Abstract
Background and purpose — Criticism of the lateral approach (LA) for hip arthroplasty is mainly based on the risk of poor patient-reported outcomes compared to the posterior approach (PA). However, there have been no controlled studies comparing patient-reported outcomes between them. In this randomized controlled trial, we tested the hypothesis that patient-reported outcomes are better in patients who have undergone total hip arthroplasty (THA) with PA than in those who have undergone THA with LA, 12 months postoperatively.
Patients and methods — 80 patients with hip osteoarthritis (mean age 61 years) were randomized to THA using PA or the modified direct LA. We recorded outcome measures preoperatively and 3, 6, and 12 months postoperatively using the Hip Disability and Osteoarthritis Outcome Score–Physical Function Short Form (HOOS-PS) as the primary outcome. Secondary outcomes were HOOS-Pain, HOOS-Quality-Of-Life, EQ-5D, UCLA Activity Score, and limping.
Results — We found no statistically significant difference in the improvements in HOOS-PS between the treatment groups at 12-month follow-up. All secondary outcomes showed similar results except for limping, where PA patients improved significantly more than LA patients.
Interpretation — Contrary to our hypothesis, patients treated with PA did not improve more than patients treated with LA regarding physical function, pain, physical activity, and quality of life 12 months postoperatively. However, limping was more pronounced in the LA patients.
The choice of surgical approach in total hip arthroplasty (THA) is debated as one factor that may influence the patient-reported outcome (PRO) (Jolles and Bogoch Citation2004, Hendrickx et al. Citation2014, Repantis et al. Citation2015) and the risk of revision, especially revision due to dislocation (Bystrom et al. Citation2003, Arthursson et al. Citation2007, Hailer et al. Citation2012, Lindgren et al. Citation2012). Worldwide, the posterior approach (PA) and the lateral approach (LA) are 2 of the most commonly used approaches. The 2 approaches differ in several ways. The abductor muscles are partly detached during the LA procedure but are not disturbed during the PA. The detachment of the abductor muscles during LA may influence the postoperative outcomes, especially physical function. Registry-based studies have revealed small differences in PROs between the 2 approaches, favoring the PA (Amlie et al. Citation2014, Jameson et al. Citation2014, Lindgren et al. Citation2014). However, this difference has not been investigated in a randomized controlled trial (RCT). A systematic review concluded that there is a need for patient-reported evidence to support a particular surgical approach (Jolles and Bogoch Citation2004).
We conducted a prospective RCT in patients operated with the PA or the LA. The primary aim of this trial was to evaluate the efficacy of the 2 surgical approaches regarding patient-reported physical function, and the secondary aims were to evaluate their efficacy regarding patient-reported pain, physical activity, limping, and quality of life. We hypothesised that patient-reported outcomes within the first year would improve more in patients who are operated with PA rather than LA.
Patients and methods
Design
The trial was a blinded, parallel-group, controlled trial with balanced randomization (1:1) following the CONSORT guidelines. A full description of the protocol has already been published (Rosenlund et al. Citation2014).
Participants
Patients were recruited at the Department of Orthopaedic Surgery and Traumatology, Odense University Hospital and Svendborg Hospital, Funen, Denmark, between May 2012 and May 2014. The 12-month follow-up was finalized in May 2015. Inclusion and exclusion criteria are given in . All the patients had primary osteoarthritis with indication for cementless THA. They were screened for eligibility by hip surgeons and by the principal investigator (SR).
Table 1. Inclusion and exclusion criteria
Intervention
All the patients had surgery at Odense University Hospital. Preoperative templating was performed using the software TraumaCad. Templating was performed to assist in restoring an equal leg length and the femoral offset. The surgeons aimed at placing the cup within 5–15° of anteversion and 30–50° of inclination (Lewinnek et al. Citation1978).
During surgery, the patients were positioned in the lateral decubitus position. All of them received the same types of cementless components (Bi-metric stem and Exceed ABT Ringloc-x shell and metal head, size 32 mm or 36 mm). 2 teams of 3 experienced surgeons performed all the operations. One team was responsible for the PA procedure and the other for the LA procedure.
All patients recived pre- and postoperative antibiotics and thromboprophylaxis during the inhospital stay.
