Abstract
Background and purpose — Trabecular metal (TM) cups have demonstrated favorable results in acetabular revision and their use in primary total hip arthroplasty (THA) is increasing. Some evidence show that TM cups might decrease periprosthetic infection (PPI) incidence. We compared the survivorship of TM cups with that of other uncemented cups in primary THA, and evaluated whether the use of TM cups is associated with a lower risk of PPI.
Patients and methods — 10,113 primary THAs with TM cup and 85,596 THAs with other uncemented cups from 2 high-quality national arthroplasty registries were included. The mean follow-up times were 3.0 years for the TM cups and 3.8 years for the other uncemented cups.
Results — The overall survivorship up to 8 years for TM cups and other uncemented cups was 94.4% and 96.2%, respectively (p = < 0.001). Adjusting for relevant covariates in a Cox regression model the TM cups had a persistently higher revision risk than other uncemented cups (HR =1.5, 95% CI 1.4–1.7, p = < 0.001). There was a slightly higher, though not statistically significant, revision rate for PPI in the TM group (1.2, 95% CI 1.0–1.6, p = 0.09).
Interpretation — Risk of revision for any reason was higher for the TM cup than for other uncemented cups in primary THA. In contrast to our hypothesis, there was no evidence that the revision rate for PPI was lower in the TM cup patients. Regardless of the promising early and mid-term results for TM cups in hip revision arthroplasty, we would like to sound a note of caution on the increasing use of the TM design, especially in uncomplicated primary THAs, where uncemented titanium cups are considered to provide a reliable outcome.
Trabecular metal (ZimmerBiomet, Warsaw, IN, USA) is made of porous tantalum and has been shown to provide higher porosity, increased initial stability, and good bone ingrowth qualities (Bobyn et al. Citation1999, Beckmann et al. Citation2014). These advantages make it an attractive option in both primary and revision THA. While TM acetabular components are most commonly used in revision THA to manage poor bone quality and acetabular bone defects (Jafari et al. Citation2010, Kremers et al. Citation2012, Mohaddes et al. Citation2015), TM cups have also shown excellent results in primary THA (Baad-Hansen et al. Citation2011, Howard et al. Citation2011). However, there is a lack of data on whether TM cups are indeed a more reliable option for primary THA compared to other uncemented cups. In addition a recent study suggested that the use of TM acetabular components in hip revision arthroplasty might be protective against subsequent failure due to periprosthetic infection (PPI) (Tokarski et al. Citation2015). PPI is a devastating complication following THA. Infection rates around 1% after primary THA have been reported by major national registries (Lindgren et al. Citation2014, Gundtoft et al. Citation2015, Huotari et al. Citation2015). The total number of primary THAs is increasing and, in addition to this increase, studies have shown that the risk for infection has been increasing as well over recent decades (Dale et al. Citation2009, Citation2012).
There is a lack of data on whether TM cups are a more reliable option for primary THA compared with other uncemented cups. A recent study suggested that the use of TM acetabular components in hip revision arthroplasty might be protective against subsequent failure due to infection (Tokarski et al. Citation2015); however, the effect of using a TM cup on infection rates following primary THA also remains unknown.
The purpose of this collaborative registry study was to:
determine the overall revision rate of TM acetabular components used in primary THA and to compare it with that of other frequently used uncemented cups;
investigate whether the use of a TM cup in primary THA will decrease the risk of early revision due to infection compared with other uncemented cup designs.
Patients and methods
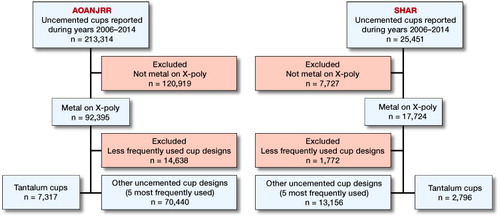
Data for this collaborative registry study were collected from the Swedish Hip Arthroplasty Register (SHAR) and the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). SHAR has been collecting data on total hip replacements since 1979 and currently has information on more than 300,000 primary hip replacements. In Sweden all orthopedic units performing hip arthroplasties report to the SHAR. The completeness of this register has been reported as 99% in primary THA. Descriptive surgical data are completed on standard forms by the responsible surgical team at each center. Several validation steps are performed on a regular basis. AOANJRR began data collection of total hip and knee arthroplasties in 1999, and includes data on more than 98% of arthroplasty procedures performed nationally since 2002 (AOANJRR 2016) AOANJRR data are validated against patient-level data provided by each state and territory health departments in Australia using a sequential, multilevel matching process reaching 94% validation on the initial pass of the validation process (AOANJRR n.d.). Data are also matched biannually with the National Death Index to obtain information on the date of death. In both registries revision is defined as a new surgical intervention when any part of the implant is removed or exchanged. This study includes data starting from January 1, 2006, which was the time point when reporting of the use of TM cups started in both registers (AOANJRR 2016).
