Abstract
Background and purpose — Most earlier publications investigating whether annual surgeon volume is associated with lower levels of adverse events (AE), reoperations, and mortality are based on patient cohorts from North America. There is also a lack of adjustment for important confounders in these studies. Therefore, we investigated whether higher annual surgeon volume is associated with a lower risk of adverse events and mortality within 90 days following primary total hip arthroplasty (THA).
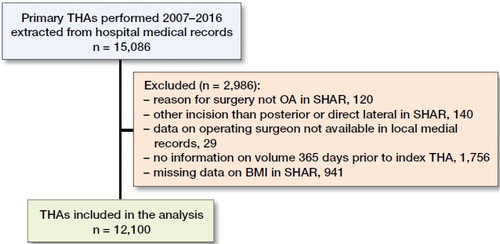
Patients and methods — We collected information on primary total hip arthroplasties (THA) performed between 2007 and 2016 from 10 hospitals in Western Sweden. These data were linked with the Swedish Hip Arthroplasty Register and a regional patient register. We used logistic regression (simple and multiple) adjusted for age, sex, comorbidities, BMI, fiation technique, diagnosis, surgical approach, time in practice as orthopedic specialist and annual volume. Annual surgeon volume was calculated as the number of primary THAs the operating surgeon had performed 365 days prior to the index THA.
Results — 12,100 primary THAs, performed due to both primary and secondary osteoarthritis by 268 different surgeons, were identified. The median annual surgeon volume was 23 primary THAs (range 0–82) 365 days prior to the THA of interest and the mean risk of AE within 90 days was 7%. If the annual volume increased by 10 primary THAs in the simple logistic regression the risk of AE decreased by 10% and in the adjusted multiple regression the corresponding number was 8%. The mortality rate in the study was low (0.2%) and we could not find any association between 90-day mortality and annual surgeon volume.
Interpretation — High annual surgical activity is associated with a reduced risk of adverse events within 90 days. Based on these findings healthcare providers should consider planning for increased surgeon volume.
In order to improve the outcomes after total hip arthroplasties (THA) and thereby reduce the burden of complications (Lawson et al. Citation2013), it is crucial to identify factors influencing adverse events (AE) associated with surgery. Earlier studies have shown that patient comorbidities, ASA classification, age, sex, BMI, and smoking increase the risk of complications and reoperations (Bozic et al. Citation2012, Lalmohamed et al. Citation2013, Arsoy et al. Citation2014, Duchman et al. Citation2015, Singh et al. Citation2015, Kallio et al. Citation2015, Bohl et al. Citation2016, Lubbeke et al. Citation2016). Procedure -related factors such as surgical approach, type of implant, fixation technique, and surgery time (Yasunaga et al. Citation2009, Lindgren et al. Citation2012) as well as hospital- and/or surgeon volume (Kreder et al. Citation1997, Solomon et al. Citation2002, Kaneko et al. Citation2014, Glassou et al. Citation2016, Kurtz et al. Citation2016, Laucis et al. Citation2016) are also suggested to influence outcomes after THA.
The association between annual volume for both hospitals and individual surgeon and AE and reoperations have been discussed during the last decade, not only for primary THAs, but also in knee arthroplasty surgery (Kreder et al. Citation2003), vascular procedures (Pearce et al. Citation1999), general surgical procedures and gynecological interventions (Muilwijk et al. Citation2007). Few studies have investigated the relation between surgeon’s annual volume and outcomes (both medical and surgical complication, reoperations, mortality, and patient-reported outcomes) following primary THAs. Most of these studies report an association between a higher annual volume and fewer AE (Kreder et al. Citation1997, Lavernia and Guzman. 1995, Katz et al. Citation2001, Citation2003, Losina et al. Citation2004, Paterson et al. Citation2010, Camberlin et al. Citation2011, Ravi et al. Citation2014, Koltsov et al. Citation2018). All of these published reports are based on patient cohorts in North America with the exception of Camberlin et al. (Citation2011) who studied a Belgian cohort of patients. There are, however, differences between countries with regards to training programs and level of surgeon activity. Second, there is a lack of publications adjusting for important confounders such as type of fixation, surgical approach, and time as orthopedic specialist. We evaluated possible associations between the surgeon’s annual volume and the risk of AE and mortality within 90 days following primary THA. We used data from a national quality register as well as hospital administrative data in Western Sweden, the second largest region in Sweden.