Posterior approach
PA was performed through an incision over the posterior part of the greater trochanter through the fascia, followed by blunt dissection of gluteus maximus. The external rotators were detached and the hip capsule incised (Hoppenfeld et al. Citation2009). The hip was dislocated by internal rotation and flexion. During closure of the wound, the capsule was repaired and the external rotators were re-inserted using a heavy absorbable suture (coated VICRYL, size 2).
Lateral approach
LA was performed through a midline incision over the greater trochanter and involved detachment of the anterior one-third of the gluteus medius insertion and gluteus minimus insertion at the tip of the greater trochanter. The hip capsule was excised on the anterior side of the joint, from the basis of the collum femoris to the acetabular rim. The hip was dislocated by external rotation, adduction and flexion. During closure of the wound, the detached parts of the gluteus medius and minimus were re-inserted using a heavy absorbable suture (coated VICRYL, size 2) to re-approximate the divided gluteus minimus and the anterior flap of gluteus medius. No capsular repair was performed. A detailed description of the approach can be found in the work by Mulliken et al. (Citation1998).
Rehabilitation
The patients were mobilized with 2 canes and allowed full weight bearing immediately postoperatively, with no movement restrictions. They received the standard rehabilitation care of the department, which has been described in detail elsewhere (Rosenlund et al. Citation2014).
Data collection
All the patients completed questionnaires preoperatively and 3, 6, and 12 months postoperatively (with 12 months as the primary endpoint).
Patient-reported outcome measures
Primary outcome
We used the disease-specific Hip Disability and Osteoarthritis Outcome Score–Physical Function Short Form (HOOS-PS) as the primary outcome. HOOS-PS is a 5-item questionnaire derived from the HOOS subscales of Activities of Daily Living (HOOS-ADL) and Sport and Recreation (Davis et al. Citation2008). The HOOS-PS score ranges from 0 (extreme symptoms) to 100 points (no symptoms). The HOOS-PS has shown good validity when compared with the HOOS-ADL, and high responsiveness in THA patients (Davis et al. Citation2009).
Secondary outcomes
We used subscales of the Hip Disability and Osteoarthritis Outcome Score (HOOS 2.0)—Pain (HOOS-Pain) and Quality Of Life (HOOS-QOL)—which range from 0 (extreme symptoms) to 100 points (no symptoms) (Thorborg et al. Citation2010, Roos Citation2012).
The patients completed the validated, transcultural, translated Danish versions of the HOOS-PS, HOOS-Pain, and HOOS-QOL (Nilsdotter et al. Citation2003, Beyer et al. Citation2008, Thorborg et al. Citation2010).
EQ-5D-3L
The Danish version of the EuroQol-5-Dimension-3-Likert-scale Health Questionnaire (EQ-5D-3L) (Brooks Citation1996, Gudex and Sørensen Citation1998) was used to assess health-related quality of life. The global health status index ranges from −0.624 (worst) to 1.0 (best) (Wittrup-Jensen et al. Citation2009). The overall health status (EQ-5D-VAS) is scored on a "thermometer-like" 100-point visual analog scale from 0 (worst imaginable) to 100 (best imaginable) (van Reenen and Oppe Citation2015) .
UCLA Activity Score
A Danish version of the University of California Los Angeles (UCLA) Activity Score was used to measure the patient-reported activity level. It uses an ordinal 10-point (1–10) Likert scale ranging from "wholly inactive: dependent on others" to "regularly participate in impact sports or heavy labour", with a higher score being better (Terwee et al. Citation2011).
Limping
We used the question about limping from the function domain of the Harris hip score (HHS). Limping is scored on an ordinal 4-point (1–4) Likert scale (no limping; slight, moderate, or severe limping), with a lower score being better. This means that a single-point change will make the patient change category e.g. from no limping to slight limping. The question was used to evaluate the patient’s own perception of his/her gait function (Mahomed et al. Citation2001).
Adverse events
We performed a medical audit 12 months postoperatively. The following complications were registered: deep infection, periprosthetic fracture, dislocation, aseptic loosening, nerve injury, pulmonary embolism, and deep vein thrombosis.
Data processing
All questionnaires were optimized for digital scanning to reduce typing errors (Paulsen et al. Citation2012). Manual validation was performed where ambiguous data occurred.
