Abstract
Background and purpose — Aseptic loosening is a main cause of late revision in total knee replacement (TKR). Teriparatide, a recombinant parathyroid hormone (PTH), stimulates osteoblasts and has been suggested to improve cancellous bone healing in humans. This might also be relevant for prosthesis fixation. We used radiostereometric analysis (RSA) to investigate whether teriparatide influences prosthesis fixation. Early migration as measured by RSA can predict future loosening.
Patients and methods — In a randomized controlled trial with blind evaluation, 50 patients with osteoarthritis of the knee were allocated to a teriparatide treatment group (Forsteo, 20 μg daily for 2 months postoperatively) or to an untreated control group. RSA was performed postoperatively and at 6 months, 12 months, and 24 months. The primary effect variable was maximal total point motion (MTPM) from 12 to 24 months.
Results — Median maximal total point motion from 12 to 24 months was similar in the 2 groups (teriparatide: 0.14 mm, 10% and 90% percentiles: 0.08 and 0.24; control: 0.13 mm, 10% and 90% percentiles: 0.09 and 0.21). [Authors: this is perhaps better than using "10th" and "90th", which looks ugly in print./language editor] The 95% confidence interval for the difference between group means was −0.03 to 0.04 mm, indicating that no difference occurred.
Interpretation — We found no effect of teriparatide on migration in total knee replacement. Other trials using the same dosing have suggested a positive effect of teriparatide on human cancellous fracture healing. Thus, the lack of effect on migration may have been due to something other than the dose. In a similar study in this issue of Acta Orthopaedica, we found that migration could be reduced with denosumab (Ledin et al. Citation2017). The difference in response between the anabolic substance teriparatide and the antiresorptive denosumab suggests that resorption has a more important role during the postoperative course than any deficit in bone formation.
Early stable fixation in total knee replacement (TKR) is important for prevention of late loosening (Ryd et al. Citation1995, Pijls et al. Citation2012). Radiostereometric analysis (RSA) is performed to estimate fixation by measuring the postoperative migration of the prosthesis. Bisphosphonates, which are most commonly used in treatment of osteoporosis, have been shown to prevent early migration in TKR (Hilding et al. Citation2000, Hilding and Aspenberg Citation2006) and they are associated with a lower risk of revision in epidemiological studies (Teng et al. Citation2015, Namba et al. Citation2016).
Teriparatide is a recombinant truncated parathyroid hormone (PTH). While bisphosphonates inhibit osteoclast activity, PTH stimulates osteoblasts and acts as an anabolic hormone. In animals, PTH stimulates fracture healing (Andreassen et al. Citation1999) and enhances implant fixation (Skripitz et al. 2001a,b, Gabet et al. Citation2006, Aspenberg et al. Citation2008). Teriparatide stimulates bone formation in osteoporosis treatment, and there are reasons to believe that teriparatide can accelerate fracture healing in clinical settings (Aspenberg et al. Citation2010, Citation2016).
At insertion of a total knee replacement, the metaphyseal bone is traumatized, which is likely to elicit a fracture healing response of a kind typical of metaphyseal injury (Aspenberg Citation2013, Bernhardsson et al. Citation2015). Since this healing response can be augmented by antiresorptive therapy, we speculated that an anabolic treatment using teriparatide would also augment the process, thereby improving early fixation in total knee replacement and thus reducing the risk of late loosening.
In this study, we assessed whether teriparatide administered in the postoperative phase can enhance bone healing at the interface between bone and cement after knee replacement surgery. We performed a randomized controlled trial with blind evaluation to study the effects of teriparatide, using migration (by RSA) as the primary effect variable. The specific hypothesis was that migration from 12 to 24 months would be reduced, as measured by maximal total point motion (MTPM). This time period was chosen because it appeared to have the best predictive value in the study by Ryd et al. (Citation1995).
Patients and methods
50 patients (30 women) were included in this 2-center trial. The patients were scheduled for elective cemented primary total knee replacement because of osteoarthritis. 29 of the patients were operated on in Motala and 21 in Oskarshamn, Sweden, and the operations were performed between November 2009 and June 2011. 1 patient (a woman) was excluded because of malignancy diagnosed after surgery. Inclusion criteria were the accepted clinical criteria for elective primary total knee replacement due to primary or secondary osteoarthritis and without severe disease (ASA 1–2). Exclusion criteria were rheumatic arthritis, a history of severe heart disease, renal insufficiency, hypercalcemia, metabolic bone disease (osteoporosis was not screened for), malignancy, bilateral surgery, previous radiation therapy of the skeleton, or inability to give informed consent. The patients who declined participation were not registered.