Patients and methods
Patient selection
Inclusion criteria for the study were: a primary THA either with a cemented, uncemented, or hybrid fixation technique in patients with index diagnosis osteoarthritis (OA) of the hip defined by the International Classification of Diseases (ICD)-10 codes M16.0–M16.7 or M16.9. All patients underwent surgery using a posterior or a direct lateral approach. We selected all surgeries performed in all hospitals managed by the county council of Western Sweden between 2007 and 2016 reported to the Swedish Hip Arthroplasty Register (SHAR) and the regional patient register, Vega ().
Sources of data
Hospital medical records, SHAR, and the regional patient register were used as data sources. The linking between hospital medical records and SHAR was done using the 10-digit personal identity number (PIN) (Ludvigsson et al. Citation2009), name of the hospital, and date of surgery. The linked dataset, containing information from hospital medical records and SHAR, was subsequently forwarded to the regional patient register to add all adverse events and the data were pseudonymized replacing the PIN with a unique identifier. For each operating surgeon involved, data on the year for license to practice and/or specialist certificate in orthopedics were obtained from publicly available data at the Swedish National Board of Health and Welfare’s register of licensed healthcare professionals (HOSP). The variable sources are detailed in , see Supplementary data.
Table 1. Source of included variables
The SHAR’s aim is to register all primary THAs and reoperations performed in Sweden. The coverage has been 100% over the last 25 years and the completeness of primary THAs exceeds 98% during the last 10 years (Kärrholm et al. Citation2016). Patient data, age, sex, height, weight, ICD-10 diagnoses, fixation technique, surgical approach, and type of implant are registered in the SHAR.
Vega, a regional patient register, was initiated in 2000. It is an aggregated database, containing records concerning all healthcare contacts (both public and privately funded) for all residents in the region. In 2006 this regional patient register contained records of about 12 million healthcare contacts for the population of approximately 1.3 million people. Vega provides information to the National Patient Register (NPR). The PIN is used as the unique identifier for all entries in Vega. The regional patient register contains details on: depiction of the caregiver at the point of contact such as, for example, level of hospital or elective care, diagnoses, and interventions such as, for example, type of surgery, and length of stay in the hospital.
Annual surgeon volume was defined as the number of primary THAs the operating surgeon performed in the 365 days prior to the index THA of interest (Ravi et al. Citation2014). Annual hospital volume was calculated as annual surgeon volume but based on number of primary THAs in the 365 days prior to the index THAs.
A direct acyclic graph was used to visualize and determine covariates of interest based on previous publications. The following covariates were identified as confounders and included in the multiple logistic regression analysis: age, sex, BMI, comorbidities, years in practice as orthopedic specialist at the time of the index THA, fixation technique, diagnostic indication for implantation, surgical approach, and annual hospital volume. Smoking was also identified as a confounder but was not included in the multiple logistic regression analysis due to lack of information on patient smoking habits over the whole investigated period (i.e., SHAR has not collected information during the entire investigated period).
The years in practice for each orthopedic specialist at the time of the index THA was defined as the difference between date for surgery and date of certification as orthopedic specialist.
The Elixhauser comorbidity index (ECI) is a comprehensive set of 30 comorbidities associated with substantial increase in length of stay, hospital charges, and mortality (Elixhauser et al. Citation1998, van Walraven et al. Citation2009). The ECI has been considered as a superior predictor for long-term outcomes (beyond 30 days) to the Charlson comorbidity index (Sharabiani et al. Citation2012). The period used for calculating ECI in this study was 365 days prior to the index THA. Comorbidities present in the 365 days prior to the index THA were used for calculating ECI.
An AE was defined as a readmission for a predefined set of World Health Organization International Classification of Diseases (ICD) and the Nordic Medico-Statistical Committee (NOMESCO) Classification of Surgical Procedures codes for interventions (Appendix, see Supplementary data). Death for any reason was also included in the definition of AE. The code list for AE has been elaborated by the Swedish Knee Arthroplasty Register (SKAR) in collaboration with the National Board of Health and Welfare to be used after knee replacements. Based on the same principles SHAR elaborated a code list adapted for elective hip replacements.
