Abstract
Background and purpose — Aseptic loosening is a main cause of late revision in total knee replacement (TKR). Migration of implants as measured by radiostereometric analysis (RSA) can predict future loosening. This migration is associated with bone resorption. Denosumab is a human monoclonal antibody that binds to receptors on osteoclast precursors and osteoclasts. This prevents osteoclast formation, resulting in less bone resorption in cortical and trabecular bone. We investigated whether denosumab can reduce migration of TKR, as measured with RSA.
Patients and methods — In this 2-center, randomized, double-blind placebo-controlled trial, 50 patients with osteoarthritis of the knee were treated with an injection of either denosumab (60 mg) or placebo 1 day after knee replacement surgery and again after 6 months. RSA was performed postoperatively and after 6, 12, and 24 months. The primary effect variable was RSA maximal total point motion (MTPM) after 12 months. We also measured other RSA variables and the knee osteoarthritis outcome score (KOOS).
Results — The primary effect variable, MTPM after 12 months, showed that migration in the denosumab group was statistically significantly less than in the controls. Denosumab MTPM 12 months was reduced by one-third (denosumab: median 0.24 mm, 10% and 90% percentiles: 0.15 and 0.41; placebo: median 0.36 mm, 10% and 90% percentiles: 0.20 and 0.62). The secondary MTPM variables (6 and 24 months) also showed a statistically significant reduction in migration. There was no significant difference in MTPM for the period 12–24 months. KOOS sub-variables were similiar between denosumab and placebo after 12 and 24 months.
Interpretation — Denosumab reduces early migration in total knee replacement, as in previous trials using bisphosphonates. As migration is related to the risk of late loosening, denosumab may be beneficial for long-term results.
Early stable fixation in total knee replacement (TKR) is important to prevent late loosening (Ryd et al. Citation1995, Pijls et al. Citation2012). Radiostereometric analysis (RSA) is performed to estimate fixation by measuring the postoperative migration of the prosthesis. Bisphosphonates, most commonly used in treatment of osteoporosis, have been shown to prevent early migration in TKR (Hilding et al. Citation2000, Hilding and Aspenberg Citation2006) and are associated with lower revision risk in epidemiological studies (Teng et al. Citation2015, Namba et al. Citation2016).
Denosumab is another antiresorptive, a human monoclonal antibody (IgG2) that binds with high affinity and specificity to RANKL, a type-II membrane protein, preventing activation of its receptor, RANK, on the surface of osteoclast precursors and osteoclasts. Denosumab prevents osteoclast formation and reduces both function and survival of the cell. The result is less bone resorption in cortical and trabecular bone (Kostenuik Citation2005).
Based on animal experiments, denosumab has been suggested to have a stronger effect on bone resorption around implants than bisphosphonates (Bernhardsson et al. Citation2015). Because we have previously found that antiresorptive therapy with bisphosphonates can reduce migration, we now studied whether there was a similar effect with denosumab. In this study, we assessed whether denosumab, administered postoperatively and after 6 months, could enhance bone healing in the interface between bone and cement after knee replacement, thereby reducing the risk of late loosening. We performed a 2-center, randomized, double-blind placebo-controlled evaluation to study the effects of denosumab, using migration by RSA at 12 months as the primary effect variable.
Patients and methods
50 patients (30 women) were included. The patients were scheduled for elective cemented primary total knee replacement because of osteoarthritis. The sample size (n = 50) was calculated from similar studies (Hilding et al. Citation2000, Ledin et al. Citation2012) to detect a mean difference in maximal total point motion (MTPM) of 0.2 mm with an SD of 0.2 (α = 0.05 and 80% power).
33 patients were operated in Motala and 17 in Oskarshamn, Sweden. The surgery was performed between January 2012 and March 2014. The inclusion criteria were men or postmenopausal women, 55–80 years of age, with idiopathic osteoarthritis of the knee.
Exclusion criteria were use of bisphosphonates or other drugs that influence bone (e.g. anti-osteoporotic agents, glucocorticoids, or anti-epileptics) in the year before randomization; cardiac disease limiting physical activities; ASA class 3 or 4; active malignant disease; previous radiation therapy; metabolic disease affecting the skeleton (other than osteoporosis); rheumatic disease; hypocalcemia; hypersensitivity to denosumab or any of its excipients; or simultaneous bilateral surgery. Inability to give informed consent because of communication problems (drug abuse, language, or behavior problems) was also an exclusion criterion. The patients who declined to participate in the study were not registered.