The patients who chose to participate in the study () were allocated to treatment by a study nurse who opened a sealed envelope on the first postoperative day. These were prepared by PA using shuffling of envelopes before numbering them. Randomization was stratified for sex and performed in blocks of 6 (with some blinded exchange between blocks) which randomized half of the patients to receive subcutaneous teriparatide (Forsteo), 20 μg daily over 2 months postoperatively, starting on the first postoperative day. The other half of the patients received no extra treatment ().
Table 1. Patient characteristics
We could not afford to make placebo injection devices, and the patients could not be blinded as to treatment, but evaluation was done blind.
Surgery
The surgery was performed by HL in 29 cases and by 2 other surgeons in 20 cases. All operations were done under spinal anesthesia. We used the Nexgen CR all-poly tibial knee prostheses (Zimmer), which was inserted after pulsed lavage and cemented with Palacos R + G cement (Heraeus Medical AB, Sollentuna, Sweden). 2 g cloxacillin was given just before and twice after the operation. Low-molecular-weight heparin (Innohep, 4,500 IE subcutaneously) was used for 14 postoperative days.
For RSA measurements, 6 tantalum beads (0.8–1.0 mm) were inserted in the all-poly tibial component and 6 in the tibial bone metaphysis. A tourniquet was used during the whole operation.
Evaluation
The radiostereometric examinations were performed on the second postoperative day, and after 6, 12, and 24 months. The analysis of the RSA data was done using the UmRSA computer program (RSA Biomedical AB, Umea, Sweden), by a blinded technician or research nurse.
Statistics
The sample size (n = 50) was calculated from similar studies (Hilding et al. Citation2000, Ledin et al. Citation2012) to be able to detect a mean difference in maximum total point motion (MTPM) of 0.2 mm with an SD of 0.2 (α = 0.05 and 80% power).
The primary effect variable was migration measured by RSA (MTPM) during the second year (from 12 to 24 months). The variable was log-transformed. The Kolmogorov-Smirnov test did not reject the hypothesis of normal distribution. Analysis of variance was performed with patient sex and site as covariates. 95% confidence intervals (CIs) in mm were calculated from the CI for log-transformed values by use of the non-transformed mean value for the controls (values for the ratio of teriparatide/control multiplied by the mean for the control in mm).
Secondary outcomes were other RSA variables. MTPM values were analyzed as above. Other variables were analyzed without transformation. We used IBM SPSS version 23 for statistical calculations.
There were no changes to the protocol.
Ethics and registration
The study was approved by the Regional Ethics Committee in Linköping on June 17, 2009 (reference number M106-09; EudraCT number 2009-011784-37). Protocol information is available at www.clinicaltrialsregister.eu. The trial was performed in compliance with the Declaration of Helsinki. Patient data are to be stored at the Department of Orthopedics, Aleris Specialist Care Motala AB, Motala, Sweden for 10 years.
Funding and potential conflicts of interest
The study was funded by the Swedish Research Council (VR 02031-47-5), the Medical Research Council of Southeast Sweden (FORSS-37511), and Linköping University.
PA has shares in Addbio AB, a company developing bisphosphonate coatings for implants. PA has also received institutional research support from Eli Lilly Corp. and Amgen.
Results
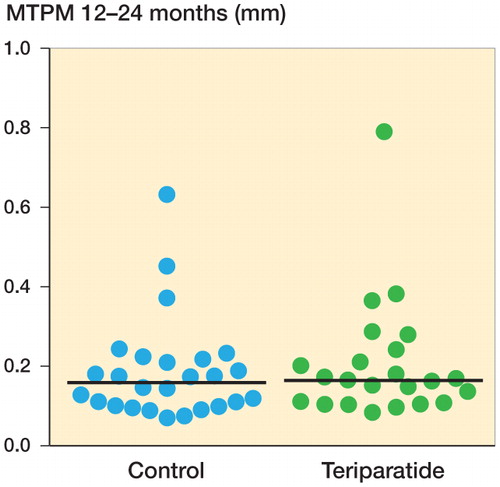
The primary effect variable, migration (MTPM from 12 to 24 months), was similar in the 2 groups (median for teriparatide: 0.14 mm, 10% and 90% percentiles: 0.08 and 0.24; median for control: 0.13 mm, 10% and 90% percentiles: 0.09 and 0.21). The 95% confidence interval for the difference between group means was −0.03 to 0.04 mm ().