Study population
Between January 2006 and December 2014, 25,451 and 213,314 operations performed with an uncemented acetabular component were reported to the SHAR and the AOANJRR, respectively. During this study period 10,113 primary THAs performed with a TM design (Trabecular Metal Tantalum or Continuum (ZimmerBiomet, Warsaw, IN, USA) were registered in SHAR (n = 2,796) and AOANJRR (n = 7,317). The 5 most commonly used uncemented acetabular components from each register (uncemented n = 83,596, SHAR n = 13,156, AOANJRR n = 70,440) were identified (). The patient selection is described in a flowchart ().
Table 1. Trabecular metal cups and 5 other most commonly used uncemented cups in primary THA in AOANJRR and SHAR. Values are frequency and (%)
Characteristics of the study population
The average age of the patients at the time of the primary operation was 68 (11–100) years in both groups. There were 44% and 43% males in the TM and the uncemented control groups, respectively (). Primary osteoarthritis was the most common reason for surgery both in the TM group (85% of all operations) and in the uncemented control group (87% of all operations) ().
Table 2. Demographics by registry. Values are frequency and (%) unless otherwise stated
Operative data
In the TM group, uncemented stems were more frequently used (65% of all TM cups) compared with the uncemented control group (52% of all uncemented cups).
In the TM group, large femoral heads (> 32 mm) were implanted in 40% (n = 4,015) of the cases. The corresponding proportion in the uncemented control group was 26% (n = 21,603). The average follow-up was 3.0 (0–9) years in the TM group and 3.8 (0–9) years in the uncemented control group.
Statistics
The time to first revision was defined using Kaplan–Meier estimates of survivorship. Survival curves were excluded when numbers at risk in any of the groups were below 100 cases. All analyses were performed using R statistical software (RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, USA). The log rank test was used to compare survival at 8 years. To reduce the risk of possible selection bias towards more difficult cases being treated with TM cups we adjusted the estimated relative revision risks in the Cox regression models performed in the whole study population for age, sex, diagnosis, femoral head size, and fixation of the stem. In addition, all analyses were repeated following stratification of the study population to include only patients with a primary diagnosis of osteoarthritis, who received a cemented stem, and whose femoral head size was 32 mm (SHAR, n = 708 and AOANJRR, n = 16,824). This was done to remove the possibility of confounding by head size, stem fixation, and primary diagnosis. In this additional subgroup analysis the estimated relative revision risks were adjusted for age and sex. Schoenfeld’s residuals were used to control for proportional hazards assumption. The primary outcome in all analyses was revision for any reason and the secondary outcome was revision for periprosthetic infection. Revision was described as a change or removal of at least 1 component. It is possible that individual cup types might interact in different ways with the femoral stem component, and therefore revisions where the stem only was exchanged were also included. Survival data are presented as percentages with 95% confidence interval (CI). Cox regression analysis is presented with hazard ratio (HR) and 95% CI.
Ethics, funding, and potential conflict of interest
Ethical approval was obtained from the Local Ethical Review Board in Gothenburg, Sweden (669-16 dd 29/09/2016). Date of issue September 29, 2016. The AOANJRR has been approved to use its own data as Federal Quality Control Activity and therefore an individual IRB approval is not needed for publications on de-identified analysis. No funding directly related to this study was received. No competing interests declared.
Results
Implant survival
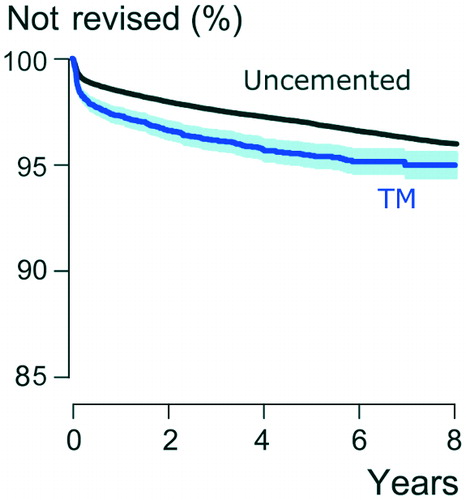
In Kaplan–Meier analysis, the up to 8-year survivorship of the TM group was 94.4% (CI 92.8–96.0) and that of the uncemented control group 96.2% (CI 96.0–96.4) (p = < 0.001) (). After adjustment for age, sex, indication for primary THA, femoral head size, and stem fixation, the TM group had a 1.5 (CI 1.4–1.7, p = < 0.001) times higher risk for revision compared with the uncemented control group. In the subgroup analysis, which included only patients with a primary diagnosis of osteoarthritis, cemented stem, and femoral head size 32 mm (total n = 17,532; TM n = 1,222; other uncemented cups n = 16,310), TM cups were also revised more often than other uncemented cups (HR =2.3, p = < 0.001, CI 1.6–3.2).