The patients who chose to participate in the study () were randomized () to treatment with either denosumab (Prolia, 60 mg; Amgen AB, Solna Sweden) or placebo. A randomization list was produced by the study monitor. The list was sent to Amgen but not to the investigators, who were kept blind. Amgen labeled syringes containing either denosumab or placebo with consecutive numbers. 4 sets of paired and consecutively numbered prefilled syringes were prepared (the pairs consisted of syringes for the first and second injection). 1 set was for men who were operated on in Motala (n = 10), 1 set was for women in Motala (n = 15), 1 set was for men in Oskarshamn (n = 10), and 1 set was for women in Oskarshamn (n = 15). Due to a faster inclusion rate in Motala, 33 patients ended up being operated there and 17 were operated in Oskarshamn. The numbers were calculated from inclusion rates in a previous, similar trial on teriparatide, which is published in the current issue of Acta Orthopaedica (Ledin et al. Citation2017). The treatment was administered on the first postoperative day after knee replacement surgery and again after 6 months.
Table 1. Patient characteristics
Surgery
The surgery was performed by HL in 33 cases and by 2 other surgeons in 17 cases. All operations were done under spinal anesthesia. We used the Nexgen CR all-poly tibial knee prostheses (Zimmer), inserted after pulsed lavage and cemented with Palacos R + G cement (Heraeus medical AB, Sollentuna, Sweden). 2 g of cloxacillin was given just before the operation and twice after. Low-molecular-weight heparin (Innohep, 4,500 IE subcutaneously) was used for 14 days postoperatively. For RSA measurements, 6 tantalum beads (0.8–1.0 mm) were inserted in the all-poly tibial component and 6 in the tibial bone metaphysis. We used a tourniquet during the whole operation.
Evaluation
The radiostereometric examinations were performed on the second postoperative day, and after 6, 12, and 24 months. The blind analysis of the RSA data was done using the UmRSA computer program (RSA Biomedical AB, Umea, Sweden) by 2 specifically trained research nurses in Motala. The patient-reported function and symptoms, knee osteoarthritis outcome score (KOOS) data, were collected preoperatively and after 12 months and 24 months by the same research nurses. There were no changes to the protocol. Unblinding was done after all the data had been locked.
Statistics
The primary effect variable was migration measured by RSA (MTPM) after 12 months. The variable was log-transformed. The Kolmogorov-Smirnov test did not reject the hypothesis of normal distribution. Analysis of variance was performed with patient sex and site as covariates. 95% confidence intervals (CIs) in mm were calculated from the CI for log-transformed values by use of the non-transformed mean value for the controls (values for the ratio of denosumab/placebo multiplied by the mean for placebo in mm).
Secondary outcomes were other RSA variables. MTPM values were analyzed as above. Other RSA variables were analyzed without transformation. KOOS data were tested with the Mann-Whitney test for all sub-variables. We used SPSS version 23 for statistical calculations.
Ethics and registration
The study was approved by the Regional Ethics Committee in Linköping on March 30, 2010: reference number 2011/114-31; EudraCT number 2011-000693-57. Protocol information is available at www.clinicaltrialsregister.eu. The trial was performed in compliance with the Declaration of Helsinki. Patient data are to be stored at the Department of Orthopedics, Aleris Specialist Care Motala AB, Motala, Sweden for 10 years. The study was monitored by Forum Östergötland, Linköping, Sweden.
Funding and potential conflict of interests
PA has shares in Addbio AB, a company developing bisphosphonate coatings for implants. He has also received institutional research support from Eli Lilly Corp and Amgen. There are no other potential conflicts of interests.
Results
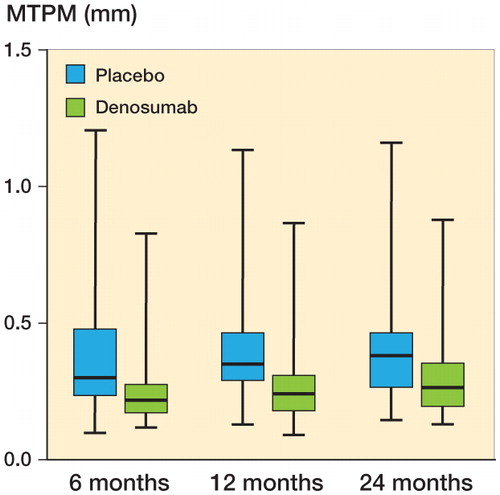
The primary effect variable, MTPM after 12 months, showed that migration in the denosumab group was reduced by one-third compared to the controls. Median MTPM at 12 months for denosumab was 0.24 mm (10% and 90% percentiles: 0.15 and 0.41); for placebo, it was 36 mm (10% and 90% percentiles: 0.20 and 0.62). The 95% confidence interval for the difference between group medians was −0.03 to −0.16 mm (p = 0.01) (). The secondary MTPM variables (at 6 and 24 months) also showed a statistically significant reduction in migration (p = 0.02 for both) ().