Sample size
The sample size calculation was based on the primary outcome HOOS-PS, using 1 preoperative assessment and 3 follow-up assessments, and an estimated correlation between follow-up measurements of 0.5. We considered the minimal clinically important difference (MCID) to be 10 points between treatment groups at the 12-month follow-up (Ehrich et al. Citation2000, Roos and Lohmander Citation2003, Terwee et al. Citation2009, Roos Citation2012) and used a standard deviation of 16.7 preoperatively and 16.1 postoperatively (Davis et al. Citation2009). To achieve a statistical power of ß = 0.80 and α = 0.05, 29 patients were needed in each treatment group. To account for possible dropouts, 40 patients were included in each treatment group.
Randomization
Balanced 1:1 block randomization was performed using a computer-generated list, with 4 blocks of 20 patients in each. The sequence was generated by a third person who was not involved in the trial (JL). The sequence was written on paper and placed in sealed opaque consecutively numbered envelopes by a secretary not involved in the trial. The envelopes were opened in the order given, and the patient was allocated to a treatment group and scheduled for surgery.
Blinding
The patients were blinded to treatment and informed, prior to participation, that the type of intervention would not be revealed to them. The reason for blinding was explained to them. Due to the nature of the intervention, the surgeons, ward doctors, nurses and physiotherapists were not blinded. However, they were all well aware of the importance of not discussing the intervention with the patients. Since all the patients were treated with the same rehabilitation protocol, there was no need to discuss the specific intervention. The principal investigator was kept blind throughout the trial and the statistical analyses.
Ethics, registration, funding and conflict of interest
The trial was approved by the Danish Data Protection Agency and the Danish Regional Committee on Biomedical Research Ethics (Southern Denmark) (project-ID: S-20120009). It was also registered at ClinicalTrials.gov (identifier: NCT01616667).
The trial was supported by the Danish Rheumatoid Association, University of Southern Denmark, Region of Zealand, Region of Southern Denmark, the Bevica Foundation, the Bjarne Jensen Foundation, and Odense University Hospital. None of the trial sponsors played any role in the trial design, data collection, data analysis, or interpretation; nor did they have any influence on the writing of the manuscript or the decision to submit the manuscript for publication.
The trial received funding from public and private foundations. One author (SO) has received grants from biomedical companies not related to this trial. No other competing interests declared.
Statistics
Descriptive statistics are reported with mean and standard deviation (SD) or median and interquartile range (IQR).
To evaluate the treatment effect (the mean difference in improvement between the PA and LA treatment groups at 12 months), we used mixed linear model analysis (with repeated measures) and point estimates (Rabe-Hesketh and Skrondal Citation2008). This model included the interaction between treatment and elapsed time, adjusted for preoperative values and assuming that data were missing completely at random (MCAR) (Rabe-Hesketh and Skrondal Citation2008). Where data were missing preoperatively, the mean or median preoperative value from all the other patients was imputed (used on 3 patients: 2 UCLA Activity Scores and 1 EQ-5D-VAS). Given the small amount of data imputation, it did not change the results. Otherwise, no data imputation was performed. We used the absolute score on the outcome variable as the dependent variable with patient as the random effect factor, and based the model on the restricted maximum likelihood estimate. The independent variables were the preoperative values, treatment group, time as a categorical variable, and the interaction between treatment group and time. Model assumptions were checked with residual plots for each dependent variable and were found to be acceptable.
The UCLA Activity Score and Limping Score were a priori considered as ordinal data and treated accordingly. However, because of the limited sample size and the many categories in each outcome, we modelled the UCLA Activity Score and Limping Score as numerical scales and used mixed linear model analysis as described above.
We performed the primary analyses using intention-to-treat (ITT) analysis followed by a per-protocol analysis. Effect size (using Cohen’s d) was calculated for continuous dependent variables. Effect sizes of 0.2, 0.5, and 0.8 were defined as being small, medium, and large, respectively (Cohen Citation1992).
The statistical analyses were performed using Stata 13.1 software, with a significance level of 0.05.
Results
499 patients aged 45–70 years received primary THA in the study period (). Of these, 151 were not eligible. Of the remaining 348 patients, 208 were excluded. 140 patients were eligible but 31 declined to participate and, by mistake, 29 were not asked to participate. 80 patients were randomized and 77 and 69 patients were available for the ITT analysis and per-protocol analysis, respectively.
