Abstract
Background and purpose — The incidence of surgical site infections (SSIs) in trauma/orthopedic surgery varies between different body parts. Antibiotic prophylaxis (e.g., with cefazolin) lowers infection rates in closed fracture surgery and in primary arthroplasty. For prophylactic antibiotics to prevent infections, sufficient concentrations at the target site (location of surgery) are required. However, dosage recommendations and the corresponding efficacy are unclear. This review assesses target site cefazolin concentrations and the effect of variation in dose and location of target site during orthopedic extremity surgery.
Methods — For this meta-analysis and systematic review, the literature was searched using the following keywords: “cephalosporins,” “orthopedic,” “extremity,” “surgical procedures,” and “pharmacokinetics”. Trials measuring target site antibiotic concentrations (bone, soft tissue, synovia) during orthopedic surgery after a single dose of cefazolin were included.
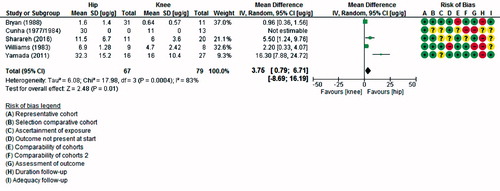
Results — The search identified 14 studies reporting on concentrations in the shoulder (n = 1), hip (n = 8), knee (n = 8), or foot (n = 1). A large variation was seen between studies, but the pooled results of 4 studies showed higher concentrations in hip than in knee (mean difference: 4 ug/g, 95% CI 0.8–7). Articles comparing different doses of cefazolin reported higher bone concentrations after 2 g than before, but pooling results did not lead to a statistically significant difference.
Interpretation — Although not all results could be pooled, this study shows that cefazolin concentrations are higher in the hip than in the knee. These findings suggest that the dose of prophylactic cefazolin might not be sufficient in distal parts of the extremity. Further research should investigate whether a higher dose of cefazolin can lead to higher concentrations and fewer SSIs.
A surgical site infection (SSI) is one of the most common complications of extremity surgery, especially when implants are involved. The infection rate ranges from 1.3% to 10% in hip and knee procedures (Agodi et al. Citation2017, De Jong et al. Citation2017) to 12% to 25% (Backes et al. Citation2014, Feilmeier et al. Citation2014, Wiewiorski et al. Citation2015) in foot and ankle surgery. Antibiotic prophylaxis is widely used and has been shown to lower infection rates in closed fracture surgery (Burnett et al. Citation1980, Boxma et al. Citation1996), as well as in primary arthroplasty (AlBuhairan et al. Citation2008, Voigt et al. Citation2015). Because of their broad-spectrum effect on methicillin-sensitive staphylococci and streptococci and relatively low costs, first-generation cephalosporins (e.g., cefazolin, cephradine, or cephalexin) are the recommended prophylactics in orthopedic/trauma surgery (Mangram et al. Citation1999, Bauer et al. Citation2017). However, there is limited evidence to support dosage recommendations in this field. The studies that form the foundation for the dosage, as mentioned in several international guidelines on surgical prophylaxis, do not include patients undergoing fracture/implant surgery (Bratzler et al. Citation2013, Moine and Fish Citation2013, Brill et al. Citation2014).
For prophylactic antibiotics to prevent infections it is necessary to achieve concentrations that exceed the minimum inhibitory concentration (MIC) of the targeted pathogen for at least the time between incision and closure of the wound (Burke Citation1961). The MIC is the serum concentration that an antibiotic should exceed to inhibit a certain pathogen (e.g., MIC of cefazolin for S. aureus is 0.5–2 µg/L, meaning that a cefazolin concentration in serum of 0.5–2 ug/L is necessary for adequate inhibition of S. aureus). Because drugs are not evenly distributed through the body, it is important to know that an antibiotic achieves sufficient concentrations not only in serum but also at the target site (location of surgery) (Müller et al. Citation2004). Data on target-site concentrations of cefazolin during orthopedic surgery of the extremities could provide us with the necessary information to assess and improve the efficacy of prophylactic cefazolin.
The aim of this systematic review was to answer the following questions:
What are the target-site concentrations of cefazolin in the extremities during orthopedic surgery?
What is the influence of location of the target site and dose of cefazolin on the target site concentration?