The codes were classified into the following groups; A = surgical procedure codes that include reoperations of THA implants and other procedures that may represent a complication, DA = diagnostic codes that imply surgical complications, DB/DB 2 = diagnostic codes that cover hip-related diseases that may have been used for complications after THA surgery, DC = diagnostic codes covering cardiovascular events that may be related to the surgery, DM/DM 2 = diagnostic codes concerning other medical events not related to the THA surgery but that may be related to the surgery if they occur shortly afterwards. A, DA, BD, and BD 2 in the Appendix are surgical complications (i.e., hip-related complications) and DC, DM, and DM 2 medical complications (i.e., serious cardiovascular or medical complications).
Statistics
SPSS version 25 (IBM Corp, Armonk, NY, USA) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis. We used both simple and multiple logistic regression. Data from the regressions are presented with regression coefficient (β-coefficient), 95% confidence interval (CI), and p-value. P-value for statistical significance was set at < 0.05. A predictive model was created to analyze risk of AE and mortality. Predicted risk was calculated using a fitted simple logistic regression model. Prediction intervals (PI) were calculated to see the prediction strength with a 95% prediction interval. The predicted risk of AE within 90 days is presented unadjusted with arbitrarily determined limits (0, 10, 20, 30, 40, and 50) for annual surgeon volume in .
Table 4. Predicted risk of AE within 90 days for annual surgeon volume of primary THAs
A sensitivity analysis was performed according to guidelines in statistical analysis of arthroplasty data to evaluate the consequence of violating the assumption of independent observation (i.e., analysis when the second hip was excluded in patients with bilateral THAs) (Ranstam et al. 2011).
Patients operated with simultaneous bilateral THAs were captured as 1 surgery in the study. As Ranstam et al. (2011) concluded based on a literature survey, there is little practical consequence of analyzing bilateral prostheses—at least with knee and hip data. We expect that the dependency structure of 2 hips from the same patients is stronger and of more consequence that the dependency structure of different patients having the same surgeon. THA surgery is a highly standardized procedure and as such we do not expect surgeon-related base risks and modelling approaches did not indicate such results.
Our primary outcome was AE within 90 days following the index THA surgery and our secondary outcome was mortality within 90 days following the index THA surgery.
Ethics, funding, and potential conflicts of interests
The study was approved by the Central Ethical Review Board in Stockholm (DNR Ö 9-2016). A research grant for the project was received from Skaraborgs Hospital research foundation. There is no conflict of interest.
Results
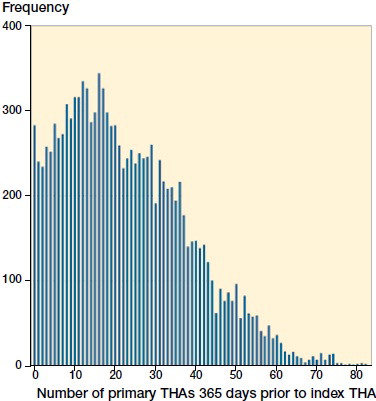
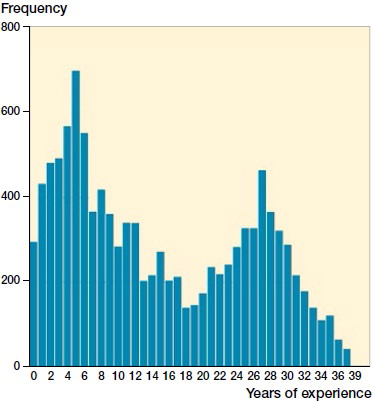
268 different surgeons performed the 12,100 operations of which 8% (989) were performed by orthopedic trainees. The median years in practice as an orthopedic specialist at the time of the index THA was 12 (0–40) (). The median annual surgeon volume was 23 primary THAs (0–82) 365 days prior to the THA of interest ().
Figure 2. Distribution of the experience of the surgeon at the time of the index THA. Experience is computed as years between orthopedic specialist certification and surgery. Note: There are 2 THAs for year 39 and 1 for year 40, not visible in the graph.