Figure 1. Flow of patients included in the trial. THA: total hip arthroplasty; OA: osteoarthritis; ITT: intention-to-treat analysis. a All concepts that were not cementless Bi-metric stem and Exceed ABT Ringloc-x shell. b Dropout analysis was performed for these patients.
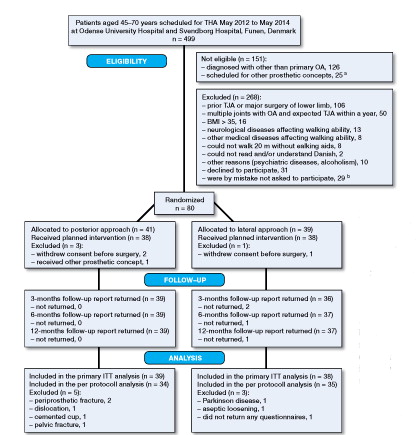
The dropout analysis showed that statistically significantly more females and patients with ASA class 2 were present in the group of patients who were not included (Appendix A, see Supplementary data).
8 patients (3 in the LA group and 5 in the PA group) did not follow the protocol for various reasons ().
Preoperative patient characteristics and surgically related outcomes are presented in . There was no difference between the groups regarding femoral head size, but more patients in the LA group received a lateralized stem.
Table 2. Demographic and clinical characteristics of patients in each treatment group
Patient-reported outcomes
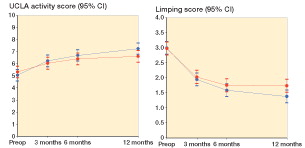
Within- and between-group change scores are given in . We found a statistically non-significant difference in the mean change score in HOOS-PS of −3.3 (95% CI: −8.7 to 2.1). Thus, the improvement in physical function 12 months postoperatively following PA treatment was not any better than the improvement following LA treatment. The same applies to all the earlier time points (). Furthermore, there was no additional improvement in the PA group (compared to the LA group) in the secondary outcomes at any time-point (HOOS-Pain, HOOS-QOL, UCLA Activity Score, EQ-5D-3L, and EQ-5D-VAS) ( and , and ). A significant between-group difference in favor of PA was observed for limping (0.4, 95% CI: 0.0–0.7) ( and ). Collectively, all outcome measures tended to have a higher numeric improvement for PA. The effect size (Cohen’s d) for the primary outcome at 12 months was small (0.3, 95% CI: −0.2 to 0.7) ().
Figure 2. HOOS-PS (top panel), HOOS-Pain (middle panel) and HOOS-QoL (quality of life; bottom panel), by time and treatment group. Mean scores with 95% CI.
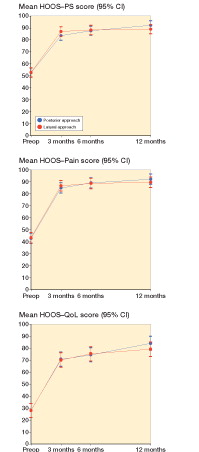
Table 3. Mean difference in patient-reported outcomes within and between treatment groups preoperatively to 12-month follow-up (intention to treat). Values are mean (95% CI) unless otherwise indicated
The per-protocol analysis changed the results on limping to a statistically non-significant improvement in favor of PA. All other results remained unchanged (Appendix B, see Supplementary data). A significant within-group improvement in HOOS-PS was observed in both treatment groups: 39 (95% CI: 35–44) and 36 (95% CI: 30–42) for PA and LA, respectively. The greatest improvement in all outcomes occurred within the first 3 months ().
Figure 3. EQ-5D-3L (left panel) and EQ-5D-VAS (right panel) by time and treatment group. Mean scores with 95% CI.
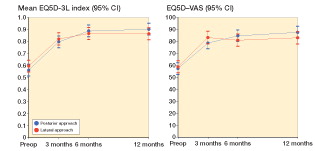
Figure 4. UCLA Activity Score and Limping Score, by time and treatment group. Mean scores with 95% CI.
A number-needed-to-treat analysis is available in Appendix C (see Supplementary data).