Figure 2. RSA maximal total point motion (MTPM) migration from 1 to 2 years, in mm (the primary effect variable). Horizontal lines indicate median values.
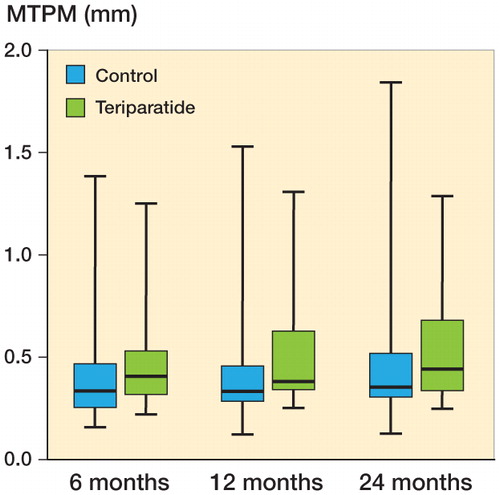
Migration (MTPM) and other variables showed no significant differences at any time point (Table 2, see Supplementary data) (). By comparison with other, similar studies, we especially noted the absence of any substantial difference at 1 year.
Figure 3. RSA maximal total point motion (MTPM) migration, in mm (secondary variables). Line in boxes indicate median values, boxes interquartile range, and whiskers total range.
The error of measurement for MTPM, based on double examinations in all patients in Motala at 1 year postoperatively (with patients raising between exposures; n = 28) was 0.091 mm (defined as SD(diff) × 2−0.5 × 1.96).
There were no suspected unexpected serious adverse reactions (SUSAR).
Discussion
Contrary to expectation, we were unable to show any effects of teriparatide treatment on prosthesis migration at any time point. Moreover, the confidence interval for the difference between group means excluded any meaningful differences. This contrasts with the effect of antiresorptive treatment, which has previously been shown to reduce migration in repeated studies with very much the same design as this one. Moreover, in our accompanying paper (Ledin et al. Citation2017), we have shown that another antiresorptive treatment (denosumab) also reduces migration (in the same settings as this study on teriparatide). The difference in response between teriparatide and antiresorptive drugs suggests that resorption plays a more important role during the postoperative course than any deficit in bone formation.
The effects of teriparatide on fracture healing are debated. There have been several studies suggesting a positive effect of the same dose regimen as we used, on radiological healing of wrist fractures (Aspenberg and Johansson Citation2010, Aspenberg et al. Citation2010) and also on the functional outcome of trochanteric hip fractures (Huang et al. Citation2015, Aspenberg et al. Citation2016). However, these results are all based on post hoc testing, and should be interpreted with caution (Aspenberg Citation2013). There has also been 1 study on proximal humeral fractures showing no obvious effect of teriparatide (Johansson Citation2016).
Prosthesis fixation has many properties in common with the healing of cancellous bone in metaphyses. Based on human biopsies (Aspenberg and Sandberg Citation2013) and animal experiments (Bernhardsson et al. Citation2015), injured cancellous bone appears to heal by rapid activation of mesenchymal cells present in the bone marrow (Sandberg et al. in press), and this process responds strongly to PTH in animal models (Skripitz and Aspenberg Citation2001a,Citationb). These notions made us believe that teriparatide would improve implant fixation in humans also.
Our findings regarding migration have rather limited relevance for weighing the evidence for or against a positive effect of teriparatide on fracture healing. Even though we believe that the response to implantation has similarities to cancellous fracture healing, the lavage and cementation creates an environment rather dissimilar to a fracture. In contrast, uncemented prostheses are dependent on the healing response to trauma to produce ingrowth of bone into pores. It is possible that the anabolic effects of teriparatide would have a positive effect here.
Supplementary data
Table 2 is available as supplementary data in the online version of this article http://dx.doi.org/10.1080/17453674.2017.1300745.
HL: participation in design, performance of and responsibility for all data collection and analysis, writing of the first draft, and revision and approval of the manuscript. LG: participation in design, responsibility for data collection in Oskarshamn, and revision and approval of the manuscript. TJ: participation in design, correspondence with the Swedish drug agency, and approval of the manuscript. PA: design, analysis, statistics, and writing, revision, and approval of the manuscript.