Mean age for all patients was 69 years (SD 11) and the proportion of females was 58%. Primary OA was the most common diagnosis (94%). Some 68% of the patients received a cemented THA followed by uncemented (); 45% of the patients had no comorbidities according to ECI 365 days preceding the index surgery ().
Table 2. Patient characteristica and surgical data
Table 3. Elixhauser comorbidity index 365 days prior to the index THA
Outcomes
Readmissions for any cause within 90 days occurred in 1,019 patients (8%) and with the AE definition used (see Appendix) the rate decreased to 818 (7%). In all, 69% of all AE could be classed as surgical complications and 31% as medical complications. For AE within 90 days the simple logistic regression showed a statistically significant reduced risk with increasing annual surgeon volume (regression coefficient = 0.990, CI 0.986–0.995). The corresponding numbers in the multiple regression were: regression coefficient = 0.992, CI 0.987–0.998. According to the predictive model the risk of an AE decreased by more than 35% if the surgeon had performed 50 or more THAs compared with 0 THAs during the 365 days preceding the index surgery ().
A total of 28 patients died within 90 days. The annual surgeon volume did not influence the risk of mortality in the simple regression (regression coefficient = 0.999, CI 0.974–1.022) or the multiple regression (regression coefficient = 1.000, CI 0.978–1.031). The prediction interval for mortality could not be calculated due to the low mortality rate.
The result of the sensitivity analysis is similar to the result including both hips. 1,093 surgeries were excluded and the sensitivity analysis contained 11,007 THAs. 70 patients were operated with simultaneous bilateral THAs. Data for the sensitivity analysis are not shown.
Discussion
We found that higher caseloads of annual THAs were associated with decreased level of AE within 90 days after surgery. This finding is supported by previous publications (Lavernia and Guzman Citation1995, Kreder et al. Citation1997, Katz et al. Citation2001, Losina et al. Citation2004, Paterson et al. Citation2010, Camberlin et al. Citation2011, Ravi et al. Citation2014). Based on previous publications it is difficult to understand what the optimal annual surgeon volume is in order to achieve low levels of AE and reoperation. Furthermore, annual surgeon volume can vary over time and by calculating the annual surgeon volume as the number of primary THAs performed 365 days prior to the index surgery we were able to capture this variation. This method has been used by Ravi et al. (Citation2014) in their study and might be a more correct estimation than using all THAs during a calendar year where all surgeries are attributed with the same volume regardless of whether the actual surgery being analyzed is the first or the last one during the measured year.
90-day mortality is rare following primary THA surgery in Sweden. The 0.2% mortality rate in our study is lower than the average mortality rates following primary THAs in 2 published systemic reviews (0.7% and 0.5%) (Singh et al. Citation2011, Berstock et al. Citation2014). Berstock et al. (Citation2014) included 7 studies on mortality within 90 days in their systemic review and in these the mortality rates varies between 0.1% and 0.7%. Ravi et al. (Citation2014) (not included in any of the systemic reviews) did not find any obvious relation between mortality within 90 days and surgeon volume despite higher mortality rates. An explanation of the lower mortality rates in our study compared with the systemic reviews might be that the Swedish THA patients are healthier than patients included in systemic reviews (i.e., a selection bias of patients undergoing THA surgery between countries and hospitals). Hence, mortality rates between different studies might not be generalized depending on differences in the organization of healthcare and individual surgical practices.
Our study has some limitations. We have not adjusted the multiple logistic regression for smoking, despite the knowledge of its negative influence on AE (Singh et al. Citation2015, Duchman et al. Citation2015). In our dataset, during the years 2013–2016, around 5% of patients were reported as smokers (information from the SHARs PROM program). Furthermore, data are missing on 17% included procedures, and finally the frequency of smoking is decreasing during the years 2013–2016. In spite of the fact that we have some information on smoking behavior in our study, we decided not to include smoking in the regression analysis because of the high amount of missing values. A second limitation is that only primary THAs performed within the region of Western Sweden were included. Some of the surgeons involved in the study might have had a temporary or partial employment, having performed primary THAs outside the investigated region. In Sweden there is no central dataset on surgeons, regarding their employment and activity. We presumed that the limited number of surgeons operating on cases outside the region of Western Sweden not would influence our conclusions. Finally, we share the same limitation as in all observational studies using administrative data. Both change of practice during the study period and local trends but also differences in registration might occur between the included hospitals. The regional patient register we used is not validated on its own but it provides data to the NPR. The Swedish National Inpatient Register (IPR) is part of the NPR. The IPR has been validated and contains 99% of all hospital discharges (Ludvigsson et al. Citation2011). In this study we used a definition of adverse events requiring hospital admission. Hence, we believe our data are robust and our conclusions are valid.