Methods
Search strategy and criteria
This meta-analysis and systematic review was performed according to the PRISMA statement (Moher et al. Citation2009) and registered in PROSPERO (no. CRD42018093697). A search was performed in MedLine (PubMed), EMBASE (Ovid), and the Cochrane Library. A clinical librarian was consulted on the search strategy. The search included the following keywords: “cephalosporins,” “orthopedic,” “extremity,” “surgical procedures,” and “pharmacokinetics” (see Supplementary data). The last search was run on January 15, 2018. In addition to the databases, bibliographies were checked for additional articles.
Eligible for inclusion were randomized controlled trials or prospective cohort studies investigating “target site” antibiotic concentrations in human, adult subjects who received prophylactic cefazolin in a single, intravenously administered dose before orthopedic/trauma surgery of the extremity. “Target site” concentrations were defined as concentrations measured in soft tissue, bone, synovia, or wound/drain fluid at or near the site of surgery. To be able to compare dosages, we chose to limit the type of administered prophylaxis to cefazolin only, the most widely studied first-generation cephalosporin. No publication date or language restrictions were imposed.
Study selection
All identified studies were screened for relevance based on title and abstract by 2 reviewers (TS and FS). The remaining studies were independently screened for eligibility based on full-text reading by the same reviewers and were included if none of the exclusion criteria were met. Studies were excluded based on intervention (cephalosporin that was not cefazolin), study design (reviews or articles only available as abstract), population (when included patients received therapeutic antibiotics up to a week before surgery or had peripheral vascular disease), and outcome (solely serum concentrations measured). Conflicts were discussed until consensus was reached.
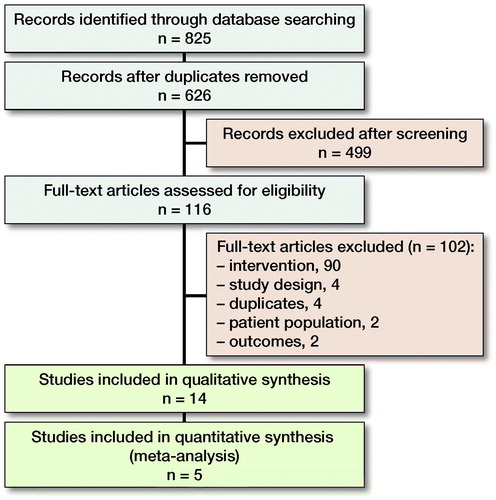
The literature search identified 825 articles, of which 626 were assessed for eligibility and a final number of 14 studies were included in the systematic review after full text screening (, ). 5 studies were also included in the meta-analysis (3 in the comparison of different target sites, 1 in the comparison of different cefazolin dosages, 1 in both).
Table 1. Study characteristics. Characteristics are reported for intervention groups (patients receiving cefazolin i.v. only) unless otherwise reported
Data collection
Data were extracted using a customized extraction sheet (based on the Cochrane data extraction template), which was pilot-tested on 5 articles randomly selected from the included articles and adjusted accordingly. One reviewer (FS) extracted the data and the other reviewer (TS) verified it. Duplicate publications were filtered out by juxtaposing author names and carefully reviewing study designs and treatment combinations. In the case of multiple publications on one trial, all published information was combined to ensure comprehensiveness of data. Collected information included (1) study characteristics (study design, number of patients included, in/exclusion criteria, and year published), (2) patient characteristics (sex, age, weight, type of procedure, given demographic or disease specifics), (3) type of intervention(s) (dose, timing of administration, comparative), (4) outcome (site of measurement, timing of measurement, unit presented as, type of analysis, results).
Study quality
Studies were screened for quality and risk of bias using the Newcastle–Ottawa Scale (NOS), designed to assess the quality of nonrandomized cohort studies (Wells et al. Citation2013). Using this scale studies were judged on 9 items within 3 domains (selection, comparability, and outcome) as either good, poor, or unclear, a “good” score counting as one point, with a maximum of 9 points. The rating sheet was adjusted to this review using topic-specific rating criteria (shown in Appendix). Quality screening was performed by one reviewer (FS) and subsequently checked by another reviewer (TS).
Due to heterogeneity of location of measurements, cefazolin dosages and measurement methods, assessment of publication bias—using for example a funnel plot—was not possible.