Adverse effects
5 patients in the PA group had adverse events. 2 had a periprosthetic fracture within 1 month of surgery (1 accidentally fell; the other had a peroperative fissure in the proximal femur, probably leading to the periprosthetic fracture). Both patients had a revision and became well-functioning. 1 had a dislocation 3 weeks postoperatively, was treated with closed reduction, and became well-functioning. 2 patients had a deep vein thrombosis and were treated medically. 1 patient in the LA group had a revision due to aseptic loosening of the stem. He became relatively well-functioning despite sequelae with a drop foot.
Discussion
The main aim of this trial was to investigate whether the efficacy—evaluated by improvement in patient-reported outcome—was better in patients who underwent THA with PA rather than THA with LA, 12 months postoperatively.
Contrary to our hypotheses, no statistically significant difference in improvement in physical function was observed between the patients in the 2 groups. The same applied to the secondary outcomes except for limping, where patients in the PA group improved more than the patients in the LA group.
There was, however, a systematic tendency towards higher numeric improvements in the PA group, ranging from 2.6 to 4.9 points on HOOS-Pain, HOOS-PS and HOOS-QOL, than in the LA group. These findings are in agreement with a cohort study of 852 THA patients operated with LA or PA, with 1–3 years of follow-up. That study showed a statistically significant difference of 4.0 points on the HOOS-ADL subscale in favor of the PA group (Amlie et al. Citation2014). Also, a randomized study with 3-month follow-up showed this tendency in both surgeon-reported and patient-reported outcomes (Witzleb et al. Citation2009), although the differences were not statistically significant.
We found a between-group difference in general health status (EQ-5D-3L) of −0.04 (95% CI: −0.1 to 0.03). This corresponds to findings from a registry-based study of 42,233 THA patients with 6 years of follow-up. That study showed a statistically significantly better EQ-5D outcome (by 0.03) in favor of the PA group (Lindgren et al. Citation2014). However, the differences in both HOOS and EQ-5D between the 2 approaches were small, and it can be questioned whether the differences are above the minimal clinically important difference (MCID) (Ehrich et al. Citation2000, Roos and Lohmander Citation2003, Terwee et al. Citation2009, Larsen et al. Citation2010, Roos Citation2012, Coretti et al. Citation2014). The effect size (Cohens’ d) also supports this notion, as the effect size for all outcomes was small to negligible.
We based the sample size calculation on the HOOS-PS subscale with 10 points as the MCID between groups. This decision was based on published evidence and related RCT studies using the HOOS questionnaire as the primary outcome measure (Ehrich et al. Citation2000, Roos and Lohmander Citation2003, Terwee et al. Citation2009, Roos Citation2012, Skou et al. Citation2012, Villadsen et al. Citation2014). We cannot rule out the possibility of type-II error in our study, if the MCID is less than 10 points as we used. A post-hoc sample size calculation showed that to detect a statistically significant between-group difference in HOOS- Physical Function of 3.3 points, using the standard deviations from our trial, 183 patients would be required in each treatment group. However, we consider that the statistically non-significant between-group difference of ∼3 HOOS points in all the HOOS subscales included, in favor of PA, was not clinically relevant—based upon current evidence (Frobell et al. Citation2008, Roos Citation2012, Villadsen et al. Citation2014, Kise et al. Citation2015, Kise et al. Citation2016). This does not imply that there could be no other clinically significant differences than those investigated in our study.
We found that patients in the PA group reported having less limping than patients in the LA group, which may be explained by the disturbance of the abductor muscles (Masonis and Bourne Citation2002, Edmunds and Boscainos Citation2011, Amlie et al. Citation2014). The difference was, however, less than 1 point. A single-point change is what differentiates 2 categories, e.g. the difference between no limping and slight limping on the 4-point Likert scale. The difference was not statistically significant in the per-protocol analysis. Our results are in line with those from a cohort study that found twice as many patients in the LA group with self-reported limping at 1–3 years postoperatively (Amlie et al. Citation2014). That study also found that the difference in mean score between patients with and without self-reported limping ranged from 17 to 35 on the 5 HOOS subscales, in favor of the non-limping patients. In contrast to our results, a Cochrane review based on 4 non-randomized cohort studies found no differences in limping between patients operated with LA or with PA, as measured with the Trendelenburg test (Jolles and Bogoch Citation2004). Also, the study by Winther et al. (2015) could not detect any difference in hip muscle strength at 3 months between the PA patients and the LA patients. However, the patients’ perception—and hence response to the question—of limping may not match an objective measurement of limping.