We thank Carmen Henriksson, Anna Haraldsson, Ulf Kallander, Ing-Marie Vallin, and Stefan Stjärne for technical assistance.
IORT_A_1300745_SUPP.PDF
Download PDF (24.1 KB)- Andreassen T T, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res 1999; 14(6): 960–8.
- Aspenberg P. Special Review: Accelerating fracture repair in humans: a reading of old experiments and recent clinical trials. BoneKEy reports 2013; 2: 244.
- Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 2010; 81(2):234–6.
- Aspenberg P, Sandberg O. Distal radial fractures heal by direct woven bone formation. Acta Orthop 2013; 84(3): 297–300.
- Aspenberg P, Wermelin K, Tengwall P, Fahlgren A. Additive effects of PTH and bisphosphonates on the bone healing response to metaphyseal implants in rats. Acta Orthop 2008; 79(1): 111–5.
- Aspenberg P, Genant H K, Johansson T, Nino A J, See K, Krohn K, Garcia-Hernandez P A, Recknor C P, Einhorn T A, Dalsky G P, Mitlak B H, Fierlinger A, Lakshmanan M C. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010; 25(2): 404–14.
- Aspenberg P, Malouf J, Tarantino U, Garcia-Hernandez PA, Corradini C, Overgaard S, Stepan JJ, Borris L, Lespessailles E, Frihagen F, Papavasiliou K, Petto H, Caeiro JR, Marin F. Effects of teriparatide compared with risedronate on recovery after pertrochanteric hip fracture: results of a randomized, active-controlled, double-blind clinical trial at 26 weeks. J Bone Joint Surg Am 2016; 98(22): 1868–78
- Bernhardsson M, Sandberg O, Aspenberg P. Experimental models for cancellous bone healing in the rat. Acta Orthop 2015; 86(6): 745–50.
- Gabet Y, Muller R, Levy J, Dimarchi R, Chorev M, Bab I, Kohavi D. Parathyroid hormone 1-34 enhances titanium implant anchorage in low-density trabecular bone: a correlative micro-computed tomographic and biomechanical analysis. Bone 2006; 39(2): 276–82.
- Hilding M, Aspenberg P. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 2006; 77(6): 912–6.
- Hilding M, Ryd L, Toksvig-Larsen S, Aspenberg P. Clodronate prevents prosthetic migration: a randomized radiostereometric study of 50 total knee patients. Acta Orthop Scand 2000; 71(6): 553–7.
- Huang T W, Yang T Y, Huang K C, Peng K T, Lee M S, Hsu R W. Effect of teriparatide on unstable pertrochanteric fractures. BioMed research international 2015; 2015: 568390.
- Johansson T. PTH 1-34 (teriparatide) may not improve healing in proximal humerus fractures. A randomized, controlled study of 40 patients. Acta Orthop 2016; 87(1): 79–82.
- Ledin H, Aspenberg P, Good L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 2012; 83(5): 499–503.
- Ledin H, Good L, Aspenberg P. Denosumab reduces early migration in total knee replacement: a randomized controlled trial in 50 patients. Acta Orthop 2017. [Ahead of print]
- Namba R S, Inacio M C, Cheetham T C, Dell R M, Paxton E W, Khatod M X. Lower total knee arthroplasty revision risk associated with bisphosphonate use, even in patients with normal bone density. J Arthroplasty 2016; 31(2): 537–41.
- Pijls B G, Valstar E R, Nouta K A, Plevier J W, Fiocco M, Middeldorp S, Nelissen R G. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop 2012; 83(6): 614–24.
- Ryd L, Albrektsson B E, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 1995; 77(3): 377–83.
- Sandberg O H, Aspenberg P. Inter-trabecular bone formation: a specific mechanism for healing of cancellous bone. Acta Orthop 2016; 87(5): 459–65.
- Skripitz R, Aspenberg P. Early effect of parathyroid hormone (1-34) on implant fixation. Clin Orthop Rel Res 2001a; (392): 427–32.
- Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br 2001b; 83(3): 437–40.
- Teng S, Yi C, Krettek C, Jagodzinski M. Bisphosphonate use and risk of implant revision after total hip/knee arthroplasty: a meta-analysis of observational studies. PloS one. 2015; 10(10):e 0139927.