Meier survival curves with re-revision for any reason as the endpoint. Whiskers show 95% confidence intervals; p = 0.06 (derived from Mantel-Haenszel log-rank test). Numbers at risk, for TM and MARR, are given above the x-axis.
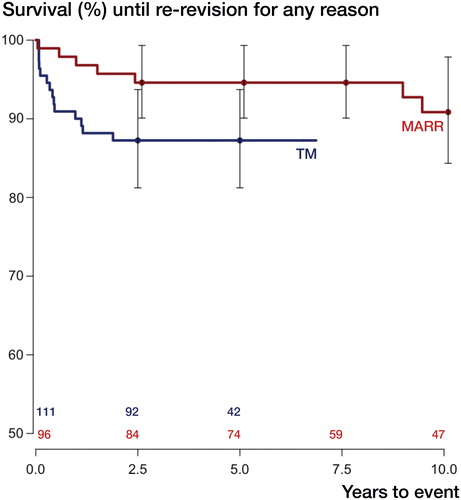
Table 3. Description of the subgroup of re-revised patients
10 of 14 hips in the TM group were re-revised due to recurrent dislocation whereas none of the 9 re-revisions in the MARR group were performed on this indication. All 10 patients who were re-revised due to dislocation had been operated with a direct lateral approach at the index procedure; 9 of 10 had received a snap-fit liner at the index procedure. 7 of these had an inner diameter of 28 mm and 2 accommodated 32-mm heads. When revised with a dual-mobility cup cemented into a TM cup or a MARR at the index procedure, no re-revision due to dislocation occurred in either group. 1 re-revision was performed due to infection in the MARR group.
There was a statistically significant difference in the use of morselized bone graft in the 2 groups investigated: 30 hips (29%) in the TM group required bone grafting as compared to 67 hips (76%) in the MARR group (p < 0.001). Duration of surgery was shorter in the TM group (130 min in the TM group as opposed to 165 min in the MARR group; p = 0.003). Perioperative blood loss accumulated to median 600 mL in the TM group and 1,100 mL in the MARR group (p = 0.05), but data on procedure duration and blood loss were missing for most of the MARR group.
Discussion
Main findings
In our study population consisting of 207 patients with 207 hips analyzed, there was no clinically relevant or statistically significant difference in the 6-year survival rates of TM cups and MARR with re-revision due to aseptic loosening as the endpoint. Exploratory analyses indicated that none of the covariates included (age, sex, acetabular defect size, and bone graft) were associated with a statistically significant influence on the primary or secondary outcomes (Tables 6 and 7, Supplementary data). Implant survival was satisfactory in both groups, given the fact that many patients presented with challenging acetabular defect situations.
Our findings support the results of Unger et al. (Citation2005) who published a consecutive series of 60 hips revised using TM cups. During follow-up (mean 42 months), only 1 out of 60 TM cups was subjected to re-revision due to aseptic loosening. However, in that series 7 hips dislocated, 5 of which required re-revision. Flecher et al. (Citation2008) included only Paprosky type-III defects in their study on 23 hips and found no re-revision during follow-up (mean 35 months). Kremers et al. (Citation2012) found a lower rate of re-revision after using a TM cup in comparison to other uncemented cup designs, but their results were not statistically significant. There was no re-revision due to aseptic loosening in 292 hips treated with a TM cup. In accordance with our findings, these authors found a higher risk of revision due to recurrent dislocation. Lakstein et al. (Citation2009) followed 53 hips that were revised with a TM cup in cases with only 50% or less host bone contact, and described a 4% failure rate during follow-up to 45 months. They also reported 4 hips with recurrent dislocations. A recent study by Konan et al. (Citation2016) retrospectively identified 46 patients with a Paprosky II or III defect and found a 10-year survival rate of 96% (CI: 93–99) with re-revision of the cup as the endpoint. Even in that study population, 2 patients had to undergo re-revision due to recurrent dislocations.
In a registry study on 2,460 hips, Mohaddes et al. (Citation2015) showed that TM cups had a satisfactory 5-year survival of 92%, which did not differ statistically significantly from implant survival in the group of revisions performed using uncemented Trilogy cups or cemented Lubinus cups. Due to its nationwide registry setting, a weakness of that study was that it did not give information on acetabular defect sizes, so the question of whether the treatment groups were comparable remains unanswered.
Our finding of comparable outcomes in terms of re-revision due to aseptic loosening in the 2 groups (TM cups and MARRs) contrasts with a systematic review on that topic. Beckmann et al. (Citation2014) found an odds ratio of 0.33 (CI: 0.16–0.67; p = 0.002) for failure when they compared TM cups with reinforcement rings of various types. However, in that review TM cups were compared to a pooled population of Müller, Burch-Schneider, and Ganz rings, and annual survival rates such as those calculated in that review indicate a constant failure rate—although it is unclear whether this assumption was correct.