Strengths and limitations
Regarding strengths, the study was a thoroughly executed, randomized controlled trial following the CONSORT statement. We randomized the patients to a specific approach that automatically included a specific team of surgeons with skills in that particular approach, thus avoiding bias due to a learning curve. To avoid the risk of comparing surgeon skills or surgeon preference rather than the surgical procedure, 3 equally experienced surgeons participated in each team.
Regarding limitations, the study was designed as a superiority trial. We cannot therefore conclude that LA is not inferior to PA. If the clinically relevant difference was less than 10 points on the HOOS subscales, the study may have been underpowered to show this with a statistically significant value. Furthermore, this trial was designed to investigate group differences between PA and LA rather than at the individual level, which has been suggested by Katz et al. (2015). At the individual level, we found that 23 patients in the LA group had a HOOS-PS score of over 88 points—as compared to 27 in the PA group, giving a non-significant absolute risk reduction of 7% (95% CI: −14 to 28). The number needed to treat (NNT) was 14 in favor of PA, but the result was not statistically significant based on the present sample size (Appendix C, see Supplementary data).
Blinding of both the patients and the principal investigator performing the analyses was maintained almost completely throughout the trial, due to the nature of the self-reported outcomes and through the discretion of the hospital personnel. We are aware that a few patients may have guessed their intervention. However, we did not systematically measure the success of blinding.
Disadvantages related to THA surgery, such as dislocation rates and revision rates, are relevant to include in the investigation of potential differences between the 2 surgical approaches, but this would require a longer follow-up and a study design with a larger sample size (Jolles and Bogoch Citation2004). These issues should be addressed before making any firm conclusion about the superiority of one approach over the other.
Generalizability
Not all eligible patients (43%) were included in the trial, which may have reduced the external validity. The strict exclusion criteria may have limited any generalizability of the results to other patient groups. However, 80% of all THA patients are diagnosed with primary osteoarthritis (Overgaard Citation2013), so the patients in this trial represent the vast majority of all THA patients.
Conclusion
We found no superior efficacy of using the PA compared with LA, as evaluated from patient-reported physical function, pain, physical activity, and quality of life. However, patients operated using the PA had less self-reported limping at 12 months. A future multicenter non-inferiority RCT would be valuable to investigate whether there are significant differences in revision rates between THAs performed with the LA and with the PA.
Supplementary data
Appendices A–C are available as supplementary data in the online version of the article http://dx.doi.org/10.1080/17453674.2017.1291100.
We thank the patients for their willingness to participate. We also thank the project nurse, Annie Gam-Pedersen, for managing all the logistics related to the patients. We are grateful to the staff of the Department of Orthopaedic Surgery and Traumatology at Odense University Hospital for providing the equipment and facilities needed to complete the trial. Lastly, we are grateful to all the surgeons who participated in this trial.
SR, AHL, CJ, LB, and SO were responsible for the concept and design of the trial. SR was responsible for inclusion of patients and collection of data on all patients included, analysis of data, and drafting of the manuscript. All the authors were responsible for interpretation of the data and performed critical revision of the manuscript.
IORT_A_1291100_SUPP.PDF
Download PDF (43 KB)- Amlie E, Havelin L I, Furnes O, Baste V, Nordsletten L, Hovik O, et al. Worse patient-reported outcome after lateral approach than after anterior and posterolateral approach in primary hip arthroplasty. A cross-sectional questionnaire study of 1,476 patients 1-3 years after surgery. Acta Orthop 2014; 85 (5): 463–9.
- Arthursson A J, Furnes O, Espehaug B, Havelin L I, Soreide J A. Prosthesis survival after total hip arthroplasty-does surgical approach matter? Analysis of 19,304 Charnley and 6,002 Exeter primary total hip arthroplasties reported to the Norwegian Arthroplasty Register. Acta Orthop 2007; 78 (6): 719–29.
- Beyer N, Thorborg K, Vinther A. Translation and Cross-Cultural Adaptation of the Danish Version of the Hip Dysfunction and Osteoarthritis Outcome Score 2.0 (HOOS 2.0). 2008; http://koos.nu/HOOSdansk.pdf 4 December, 2015.