One strength is that we could control for the surgeon’s experience (i.e., years as orthopedic specialist) at the time of the index surgery. The Swedish National Board of Health and Welfare register of licensed healthcare professionals has the exact date of certification for all doctors applying for licenses to practice and orthopedic specialist certification. We decided to include years in practice in the regression model. We have previously shown that surgeons with longer experience operate on patients with different diagnoses, patient characteristics, and using other implants compared with less experienced surgeons (Jolbäck et al. Citation2018). Years as a recognized specialist in orthopedics might also be considered as a proxy for surgical skills accumulated by the experience of previous procedures during the surgeon’s career. But, also, the knowledge gained and experience of preparing patients both physically and mentally prior to the surgery can be of importance. More experienced surgeons are likely to make more appropriate decisions regarding the indication for surgery, the operative details (technical aspects), and other perioperative factors that could result in an improved outcome. By including the years in practice at the time of the index surgery in the analysis we were able to adjust for the above confounders. Another strength of the study is that we have been able to adjust for both surgical approach and type of fixation. To our knowledge, this is the first publication analyzing the risk of adverse events and mortality based on annual surgeon volume, adjusting for important confounders such as type of fixation, surgical approach, and time as orthopedic specialist. Finally, we used an administrative database registering all healthcare including readmission to hospitals in the whole of Sweden for the inhabitants of Western Sweden. This means that the risk of not collecting all readmissions within 90 days following the index THA is near to non-existent.
Analyzing 12,100 surgeries reported to the SHAR, we conclude that high annual surgical activity is associated with a reduced risk of AE within 90 days following primary THAs. Based on these findings, healthcare providers should consider planning for an increased surgeon volume.
Supplementary data
and the Appendix are available as supplementary data in the online version of this article, http://dx.doi.org/10.1080/17453674.2018.1554418
Supplemental Material
Download PDF (31.7 KB)PJ had the original idea for the study, processed the data, and prepared the first version of the manuscript. All authors took part in the planning of the study, analysis, and interpretation of the data, and in writing of the manuscript. All authors read and approved the final manuscript.
Acta thanks Sarah Whitehouse for help with peer review of this study.
- Arsoy D, Woodcock J A, Lewallen D G, Trousdale R T. Outcomes and complications following total hip arthroplasty in the super-obese patient, BMI > 50. J Arthroplasty 2014; 29(10): 1899–905.
- Berstock J R, Beswick A D, Lenguerrand E, Whitehouse M R, Blom A W. Mortality after total hip replacement surgery: a systematic review. Bone Joint Res 2014; 3(6): 175–82.
- Bohl D D, Sershon R A, Fillingham Y A, Della Valle C J. Incidence, risk factors, and sources of sepsis following total joint arthroplasty. J Arthroplasty 2016; 31(12): 2875–9.e2.
- Bozic K J, Lau E, Kurtz S, Ong K, Rubash H, Vail T P, Berry D J. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am 2012; 94(9): 794–800.
- Camberlin C, Vrijens F, De Gauquier K, Devriese S, Van De Sande S. Provider volume and short term complications after elective total hip replacement: an analysis of Belgian administrative data. Acta Orthop Belg 2011; 77(3): 311–19.
- Duchman K R, Gao Y, Pugely A J, Martin C T, Noiseux N O, Callaghan J J. The effect of smoking on short-term complications following total hip and knee arthroplasty. J Bone Joint Surg Am 2015; 97(13): 1049–58.
- Elixhauser A, Steiner C, Harris D R, Coffey R M. Comorbidity measures for use with administrative data. Med Care 1998; 36(1): 8–27.