Infection
In the TM group, including all TM cups, 79 of all 360 revisions were performed due to infection, whereas, in the uncemented control group 27% (n = 561) of all revisions were performed due to infection (). There was a slightly higher, though not statistically significant, revision rate due to infection in the TM group compared with the uncemented control group (HR =1.2, CI 1.0-–1.6, p = 0.09) (). In the TM group, in 33% of cup revisions the stem was revised simultaneously (n = 120), whereas in groups of other uncemented cups 41% of cup revisions had simultaneous stem revision (n = 848).
Figure 3. Kaplan–Meier survival for TM cups and other uncemented cups in primary THA with revision for infection as the end-point. 95% CI levels presented around the curves in light blue and light grey.
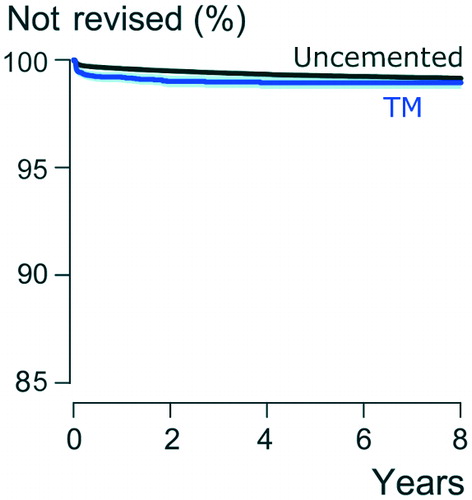
Table 3. Reasons for revision and type of revision by registry. Values are frequency and (%) unless otherwise stated
Discussion
Since the introduction of trabecular metal in 1997, TM cups have shown promising results in acetabular revisions (Siegmeth et al. Citation2009, Jafari et al. Citation2010, Davies et al. Citation2011, Skyttä et al. Citation2011, Mohaddes et al. Citation2015). Mid- to long-term survivorship of TM cups has been promising in primary THAs as well, where the use of a TM cup has been increasing lately (Macheras et al. Citation2009, Baad-Hansen et al. Citation2011, Howard et al. Citation2011, Wegrzyn et al. Citation2015, De Martino et al. Citation2016). In this collaborative register-based study we found that the risk for revision for the TM cups was 50% higher in the unrestricted analysis and 128% higher in the restricted analysis than that of the 5 most frequently used uncemented cup designs after primary THA revision for any reason as the end-point. In addition, there was weak evidence of association between the use of a TM cup and higher risk for revision for PPI compared with other uncemented designs.
Our findings that TM cups have a higher revision rate compared with the control group of other uncemented cups is in contrast to some earlier studies. When adjusted for confounding factors in the Cox model, the TM cups still had a 1.5 times higher risk for revision for any reason when compared with the 5 most commonly used uncemented cup designs. Previous studies have reported excellent results with tantalum cups used in primary THA (Jafari et al. Citation2010, Noiseux et al. Citation2014, De Martino et al. Citation2016), or have not found any statistically or clinically significant difference in the survival results with TM cups compared with uncemented titanium cups in primary THA (Baad-Hansen et al. Citation2011, Mohaddes et al. Citation2015). Wegrzyn et al. (Citation2015) presented 100% survivorship for the TM cup in a randomized controlled trial at 10 years, and they also observed statistically significantly fewer radiolucencies around the TM cups compared with other uncemented titanium cups. We were not able to assess radiographs and therefore could not study radiolucencies.