- Brooks R. EuroQol: the current state of play. Health Policy 1996; 37 (1): 53–72.
- Bystrom S, Espehaug B, Furnes O, Havelin L I, Norwegian Arthroplasty R. Femoral head size is a risk factor for total hip luxation: a study of 42,987 primary hip arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop Scand 2003; 74 (5): 514–24.
- Cohen J. A power primer. Psychol Bull 1992; 112 (1): 155–9.
- Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res 2014; 14 (2): 221–33.
- Davis A M, Perruccio A V, Canizares M, Tennant A, Hawker G A, Conaghan P G, et al. The development of a short measure of physical function for hip OA HOOS-Physical Function Shortform (HOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008; 16 (5): 551–9.
- Davis A M, Perruccio A V, Canizares M, Hawker G A, Roos E M, Maillefert J F, et al. Comparative, validity and responsiveness of the HOOS-PS and KOOS-PS to the WOMAC physical function subscale in total joint replacement for osteoarthritis. Osteoarthritis Cartilage 2009; 17 (7): 843–7.
- Edmunds C T, Boscainos P J. Effect of surgical approach for total hip replacement on hip function using Harris Hip scores and Trendelenburg’s test. A retrospective analysis. Surgeon 2011; 9 (3): 124–9.
- Ehrich E W, Davies G M, Watson D J, Bolognese J A, Seidenberg B C, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000; 27 (11): 2635–41.
- Frobell R, Lohmander S, Roos E, Roos H, Ranstam J. Statistical Analysis Plan (SAP) for the KANON-Study. Journal [serial online]. 2008 Date: [20 screens]. Available from: https://lup.lub.lu.se/search/publication/1276774.
- Gudex C M, Sørensen J. Et generisk mål for helbredstilstand. Månedsskrift for praktisk lægegerning 1998; 76 (10): 1339–45.
- Hailer N P, Weiss R J, Stark A, Karrholm J. The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis. Acta Orthop 2012; 83 (5): 442–8.
- Hendrickx C, De Hertogh W, Van Daele U, Mertens P, Stassijns G. Effect of percutaneous assisted approach on functional rehabilitation for total hip replacement compared to anterolateral approach: study protocol for a randomized controlled trial. Trials 2014; 15: 392.
- Hoppenfeld S, deBoer P, Buckley R. Surgical Exposures in Orthopaedics. The Anatomic Approach. The Hip, Fourth Edition. Lippinoctt Williams & Wilkins 2009.
- Jameson S S, Mason J, Baker P, Gregg P J, McMurtry I A, Deehan D J, et al. A comparison of surgical approaches for primary hip arthroplasty: a cohort study of patient reported outcome measures (PROMs) and early revision using linked national databases. J Arthroplasty 2014; 29 (6): 1248–55 e1.
- Jolles B M, Bogoch E R. Posterior versus lateral surgical approach for total hip arthroplasty in adults with osteoarthritis. Cochrane Database Syst Rev 2004; (1): CD003828.
- Kise N J, Roos E, Risberg M A, Ranstam J. STATISTICAL ANALYSIS PLAN (SAP) FOR: The Odense-Oslo Meniscectomy versus Exercise (OMEX) study. A randomized controlled trial for treatment of degenerative meniscus tears in middle-aged patients with a 2-year follow-up. Journal [serial online]. 2015 Date: [24 screens]. Available from: http://www.sdu.dk/om_sdu/institutter_centre/iob_idraet_og_biomekanik/forskning/forskningsenheder/fof/forskningsprojekter/omex.
- Kise N J, Risberg M A, Stensrud S, Ranstam J, Engebretsen L, Roos E M. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ 2016; 354: i3740.
- Larsen K, Hansen T B, Soballe K, Kehlet H. Patient-reported outcome after fast-track hip arthroplasty: a prospective cohort study. Health Qual Life Outcomes 2010; 8: 144.
- Lewinnek G E, Lewis J L, Tarr R, Compere C L, Zimmerman J R. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am 1978; 60 (2): 217–20.