Statistics
All statistical analyses were performed using Review Manager (RevMan, Version 5.3, the Nordic Cochrane Centre, Copenhagen, the Cochrane Collaboration, 2014). Mean target site antibiotic concentrations were compared between different locations of the target site and between different dosages of cefazolin. To compare these groups, while taking the heterogeneity between studies into account, only studies describing 2 different doses/measuring sites were included in the meta-analysis. Only concentrations measured in bone were included for meta-analysis, since soft tissue samples were not comparable (different locations). Antibiotic concentrations were expressed as weighted mean difference and the corresponding 95% confidence interval (CI). Statistical analyses were performed using a random-effects model, considering the heterogeneity of included trials (Borenstein et al. Citation2010). The I2 was used as a measure for consistency of data. To incorporate the heterogeneity in the estimation of difference in concentrations between hip and knee, a 95% prediction interval (PI) was also computed. Where the CI only represents the average of study effects, the PI presents the range of expected results for 95% of similar studies that might be conducted in the future (Higgins et al. Citation2009). However, as Partlett and Riley (Citation2017) recently concluded, the PI performs best when heterogeneity is high but when there is also a minimum of 5 included studies. With a smaller amount of studies, the PI tends to be too wide, and should not be interpreted as true effect. Statistical significance was defined as p < 0.05.
Results
Included trials were mostly prospective cohorts (1, 3–5, 7–13, number of refs refers to numbers given in ), except for 3 randomized controlled trials (2, 6, 14). Most studies included patients undergoing elective total hip replacement (n = 3) (3, 8, 9), total knee replacement (n = 4) (1, 4, 6, 14) or both (n = 5) (2, 4, 10, 12, 13) (of which ref no. 4 compared their results in the knee with results in the hip from a previously reported trial (3) in their most recent article) and 1 each included patients with a femur fracture (11), shoulder surgery (7), or bunionectomy (5). gives an overview of the different target sites and given dose of cefazolin described by each study.
Table 2. Cefazolin dose and site of measurement of included studies
All studies reported target site antibiotic concentrations in bone and some also measured concentrations in soft tissue (n = 4) (6–8, 14) or synovial fluid (n = 1) (4). Target site concentrations were reported as a mean value in 10 studies (1, 2, 5, 7–10, 12–14), as mean peak value in 2 studies (3, 4), as separate values per patient in one study (11), and as percentage of patients with values above the MIC in 2 studies (6, 10). Due to the heterogeneity of included studies regarding cefazolin doses, sampling, and analysis methods, the majority of data could not be pooled.
Quality assessment
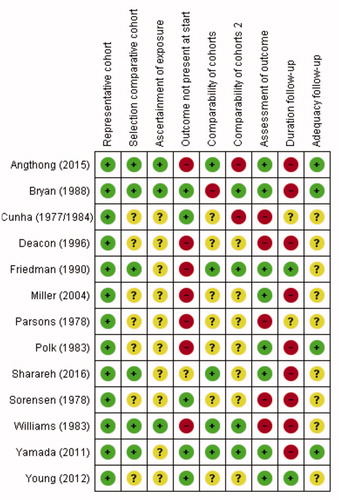
Although a minimal score on the NOS suggesting good quality has not been established, the overall risk of bias in the included studies seemed high, with only 5 studies scoring 5 or more out of 9 points on the NOS (Wells et al. Citation2013). The studies used in meta-analysis scored relatively high with 2 studies scoring seven stars and 1 each scoring 6, 5, and 4 points (, see Supplementary data).
Figure 2. Risk of bias analysis, according to Newcastle–Ottawa Scale (NOS) (Wells et alCitation2013).
+: low risk of bias/good quality,
–: high risk of bias/poor quality,
?: unknown risk of bias/unclear or not applicable (e.g., comparability of cohorts in studies where only one group received intravenously administered cefazolin).
A green “+” matches with a star on the NOS.