- Glassou E N, Hansen T, Mäkelä K, Havelin L I, Furnes O, Badawy M, Kärrholm J, Garellick G, Eskelinen A, Pedersen A B. Association between hospital procedure volume and risk of revision after total hip arthroplasty: a population-based study within the Nordic Arthroplasty Register Association database. Osteoarthritis Cartilage 2016; 24(3): 419–26.
- Jolbäck P, Rolfson O, Mohaddes M, Nemes S, Karrholm J, Garellick G, Lindahl H. Does surgeon experience affect patient-reported outcomes 1 year after primary total hip arthroplasty? Acta Orthop 2018; 89(3): 265–71.
- Kallio P J, Nolan J, Olsen A C, Breakwell S, Topp R, Pagel P S. Anesthesia preoperative clinic referral for elevated hba1c reduces complication rate in diabetic patients undergoing total joint arthroplasty. Anesth Pain Med 2015; 5(3): e24376.
- Kaneko T, Hirakawa K, Fushimi K. Relationship between peri-operative outcomes and hospital surgical volume of total hip arthroplasty in Japan. Health Policy 2014; 117(1): 48–53.
- Kärrholm J, Lindahl H, Malchau H, Mohaddes M Rogmark C, Rolfsson O. The Swedish Hip Arthroplasty Register Annual Report; 2016, 2017.
- Katz J N, Losina E, Barrett J, Phillips C B, Mahomed N N, Lew R A, Guadagnoli E, Harris W H, Poss R, Baron J A. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am 2001; 83-A(11): 1622–9.
- Katz J N, Phillips C B, Baron J A, Fossel A H, Mahomed N N, Barrett J, Lingard E A, Harris W H, Poss, R, Lew R A, Guadagnoli E, Wright E A, Losina E. Association of hospital and surgeon volume of total hip replacement with functional status and satisfaction three years following surgery. Arthritis Rheum 2003; 48(2): 560–8.
- Koltsov J C B, Marx R G, Bachner E, McLawhorn A S, Lyman S. Risk-based hospital and surgeon-volume categories for total hip arthroplasty. J Bone Joint Surg Am 2018; 100: 1203–8.
- Kreder H J, Deyo R, Koepsell T, Swiontkowski M F, Kreuter W. Relationship between the volume of total hip replacements performed by providers and the rates of postoperative complications in the state of Washington. J Bone Joint Surg Am 1997; 79(4): 485–94.
- Kreder H J, Grosso P, Williams J I, Jaglal S, Axcell T, Wal E K, Stephen D J. Provider volume and other predictors of outcome after total knee arthroplasty: a population study in Ontario. Can J Surg 2003; 46(1): 15–22.
- Kurtz S M, Lau E C, Ong K L, Adler E M, Kolisek F R, Manley M T. Hospital, patient, and clinical factors influence 30- and 90-day readmission after primary total hip arthroplasty. J Arthroplasty 2016; 31(10): 2130–8.
- Lalmohamed A, Vestergaard P, Javaid M K, de Boer A, Leufkens H G, van Staa T P, de Vries F. Risk of gastrointestinal bleeding in patients undergoing total hip or knee replacement compared with matched controls: a nationwide cohort study. Am J Gastroenterol 2013; 108(8): 1277–85.
- Laucis N C, Chowdhury M, Dasgupta A, Bhattacharyya T. Trend toward high-volume hospitals and the influence on complications in knee and hip arthroplasty. J Bone Joint Surg Am 2016; 98(9): 707–12.
- Lawson E H, Hall B L, Louie R, Ettner S L, Zingmond D S, Han L, Rapp M, Ko C Y. Association between occurrence of a postoperative complication and readmission: implications for quality improvement and cost savings. Ann Surg 2013; 258(1): 10–18.
- Lavernia C J, Guzman J F. Relationship of surgical volume to short-term mortality, morbidity, and hospital charges in arthroplasty. J Arthroplasty 1995; 10(2): 133–40.
- Lindgren V, Garellick G, Kärrholm J, Wretenberg P. The type of surgical approach influences the risk of revision in total hip arthroplasty: a study from the Swedish Hip Arthroplasty Register of 90,662 total hip replacements with 3 different cemented prostheses. Acta Orthop 2012; 83(6): 559–65.