The use of TM cups has been recommended for more demanding cases with larger acetabular bone defects because of the reliable bone ingrowth observed with this material (Bobyn et al. Citation1999). Although increased use in more complicated primary procedures might be one reason explaining the higher overall and infection revision rate of the TM cups found in this study, the evidence we have indicates that the use of these devices in Sweden and Australia has largely been in routine primary procedures. To verify this, we looked at the distribution of use by hospital in Sweden and by surgeon in Australia. In Sweden 28% of hospitals performing 40 or more uncemented primary cups per year used TM in at least 30% of all cases. In Australia 22% of surgeons have used a TM cup. For surgeons doing more than 25 procedures a year almost 14% use the TM cup in over 50% of their procedures and 11% use it in over 75%. Despite its reputation of being a specialty cup, in practice it is being used in these countries as a routine cup. Our results may indicate that this is not a beneficial trend from either the cost point of view or more importantly the outcome. However, TM cups used with large bone deficiencies in revision surgery have been reported to have excellent results (Weeden and Schmidt Citation2007, Sternheim et al. Citation2012). We do not recommend that our results be extrapolated to either complex primary or revision surgery.
Reducing the rate of infection remains an important imperative in joint replacement surgery. In a recent study by Tokarski et al. (Citation2015), TM cups used for first-time cup revisions showed lower second revision rate for infection than titanium cups. The risk of second revision due to infection in cases revised due to infection was substantially lower with a tantalum cup than with a titanium cup (3% vs. 18%, respectively). The authors commented that this might be due to better osteointegration or directly associated with tantalum’s biological properties. The data from our study indicate that if there is a beneficial effect of TM with respect to infection risk it is not evident when used for routine primary procedures. There was, in fact, a slightly higher revision rate due to PPI in the TM group compared with other uncemented cups, but this was not statistically significant (p = 0.09). As previously stated, TM cups have been recommended for use in more complex primary procedures. It is possible to speculate that the trend to increased infection may indicate that patients operated on using TM cups in this study may have been at a greater risk for infection; however, we have no evidence for this. At the very least it can be stated that in this large population-based study we have not been able to confirm that there is a beneficial effect on infection risk when TM cups are used in primary procedures.
We acknowledge a number of limitations in this study, the most important being the potential for confounding. Our major concern was that TM cups might be selectively used in more demanding cases. A number of approaches were taken to address this, including the adjustments used in the regression analyses as well as in the subgroup analysis. There are, however, a number of other reasons why we believe that selection bias is not a major factor in this study. During the study period 2006–2014, over 10% of all uncemented cups used in primary THA were TM cups. The use of the TM cup has also rapidly increased with a 5-fold increase during the study period. The TM is clearly a commonly used acetabular component with use comparable to other commonly used cementless acetabular components, indicating frequent use in routine procedures.
The lack of radiological data and patient-reported outcome measures, usual for large registry-based studies, is a further limitation. It is likely that there are unrevised patients in both groups with pain and/or poor function. Although revision is an excellent primary outcome measure for large population-based studies, the number of patients with unsatisfactory results will be larger than the revised population. There is no reason, however, to suspect that the indications for revision vary between the different populations. Another possible problem in registry collaboration is aggregating data from individual registries. Nonetheless, due to the similar data structure in both SHAR and AOANJRR we are not concerned about combining data in this study.
Although the maximum follow-up of this study is 9 years, the mean follow-up time is 3 years. This in part reflects the increased use of these devices in both Sweden and Australia in recent years. Risk of revision due to dislocation or infection is highest during the first 2 years after primary THA (Pulido et al. Citation2008, Jameson et al. Citation2011). This is well covered by the mean follow-up of this study. Determining the long-term outcomes of TM cups requires studies with longer follow-up.
In summary, we found the early and mid-term revision rate of TM cups is significantly higher than that of other uncemented cups in primary THA. Further, there was no statistically significant difference in revision rate for infection between these 2 groups. Although TM cups may be a good option in complex primary or revision hip arthroplasty, there does not appear to be any reason to use TM cups in routine primary THAs, where uncemented titanium cups provide patients with a good and reliable outcome.
All authors participated in the study design. MM and ML performed the statistical analyses. Interpretation of the results was done by IL, SEG, AE, HM, and MM. All authors contributed to the writing of manuscript.
Acta thanks Adrian Sayers and Willem Schreurs for help with peer review of this study.
- AOANJRR. AOANJRR validation process. Available from: https://aoanjrr.sahmri.com/en/data [last accessed December 12, 2017].
- AOANJRR. Annual Report 2016. Available from: https://aoanjrr.sahmri.com/annual-reports-2016 [last accessed December 12, 2017].
- Baad-Hansen T, Kold S, Nielsen P T, Laursen M B, Christensen P H, Soballe K. Comparison of trabecular metal cups and titanium fiber-mesh cups in primary hip arthroplasty: a randomized RSA and bone mineral densitometry study of 50 hips. Acta Orthop 2011; 82(2): 155–60.