Target-site concentrations
Bone concentrations
Overall, target-site antibiotic concentrations in the bone ranged from 0.64 µg/g to 88 µg/g, but the variation of concentrations between different administered dosages of cefazolin and location of measurement was quite large. All of the 10 studies reporting a MIC () reported mean target site concentrations higher than the minimum concentration for S. aureus (0.5–2 µg/mL). However, most studies also described MICs for other organisms, usually requiring higher concentrations of cefazolin. In 5 studies, mean cefazolin concentrations were higher than all of the MICs they reported for different pathogens/resistance patterns, ranging from 0.5 ug/mL for S. aureus, to 3–4 µg/mL for E. coli and Klebsiella species (4, 5, 8, 10, 11). 2 studies specifically reported the percentage of patients achieving bone concentrations higher than the MIC. Friedman et al. (Citation1990) reported that 10 of 24 patients achieved bone concentrations higher than a MIC of 4 µg/mL at 30 minutes after administering 1g of cefazolin. Sharareh et al. (Citation2016), using a MIC of 2 µg/mL, found that in the group receiving 1 g of cefazolin, 3 of 4 reached the MIC compared with 25 of 27 in the group receiving 2 g.
Soft tissue concentrations
Cunha et al. (Citation1984) took samples of the synovia of the knee and found mean peak levels of 8 mg/L, which cannot be compared with measurements in bone due to the unit (mg/L vs. ug/g) and absence of standard deviations (, see Supplementary data). Miller et al. (Citation2004) found a mean cefazolin concentration of 11 µg/g in the soft tissue of the shoulder (SD not reported), which was lower than the 36 µg/g they found in bone at the same time-point. Young et al. (Citation2013) found values between 7 µg/g and 13 µg/g in fat around the knee joint at different time-points, comparable to the values measured simultaneously in bone. Friedman et al. (Citation1990), conversely, reported a higher percentage of patients with concentrations above the MIC (4 µg/g) in soft tissue than in bone of the knee at each time-point. Parsons et al. (Citation1978) also found higher levels in the hip capsule than in bone with mean concentrations of 35 µg/g (SD 7.2) and 14 (2.3) respectively.
Table 3. Soft tissue target site cefazolin concentrations
Location of target site
Mean (peak) antibiotic concentrations for different measuring sites were ranging from 1.6 µg/g to 88 µg/g in the hip, from 0.64 µg/g to 40 µg/g in the knee, 2.4 µg/g in the foot, and 36 µg/g in the shoulder, measured at varying time-points. The results of each individual study are displayed in and (see Supplementary data). shows that although concentrations in the knee were lower than in the hip, nearly all measured concentrations were higher than the MIC of S. aureus. The concentrations that were lower or only just above the MIC were measured either more than 100 minutes after administration or in the foot. In 5 studies concentrations in the hip and knee were compared (2, 4, 10, 12, 13). All reported higher concentrations measured in the hip than in the knee, which were statistically significant in 2 (10, 12). The other 3 studies either did not compare knee and hip directly or did not perform statistical testing for this comparison.
Figure 3. Mean target site concentrations organized according to location of measurements. Mean or maximum target site concentrations of all included studies. When results were reported separately for individual patients or results were given for multiple time-points, these are depicted separately. The bar with the dotted line represents the reported MIC90 of Staphylococcus aureus (0.5–2.0 µg/L).
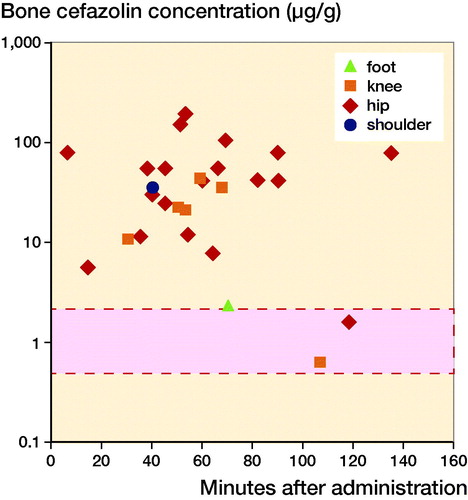
Table 4. Antibiotic concentrations organized by location of target site
Table 5. Antibiotic bone concentrations organized by dose
Of the 5 studies measuring antibiotic concentrations at different target sites, 4 (2, 10, 12, 13) could be pooled. The results from Cunha et al. (Citation1984) could not be included in the meta-analysis due to the fact that no standard deviations were provided. When pooled, target site cefazolin concentrations were significantly higher in the hip (acetabulum, femoral head, or proximal femur) than in the knee (distal femur or proximal tibia) with a mean difference of 4 µg/g (CI 0.8–7) (, see Supplementary data). Although the time-points at which concentrations were measured differed between studies, the time-points of measurements in hip and knee within each study were similar. Heterogeneity between the studies is high, with an I2 of 83%.