Figure 2. RSA maximal total point motion (MTPM) migration in mm. Data for 12 months were the primary effect variable. Line in boxes indicate median values, boxes interquartile range, and whiskers total range.
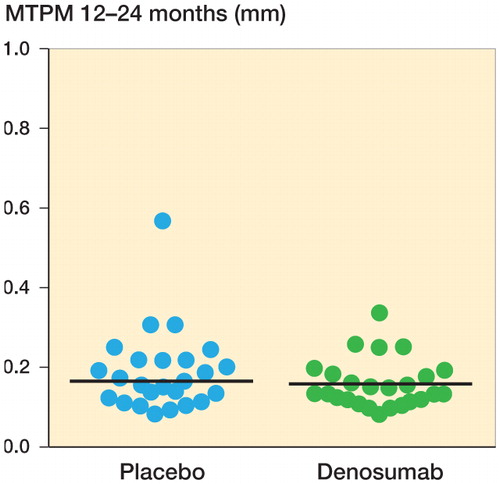
Migration by MTPM from 12 to 24 months showed no statistically significant difference (Table 2, see Supplementary data) (). The error of measurement for MTPM, based on double examinations in all patients at 1 year postoperatively (with patients raising between exposures; n = 48) was 0.095 mm (defined as SD(diff) × 2−0.5 × 1.96).
Figure 3. RSA maximal total point motion migration (MTPM) in mm between 12 and 24 months. Horizontal lines indicate median values.
There was no significant difference in migration between men and women, and between the sites, and we found no benefit in stratification: an analysis by a t-test without adjustment for sex or site yielded very similar results to those from the ANCOVA. The correlation between age and MTPM at 1 year was minimal (r = 0.0).
KOOS sub-variables showed no statistically significant difference between denosumab and placebo postoperatively, or after 12 or 24 months (Figure 4, see Supplementary data).
There were no suspected unexpected serious adverse reactions (SUSAR).
Discussion
The denosumab group had reduced early migration by one-third, as measured by the primary effect variable (MTPM at 12 months) and the secondary MTPM variables (6 and 24 months) in total knee replacement, which is similar to findings in previous trials using bisphosphonates (Hilding et al. Citation2000, Hilding and Aspenberg Citation2006). This suggests that the risk of loosening was reduced by the treatment. The total 10-year revision rate for cemented knees is normally around 4%, but revision for aseptic loosening amounts to only 1% (The Swedish Knee Arthroplasty Register 2015). It has been estimated that the 5-year revision rate increases by 7 percentage points (CI: 4.7–9.5) for every 1 mm increase in MTPM during the first postoperative year (Pijls et al. Citation2012). Assuming a linear relationship, our results would indicate that denosumab can eliminate the need for most revisions due to aseptic loosening. This estimation, however, is likely to be overoptimistic. The relationship between migration and the risk of loosening is not linear. It is even possible that there is a dichotomy in the data, similar to that for acetabular cups (Aspenberg et al. Citation2008). Therefore, reducing median values is probably less important than reducing the number of patients with excessive migration. Such an endpoint, however, would require a much larger number of patients than is normally feasible with an RSA study.
MTPM is the length of the translation vector of the point in a rigid body that has the greatest motion. MTPM always has a positive value and is not normally distributed. We used MTPM because the possible effect of a drug on migration is most likely to be detected at the point of maximum motion (Valstar et al. Citation2005). This might explain why most measures of translation and rotation did not show any statistically significant differences. The treatment effect was apparent after 6 months, and after that the changes were small (). Also, MTPM at 12 to 24 months was rather similar between the groups, apparently because the effect of denosumab mainly occurred before that time period. Hence, it is likely that the second dose of denosumab was unnecessary.