- Lindgren J V, Wretenberg P, Karrholm J, Garellick G, Rolfson O. Patient-reported outcome is influenced by surgical approach in total hip replacement: a study of the Swedish Hip Arthroplasty Register including 42,233 patients. Bone Joint J 2014; 96-B (5): 590–6.
- Lindgren V, Garellick G, Karrholm J, Wretenberg P. The type of surgical approach influences the risk of revision in total hip arthroplasty: a study from the Swedish Hip Arthroplasty Register of 90,662 total hipreplacements with 3 different cemented prostheses. Acta Orthop 2012; 83 (6): 559–65.
- Mahomed N N, Arndt D C, McGrory B J, Harris W H. The Harris hip score: comparison of patient self-report with surgeon assessment. J Arthroplasty 2001; 16 (5): 575–80.
- Masonis J L, Bourne R B. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop Relat Res 2002; (405): 46–53.
- Mulliken B D, Rorabeck C H, Bourne R B, Nayak N. A modified direct lateral approach in total hip arthroplasty: a comprehensive review. J Arthroplasty 1998; 13 (7): 737–47.
- Nilsdotter A K, Lohmander L S, Klassbo M, Roos E M. Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003; 4: 10.
- Overgaard S. Danish Hip Arthroplasty Register-Annual report of 2013. Danish Hip Arthroplasty Register-Annual report of 2013. Danish Hip Arthroplasty Register; 2013.
- Paulsen A, Overgaard S, Lauritsen J M. Quality of data entry using single entry, double entry and automated forms processing–an example based on a study of patient-reported outcomes. PLoS One 2012; 7 (4): e35087.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata, Second Edition. Stata Press 2008.
- Repantis T, Bouras T, Korovessis P. Comparison of minimally invasive approach versus conventional anterolateral approach for total hip arthroplasty: a randomized controlled trial. Eur J Orthop Surg Traumatol 2015; 25 (1): 111–6.
- Roos E M. KOOS FAQs. 2012; http://koos.nu/index.html 4 December 2015.
- Roos E M, Lohmander L S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003; 1: 64.
- Rosenlund S, Broeng L, Jensen C, Holsgaard-Larsen A, Overgaard S. The effect of posterior and lateral approach on patient-reported outcome measures and physical function in patients with osteoarthritis, undergoing total hip replacement: a randomised controlled trial protocol. BMC Musculoskelet Disord 2014; 15: 354.
- Skou S T, Roos E M, Laursen M B, Rathleff M S, Arendt-Nielsen L, Simonsen O H, et al. Total knee replacement plus physical and medical therapy or treatment with physical and medical therapy alone: a randomised controlled trial in patients with knee osteoarthritis (the MEDIC-study). BMC Musculoskelet Disord 2012; 13: 67.
- Terwee C B, Roorda L D, Knol D L, De Boer M R, De Vet H C. Linking measurement error to minimal important change of patient-reported outcomes. J Clin Epidemiol 2009; 62 (10): 1062–7.
- Terwee C B, Bouwmeester W, van Elsland S L, de Vet H C, Dekker J. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthritis Cartilage 2011; 19 (6): 620–33.
- Thorborg K, Roos E M, Bartels E M, Petersen J, Holmich P. Validity, reliability and responsiveness of patient-reported outcome questionnaires when assessing hip and groin disability: a systematic review. Br J Sports Med 2010; 44 (16): 1186–96.
- van Reenen M, Oppe M. EQ-5D-3L User Guide- Basic information on how to use the EQ-5D-3L instrument 2015; http://www.euroqol.org/about-eq-5d/publications/user-guide.html 5 December, 2015.
- Villadsen A, Overgaard S, Holsgaard-Larsen A, Christensen R, Roos E M. Postoperative effects of neuromuscular exercise prior to hip or knee arthroplasty: a randomised controlled trial. Ann Rheum Dis 2014; 73 (6): 1130–7.
- Wittrup-Jensen K U, Lauridsen J, Gudex C, Pedersen K M. Generation of a Danish TTO value set for EQ-5D health states. Scand J Public Health 2009; 37 (5): 459–66.
- Witzleb W C, Stephan L, Krummenauer F, Neuke A, Gunther K P. Short-term outcome after posterior versus lateral surgical approach for total hip arthroplasty - A randomized clinical trial. Eur J Med Res 2009; 14 (6): 256–63.