Dose of cefazolin
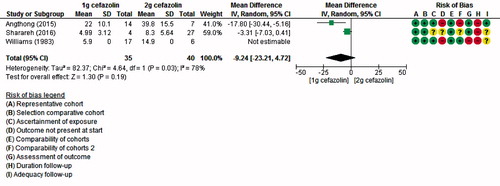
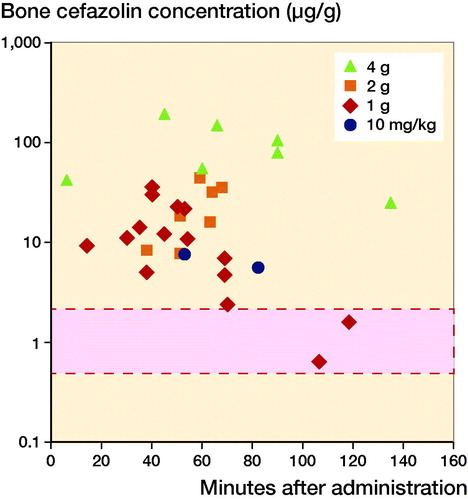
Bone concentrations were ranging from 0.64 µg/g to 36 µg/g when 1 g of cefazolin was given, 8.3 µg/g to 40 µg/g in 2 g, 10 µg/g to 88 µg/g in 4 g, and 7.7 µg/g in the study administering 10 mg/kg (, in Supplementary data). 3 studies compared target-site concentrations according to the given dose of cefazolin (either 1 g or 2 g) (1, 10, 12). All of these studies report higher levels for the group receiving 2 g of cefazolin, but only in 1 study statistical significance was achieved (1) (, see Supplementary data). shows that the concentrations lower or just above the MIC were all measured after administration of 1 g of cefazolin. Only 2 studies (1, 10) could be used for meta-analysis, because of missing standard deviations in the study by Williams et al. (Citation1983). As shown in , pooling the results did not lead to a statistically significant difference in target site concentration (mean difference –9, CI –23 to 4). Time-points of the measurements were similar both between and within the different studies. Nonetheless, heterogeneity between the studies was high with an I2 of 78%.
Figure 4. Mean target site concentrations organized according to dose of cefazolin. Mean or maximum target site concentrations of all included studies. When results were reported separately for individual patients or results were given for multiple time points, these are depicted separately. The bar with the dotted line represents the reported MIC90 of Staphylococcus aureus (0.5–2.0 µg/L).
Discussion
Because of the large clinical implications of SSIs in orthopedic trauma surgery, prophylactic antibiotics are widely initiated. However, dosage recommendations and corresponding efficacy remain unclear, partly because of the unequal distribution of drugs throughout the body. Sufficient concentrations of antibiotics at the target site (location of surgery) are required for optimal infection prevention. Therefore this meta-analysis focused on the target-site concentrations of cefazolin, the most commonly used prophylactic in orthopedic/trauma surgery.