- Beckmann N A, Weiss S, Klotz M C M, Gondan M, Jaeger S, Bitsch R G. Loosening after acetabular revision: comparison of trabecular metal and reinforcement rings. A systematic review. J Arthroplasty 2014; 29(1): 229–35.
- Bobyn J D, Stackpool G J, Hacking S A, Tanzer M, Krygier J J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg 1999; 81(5): 907–14.
- Dale H, Fenstad A M, Hallan G, Havelin L I, Furnes O, Overgaard S, et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop 2012; 83(5): 449–58.
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin L I, Engesaeter L B. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop 2009; 80(6): 639–45.
- Davies J H, Laflamme G Y, Delisle J, Fernandes J. Trabecular metal used for major bone loss in acetabular hip revision. J Arthroplasty 2011; 26(8): 1245–50.
- De Martino I, De Santis V, Sculco P K, D’Apolito R, Poultsides L A, Gasparini G. Long-term clinical and radiographic outcomes of porous tantalum monoblock acetabular component in primary hip arthroplasty: a minimum of 15-year follow-up. J Arthroplasty 2016; 31(9): 110–14.
- Gundtoft P H, Overgaard S, Schønheyder H C, Møller J K, Kjaersgaard-Andersen P, Pedersen A B. The incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop 2015; 86(3): 326–34.
- Howard J L, Kremers H M, Loechler Y A, Schleck C D, Harmsen WS, Berry D J, et al. Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg (Am) 2011; 93(17)1597–604.
- Huotari K, Peltola M, Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop 2015; 86(3):3 21–5.
- Jafari S M, Bender B, Coyle C, Parvizi J, Sharkey P F, Hozack W J. Do tantalum and titanium cups show similar results in revision hip arthroplasty? Clin Orthop Relat Res 2010; 468(2): 459–65.
- Jameson S S, Lees D, James P, Serrano-Pedraza I, Partington P F, Muller S D, et al. Lower rates of dislocation with increased femoral head size after primary total hip replacement: a five-year analysis of NHS patients in England. J Bone Joint Surg (Br) 2011; 93: 876–80.
- Kremers H M, Howard J L, Loechler Y, Schleck C D, Harmsen W S, Berry D J, et al. Comparative long-term survivorship of uncemented acetabular components in revision total hip arthroplasty. J Bone Joint Surg (Am) 2012; 94(12): e82.
- Lindgren J V, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord 2014; 15: 384.
- Macheras G, Kateros K, Kostakos A, Koutsostathis S, Danomaras D, Papagelopoulos P J. Eight- to ten-year clinical and radiographic outcome of a porous tantalum monoblock acetabular component. J Arthroplasty 2009; 24(5): 705–9.
- Mohaddes M, Rolfson O, Kärrholm J. Short-term survival of the trabecular metal cup is similar to that of standard cups used in acetabular revision surgery. Acta Orthop 2015; 86(1): 26–31.
- Noiseux N O, Long W J, Mabry T M, Hanssen A D, Lewallen D G. Uncemented porous tantalum acetabular components: early follow-up and failures in 613 primary total hip arthroplasties. J Arthroplasty 2014; 29(3): 617–20.
- Pulido L, Ghanem E, Joshi A, Purtill J J, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 2008; 466(7): 1710–15.
- Siegmeth A, Duncan C P, Masri B A, Kim W Y, Garbuz D S. Modular tantalum augments for acetabular defects in revision hip arthroplasty. Clin Orthop Relat Res 2009; 467(1): 199–205.
- Skyttä E T, Eskelinen A, Paavolainen P O, Remes V M. Early results of 827 trabecular metal revision shells in acetabular revision. J Arthroplasty 2011; 26(3): 342–5.
- Sternheim A, Backstein D, Kuzyk P R, Goshua G, Berkovich Y, Safir O, Gross A E. Porous metal revision shells for management of contained acetabular bone defects at a mean follow-up of six years: a comparison between up to 50% bleeding host bone contact and more than 50% contact. J Bone Joint Surg Br 2012; 94(2): 158–62.
- Tokarski A T, Novack T A, Parvizi J. Is tantalum protective against infection in revision total hip arthroplasty? Bone Joint J 2015; 97-B(1): 45–9.
- Weeden S H, Schmidt R H. The use of tantalum porous metal implants for Paprosky 3A and 3B defects. J Arthroplasty 2007; 22(6Suppl): 151–5.
- Wegrzyn J, Kaufman K R, Hanssen A D, Lewallen D G. Performance of porous tantalum vs. titanium cup in total hip arthroplasty: randomized trial with minimum 10-year follow-up. J Arthroplasty 2015; 30(6): 1008–13.