- Losina E, Barrett J, Mahomed N N, Baron J A, Katz J N. Early failures of total hip replacement: effect of surgeon volume. Arthritis Rheum 2004; 50(4): 1338–43.
- Lubbeke A, Zingg M, Vu D, Miozzari H H, Christofilopoulos P, Uckay I, Harbarth S, Hoffmeyer P. Body mass and weight thresholds for increased prosthetic joint infection rates after primary total joint arthroplasty. Acta Orthop 2016; 87(2): 132–8.
- Ludvigsson J F, Otterblad-Olausson P, Pettersson B U, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24(11): 659–67.
- Ludvigsson J F, Andersson E, Ekbom E, Feychting M, Kim J-L, Reuterwall C, Heurgren M, Otterblad-Olausson P. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450.
- Muilwijk J, van den Hof S, Wille J C. Associations between surgical site infection risk and hospital operation volume and surgeon operation volume among hospitals in the Dutch nosocomial infection surveillance network. Infect Control Hosp Epidemiol 2007; 28(5): 557–63.
- Paterson J M, Williams J I, Kreder H J, Mahomed N N, Gunraj N, Wang X, Laupacis A. Provider volumes and early outcomes of primary total joint replacement in Ontario. Can J Surg 2010; 53(3): 175–83.
- Pearce W H, Parker M A, Feinglass J, Ujiki M, Manheim L M. The importance of surgeon volume and training in outcomes for vascular surgical procedure. J Vasc Surg 1999; 29(5): 768-76.
- Ranstam J, Kärrholm J, Pulkkinen P, Makela K, Espehaug B, Pedersen A B, Mehnert F, Furnes O, Nara Study Group. Statistical analysis of arthroplasty data, II: Guidelines. Acta Orthopaedica 2011; 82(3): 258–67.
- Ravi B, Jenkinson R, Austin P C, Croxford R, Wasserstein D, Escott B, Paterson J M, Kreder H, Hawker G A. Relation between surgeon volume and risk of complications after total hip arthroplasty: propensity score matched cohort study. BMJ 2014; 348: g3284.
- Sharabiani M T, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care 2012; 50(12): 1109–18.
- Singh J A, Kundukulam J, Riddle D L, Strand V, Tugwell P. Early postoperative mortality following joint arthroplasty: a systematic review. J Rheumatol 2011; 38(7): 1507–13.
- Singh J A, Schleck C, Harmsen W S, Jacob A K, Warner D O, Lewallen D G. Current tobacco use is associated with higher rates of implant revision and deep infection after total hip or knee arthroplasty: a prospective cohort study. BMC Medicine 2015; 13: 283.
- Solomon D H, Losina E, Baron J A, Fossel A H, Guadagnoli E, Lingard E A, Miner A, Phillips C B, Katz J N. Contribution of hospital characteristics to the volume–outcome relationship: dislocation and infection following total hip replacement surgery. Arthritis Rheum 2002; 46(9): 2436–44.
- van Walraven C, Austin P, Jennings A, Quan H, Forster A J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009; 47(6): 626–33.
- Yasunaga H, Tsuchiya K, Matsuyama Y, Ohe K. High-volume surgeons in regard to reductions in operating time, blood loss, and postoperative complications for total hip arthroplasty. J Orthop Sci 2009; 14(1): 3–9.
Appendix 1
ICD 10 and NOMESCO codes used for definition of adverse events
DA Diagnosis for complications (ICD-10 codes). Whether they occur as main or secondary diagnosis for readmission
BD Diagnosis for hip-related complications (ICD-10 codes). Whether they occur as main or secondary diagnosis for readmission
BD2 Diagnosis for hip-related complications (ICD-10 codes). Whether they occur as main diagnosis for readmission
DC Diagnosis for serious cardiovascular complication (ICD-10 codes). Whether they occur as main or secondary diagnosis for readmission
DM Diagnosis of medical complication (ICD-10 codes). Whether they occur as main or secondary diagnosis for readmission
DM 2 Diagnosis of medical complication (ICD-10 codes). Whether they occur as main diagnosis for readmission
A Codes for hip surgery (NOMESCO). Whether they occur as main procedure during the readmission