The risk of re-revision surgery is an important issue when choosing an implant for revision surgery. Whenever MARRs are combined with bone graft, bone remodeling can usually be expected. In contrast, implantation of TM cups is achieved at the cost of bone stock, with bone stock removal and no bone stock restoration occurring from the index procedure, thus potentially complicating subsequent re-revision.
Dislocation after revision hip surgery
The problem of joint instability and dislocation is common after revision hip surgery. In our material, recurrent dislocation subsequent to revision hip surgery with insertion of a TM cup combined with a snap-fit liner accounted for a large proportion of all re-revisions in this treatment group. One could speculate that small to medium TM cups—that are used together with their recommended uncemented liners—do not distalize the center of hip rotation to the same degree as is achieved by the use of cups cemented into MARRs or larger TM cups. In contrast to other researchers’ findings, neither transfemoral approach nor smaller head size increased the risk of re-revision due to recurrent dislocation. On the contrary, 32-mm heads presented an increased risk. This increase should be interpreted with caution: It could be related to confounding factors that we cannot adequately control for, such as inadequate cup positioning in terms of abduction and anteversion angles, cranialization of the center of rotation, or soft tissue laxity.
The technique of cementing a dual-mobility cup into a TM revision shell seems to adequately address the issue of dislocation, since no revisions due to dislocation occurred in hips operated using that technique. On the other hand, the use of a dual-mobility cup might result in higher wear and could thereby lead to later aseptic loosening.
Strengths and weaknesses of the study
To the best of our knowledge, our study is the first comparative study of TM cups and MARRs to account for acetabular defect size. Since the TM concept gradually replaced the older MARR method, the treatment groups appeared to be comparable with respect to demography and acetabular defect size. The size of our cohort was larger than that reported by Unger et al. (Citation2005) (60 hips), Flecher et al. (Citation2008) (23 hips), and Batuyong et al. (Citation2014) (24 hips). Our radiographic evaluation according to Paprosky used a well-established and validated tool in the assessment of acetabular bone loss (Yu et al. Citation2013).
The study also had limitations. The TM group involved 2 different implants and, as mentioned above, re-revision due to dislocation appeared to be an issue foremost when patients were treated with the Continuum cup. Heterogeneity within the groups makes comparisons between TM and MARR difficult, although not impossible.
The fact that the TM cup was introduced in 2008 and replaced the MARR as a standard treatment led to (1) shorter observation times in the TM group compared to the MARR group, and (2) a higher proportion of deceased, i.e. censored, patients in the MARR group. This could have biased our Kaplan-Meier analysis by overestimation of the risk of re-revision in the MARR group (Gillam et al. Citation2010). However, we attempted to address this issue by calculating a competing risk scenario based on cumulative incidence functions, and by performing a sensitivity analysis that censored all patients after the maximal observation time in the TM group (7 years). The survival estimates obtained from these additional analyses did not differ substantially from those obtained by use of the traditional Kaplan-Meier method, even though the cumulative incidence functions with the endpoint re-revision for any reason indicated a small but statistically significant difference between the MARR and the TM group, which was not present in the Kaplan-Meier analysis. It can be argued that—according to the NARA guidelines for statistical analysis of arthroplasty data —"[Kaplan-Meier] estimates of implant failure are more clinically meaningful and straightforward to interpret for clinicians and patients" (Ranstam et al. Citation2011).
Gradual changes in general treatment strategies have evolved during the observation period, introducing confounders that we cannot control for. For instance, the oral use of non-steroidal anti-inflammatory drugs may have increased during the study period, as has the use of local infiltration analgesia based on ketorolac, both of which have a potential influence on implant ingrowth and bone formation.
This observational study was registry-based, and therefore lacked a defined prospective protocol for clinical and radiographic follow-up. Although radiographs obtained postoperatively were available for all patients—albeit at different time points after surgery—it was beyond the scope of this study to analyze these in depth. The lack of systematic long-term radiographic follow-up prevents us from detecting implants at risk of impending revision, and the actual number of loose implants might therefore be higher than the number of revised implants. We can only draw conclusions on the endpoint re-revision, whereas impending revision would remain unnoticed. The clinical outcome is also not taken into consideration, which means that the 2 implants being compared, while not different in terms of implant survival, could perform differently clinically.
Registration of blood loss and operating time (among other variables) was initiated during 2005, and therefore data on these parameters were missing in a large proportion of the MARR group, which makes comparisons of these variables difficult.
Finally, absence of statistical significance is not equal to the significance of absence, so our failure to detect statistically significant differences in terms of implant survival in our sample does not exclude the possibility that such differences exist in the general population.