The difference in response between the anabolic drug teriparatide, shown in our accompanying paper in this issue of Acta Orthopaedica, and antiresorptive drugs such as bisphosphonates and denosumab suggest that resorption plays a more important role during the postoperative course than any deficit in bone formation (Ledin et al. Citation2017). The primary variable of the teriparatide trial was migration from 12 to 24 months. This choice was motivated by the study of Ryd et al. (Citation1995), where this time period was described as having the highest predictive value. However, later studies have emphasized earlier time periods for prediction (Pijls et al. Citation2012), so we changed the primary effect variable when we came to the denosumab trial. In either case, teriparatide showed no effect regardless of the time period analyzed, and the results are similar to those in the control patients in both studies.
Antiresorptives in the form of bisphosphonates reduce early migration, but they are unlikely to be sufficiently powerful to also inhibit the progression of clinical, symptomatic late loosening. However, due to the different mechanisms of action, it is theoretically possible that denosumab may be of value in the treatment of established loosening (Aspenberg et al. Citation2011).
The patient-reported function and symptoms (KOOS) were similar between the groups. This was to be expected. Migration is thought to influence the risk of loosening in the long run, and even if the loosening process starts postoperatively, it probably remains subclinical for several years. Moreover, only a few patients will ever experience loosening, and the study had far too little power to detect a difference in symptoms associated with risk of future loosening in these few cases.
As early migration is related to the risk of late loosening (Ryd et al. Citation1995, Pijls et al. Citation2012), denosumab might be beneficial for long-term results.
Supplementary data
Table 2 and Figure 4 are available as supplementary data in the online version of this article http://dx.doi.org/10.1080/17453674.2017.1300746.
HL: design, data collection, analysis, statistics, and writing. LG: data collection and analysis. PA: design, analysis, statistics, and writing.
We thank Carmen Henriksson, Anna Haraldsson, Ing-Marie Vallin, and Stefan Stjärne for technical assistance. The study was funded by Amgen AB, Solna, Sweden.
IORT_A_1300746_SUPP.PDF
Download PDF (87 KB)- Aspenberg P, Wagner P, Nilsson KG, Ranstam J. Fixed or loose? Dichotomy in RSA data for cemented cups. Acta Orthop 2008; 79(4): 467–73.
- Aspenberg P, Agholme F, Magnusson P, Fahlgren A. Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone 2011; 48(2): 225–30.
- Bernhardsson M, Sandberg O, Aspenberg P. Anti-RANKL treatment improves screw fixation in cancellous bone in rats. Injury 2015; 46(6): 990–5.
- Hilding M, Aspenberg P. Postoperative clodronate decreases prosthetic migration: 4-year follow-up of a randomized radiostereometric study of 50 total knee patients. Acta Orthop 2006; 77(6): 912–6.
- Hilding M, Ryd L, Toksvig-Larsen S, Aspenberg P. Clodronate prevents prosthetic migration: a randomized radiostereometric study of 50 total knee patients. Acta Orthop Scand 2000; 71(6): 553–7.
- Kostenuik P J. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol 2005; 5(6): 618–25.
- Ledin H, Aspenberg P, Good L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion. Acta Orthop 2012; 83(5): 499–503.
- Ledin H, Good L, Johansson T, Aspenberg P. No effect of teriparatide on migration in total knee replacement. A randomized controlled trial in 50 patients. Acta Orthop 2017. [Ahead of print]
- Namba R S, Inacio M C, Cheetham T C, Dell R M, Paxton E W, Khatod M X. Lower total knee arthroplasty revision risk associated with bisphosphonate use, even in patients with normal bone density. J Arthroplasty 2016; 31(2):5 37–41.
- Pijls B G, Valstar E R, Nouta K A, Plevier J W, Fiocco M, Middeldorp S, Nelissen R G. Early migration of tibial components is associated with late revision: a systematic review and meta-analysis of 21,000 knee arthroplasties. Acta Orthop 2012; 83(6): 614–24.
- Ryd L, Albrektsson B E, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br 1995; 77(3):3 77–83.
- Teng S, Yi C, Krettek C, Jagodzinski M. Bisphosphonate use and risk of implant revision after total hip/knee arthroplasty: a meta-analysis of observational studies. PloS one. 2015; 10(10): e0139927.
- Valstar ER, Gill R, Ryd L, Flivik G, Borlin N, Karrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4): 563–72.