Conclusion
There was only a small and statistically insignificant difference in 6-year implant survival between TM cups and MARRs with re-revision for aseptic loosening as the endpoint. Recurrent dislocation was the reason for re-revision in 10 out of 14 hips in the TM group, whereas aseptic loosening was the most common failure mechanism in the MARR group. Operating times and blood loss were less when using TM cups than when using MARRs. Our findings indicate that TM cups are an interesting treatment option in acetabular revision surgery, since aseptic loosening after the use of this concept does not appear to be more common than after the use of MARRs. However, the increased risk of recurrent dislocation after the use of TM cups must be investigated further.
Supplementary data
Tables 4–7 are available as supplementary data in the online version of this article http://dx.doi.org/10.1080/17453674.2016.1248315.
AB and EF collected data under the guidance of HM. AB, EF, and NPH analyzed the radiographs. AB and NPH performed the statistical analysis. All the authors were involved in writing of the manuscript.
This study was funded in part by Zimmer. However, Zimmer and its employees had no part in planning of the study, data collection, analysis, interpretation of data, or writing of the manuscript.
IORT_A_1248315_Suppl.pdf
Download PDF (23.4 KB)- Balla V K, Bodhak S, Bose S, Bandyopadhyay A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 2010; 6(8): 3349–59.
- Batuyong E D, Brock H S, Thiruvengadam N, Maloney W J, Goodman S B, Huddleston J I. Outcome of porous tantalum acetabular components for Paprosky type 3 and 4 acetabular defects. J Arthroplasty 2014; 29(6): 1318–22.
- Beckmann N A, Weiss S, Klotz M C, Gondan M, Jaeger S, Bitsch R G. Loosening after acetabular revision: comparison of trabecular metal and reinforcement rings. A systematic review. J Arthroplasty 2014; 29(1): 229–35.
- Berry D, Müller M. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br 1992; 74-B(5): 711–5.
- Bobyn J D, Toh K K, Hacking S A, Tanzer M, Krygier J J. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty 1999; 14(3): 347–354.
- Burns A W R, Bourne R B. (vi) Economics of revision total hip arthroplasty. Current Orthopaedics 2006; 20(3): 203–7.
- Flecher X, Sporer S, Paprosky W. Management of severe bone loss in acetabular revision using a trabecular metal shell. J Arthroplasty 2008; 23(7): 949–55.
- Gillam M H, Ryan P, Graves S E, Miller L N, de Steiger R N, Salter A. Competing risks survival analysis applied to data from the Australian Orthopaedic Association National Joint Replacement Registry. Acta Orthop 2010; 81(5): 548–55.
- Konan S, Duncan C P, Masri B A, Garbuz D S. Porous tantalum uncemented acetabular components in revision total hip arthroplasty: a minimum ten-year clinical, radiological and quality of life outcome study. Bone Joint J 2016; 98-B(6): 767–771.
- Kremers H M, Howard J L, Loechler Y, Schleck C D, Harmsen W S, Berry D J, Cabanela M E, Hanssen A D, Pagnano M W, Trousdale R T, Lewallen D G. Comparative long-term survivorship of uncemented acetabular components in revision total hip arthroplasty. J Bone Joint Surg Am 2012; 94(12): e82.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89(4): 780–5.
- Labek G, Thaler M, Janda W, Agreiter M, Stöckl B. Revision rates after total joint replacement: Cumulative results from worlwide joint register datasets. J Bone Joint Surg Br 2011; 93-B(3): 293–7.
- Lakstein D, Backstein D, Safir O, Kosashvili Y, Gross A E. Trabecular Metal cups for acetabular defects with 50% or less host bone contact. Clin Orthop Relat Res 2009; 467(9): 2318–24.
- Mohaddes M, Rolfson O, Karrholm J. Short-term survival of the trabecular metal cup is similar to that of standard cups used in acetabular revision surgery. Acta Orthop 2015; 86(1): 26–31.
- Paprosky W G, Perona P G, Lawrence J M. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6-year follow-up evaluation. J Arthroplasty 1994; 9(1): 33–44.
- R Core Team. (2015). "R: A language and environment for statistical computing." http://www.R-project.org/.
- Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen A B, Mehnert F, Furnes O. Statistical analysis of arthroplasty data II. Acta Orthop 2011; 82(3): 258–67.
- Starker M, Kandziora F, Jager A, Kerschbaumer F. Acetabular reconstruction using acetabular reinforcement rings. Orthopade 1998; 27(6): 366–74.
- Swedish Hip Arthroplasty Register. Annual Report. 2013.
- Swedish Hip Arthroplasty Register. Annual Report. 2014.
- Unger A S, Lewis R J, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthroplasty 2005; 20(8): 1002–9.
- Yu R, Hofstaetter J G, Sullivan T, Costi K, Howie D W, Solomon L B. Validity and reliability of the Paprosky acetabular defect classification. Clin Orthop Relat Res 2013; 471(7): 2259–65.