A few limitations can be pointed out for this review and the available literature. First of all, the quality of the individual studies included in this systematic review varied. Most studies presented data that were collected before the year 2000, which often resulted in incomplete reporting and possibly outdated analysis methods. Also, given the fact that most studies included only elective orthopedic surgery, selection bias, by including only relatively healthy patients, may have occurred. Second, given the large heterogeneity in methods used for sampling, timing of the samples, processing, and analyzing, less than half of the results could be pooled. Third, target-site concentrations of an antibiotic may also be influenced by other patient or surgical characteristics such as renal function, obesity, bleeding, or tourniquet use. Although most of these characteristics do not seem to differ within study groups, tourniquet use could have an influence on the comparison between samples of hip and knee. Another limitation is the fact that all of the individual studies measured concentrations in samples of bone/soft tissue, the so called “whole tissue concentration.” As described by Mouton et al. (Citation2008), these concentrations are only an estimate of the “unbound” or active part of the drug and cannot be credibly compared with the MIC, which is the total (unbound plus plasma protein bound) concentration in serum. Moreover, homogenizing or grinding up whole-tissue samples leads to dilution of the drug by mixing intracellular and extracellular fluids, resulting in, depending on the type of drug, under- or overestimation of its concentrations (Mouton et al. Citation2008). Nevertheless, simply using the serum concentration as a surrogate might not be sufficient for estimating effect, since the distribution of drugs throughout the body is not homogeneous (Müller et al. Citation2004). Finally, regarding the clinical implications of antibiotic prophylaxis, more than the concentration itself, the time that the concentration is higher than the MIC (T > MIC) is important. The T > MIC should at least overlap with the “decisive period.” This is the period that starts at incision and ends after 3 hours, during which antibiotics can effectively suppress the development of a wound infection (Burke Citation1961). Instead of reporting this T > MIC, all included studies solely measured antibiotic concentrations at individual moments in time. When samples are taken at multiple time-points, they can be used to predict levels of antibiotics in tissue over a course of time, using population kinetics, like Gergs et al. (Citation2014). With population kinetics, model predicted time-concentration curves for each patient can be fabricated, which allows the evaluation of the T > MIC, and therefore a more precise estimate of clinical efficacy.
We found a large variation in target site cefazolin concentrations of the extremity between different studies. In general, the achieved concentrations in bone surpassed the minimal inhibitory concentration (MIC) for S. aureus, the most common pathogen of an SSI. However, when stratifying the results on the location of the target site and dose of cefazolin, some measurements did not reach this MIC.
Regarding the association between location of the target site and antibiotic concentrations, our study showed that the same dosage of cefazolin resulted in statistically significantly lower concentrations in the knee than in the hip. Although, with just a single study in the foot (Deacon et al. Citation1996), no definite conclusions can be drawn, this could mean that for surgery of the more distal parts of the extremity (e.g., foot/ankle), 1 g of cefazolin is not sufficient as prophylaxis. Whether or not a higher dose is beneficial in this area is yet to be determined.
As for the relationship between cefazolin dose and concentration, all 3 articles that compared different dosages found higher concentrations when 2 g was given instead of 1 g, although only 1 with statistical significance. A visualization of these concentrations, including also the studies investigating only one dose, suggests that the higher the dose, the higher the concentration (). However, even though concentrations seemed increasingly high, the clinical implications of this phenomenon have not been investigated. A ceiling effect, where higher concentrations would not lead to fewer SSIs, is likely to occur at a certain time and could pave the way for antimicrobial resistance.
This first systematic review shows that there is a large variation in target site cefazolin concentrations of the extremity between different studies. In general, the achieved concentrations in bone surpassed the minimal inhibitory concentration (MIC) for S. aureus, the most common pathogen of an SSI. Most importantly, we found that the local concentration of cefazolin is associated with the location of the target site. Although no definite conclusions can be drawn based on this study, a higher dose of cefazolin seems to produce higher whole-tissue concentrations. These insights could be helpful on the path towards more efficient use of antibiotics. In particular, this study gives rise to the question whether the dose of prophylactic cefazolin needs to be adjusted to the location of the target site. To make any recommendations for the dose of prophylactic cefazolin in orthopedic/trauma surgery of the extremity however, additional prospective research is needed. We believe that a preclinical trial, comparing multiples dosages and locations of measurement in the extremity, is necessary before further investigating the efficacy of prophylactic cefazolin in preventing SSIs.
Supplementary data
and and are available as supplementary data in the online version of this article, http://dx.doi.org/ 10.1080/17453674.2019.1577014
Supplemental Material
Download PDF (514.7 KB)TS conceived of the presented idea; FS and TS performed the search, screened and analyzed the articles; FS drafted the manuscript; TS, JCG, and RM revised the manuscript critically for intellectual content.
The authors would like to thank Faridi S. van Etten-Jamaludin, clinical librarian, for her help with the literature search.
Acta thanks Anna Stefánsdóttir for help with peer review of this study.
- Agodi A, Auxilia F, Barchitta M, Cristina M L, D’Alessandro D, Mura I, Nobile M, Pasquarella C, GISIO-SItI. Risk of surgical site infections following hip and knee arthroplasty: results of the ISChIA-GISIO study. Ann DI Ig Med Prev E DI Comunita 2017; 29(5): 422–30.
- AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br 2008; 90(7): 915–19.
- Angthong C, Krajubngern P, Tiyapongpattana W, Pongcharoen B, Pinsornsak P, Tammachote N, Kittisupaluck W. Intraosseous concentration and inhibitory effect of different intravenous cefazolin doses used in preoperative prophylaxis of total knee arthroplasty. J Orthop Traumatol 2015; 16(4): 331–4.
- Backes M, Schepers T, Beerekamp M S H, Luitse J S K, Goslings J C, Schep N W L. Wound infections following open reduction and internal fixation of calcaneal fractures with an extended lateral approach. Int Orthop 2014; 38(4): 767–73.
- Bauer M P, van de Garde E M W, van Kasteren M E E, Prins J, Vos M. SWAB Richtlijn: [peri-operatieve profylaxe. [Dutch] 2017; (January): 1–10.
- Borenstein M, Hedges L V, Higgins J P T, Rothstein H R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1(2): 97–111.
- Boxma H, Broekhuizen T, Patka P, Oosting H. Randomised controlled trial of single-dose antibiotic prophylaxis in surgical treatment of closed fractures: the Dutch Trauma Trial. Lancet 1996; 347(9009): 1133–7.
- Bratzler D W, Dellinger E P, Olsen K M, Perl T M, Auwaerter P G, Bolon M K, Fish D N, Napolitano L M, Sawyer R G, Slain D, Steinberg J P, Weinstein R A. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Heal Pharm 2013; 70(3): 195–283.
- Brill M J E, Houwink A P I, Schmidt S, Van Dongen E P A, Hazebroek E J, Van Ramshorst B, Deneer V H, Mouton J W, Knibbe C A J. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother 2014; 69(3): 715–23.
- Bryan C S, Morgan S L, Caton R J, Lunceford E M. Cefazolin versus cefamandole for prophylaxis during total joint arthroplasty. Clin Orthop Relat Res 1988; (228): 117–22.
- Burke J F. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961; 50: 161–8.
- Burnett J W, Gustilo R B, Williams D N, Kind A C. Prophylactic antibiotics in hip fractures: a double-blind, prospective study. J Bone Joint Surg Ser A 1980; 62(3): 457–62.
- Cunha B A, Gossling H R, Pasternak H S, Nightingale C H, Quintiliani R. The penetration characteristics of cefazolin, cephalothin, and cephradine into bone in patients undergoing total hip replacement. J Bone Joint Surg Am 1977; 59(7): 856–9.
- Cunha B A, Gossling H R, Pasternak H S, Nightingale C H, Quintiliani R. Penetration of cephalosporins into bone. Infection 1984; 12(2): 80–4.
- Deacon J S, Wertheimer S J, Washington J A. Antibiotic prophylaxis and tourniquet application in podiatric surgery. J Foot Ankle Surg 1996; 35(4): 344–9.
- De Jong L, Klem T M A L, Kuijper T M, Roukema G R. Factors affecting the rate of surgical site infection in patients after hemiarthroplasty of the hip following a fracture of the neck of the femur. Bone Joint J 2017; 99B(8): 1088–94.
- Feilmeier M, Dayton P, Sedberry S, Reimer R A. Incidence of surgical site infection in the foot and ankle with early exposure and showering of surgical sites: a prospective observation. J Foot Ankle Surg 2014; 53(2): 173–5.
- Friedman R J, Friedrich L, White R L, Kays M B, Brundage D M, Graham J. Antibiotic prophylaxis and tourniquet inflation in total knee arthroplasty. Clin Orthop Relat Res 1990; (260): 17–23.
- Gergs U, Clauss T, Ihlefeld D, Weiss M, Pönicke K, Hofmann G O, Neumann J. Pharmacokinetics of ceftriaxone in plasma and bone of patients undergoing hip or knee surgery. J Pharm Pharmacol 2014; 66(11): 1552–8.
- Higgins J P T, Thompson S G, Spiegelhalter D J. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172(1): 137–59.
- Hozo S P, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13.
- Mangram A J, Horan T C, Pearson M L, Silver L C, Jarvis W R. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 1999; 20(4): 250–78; quiz 279-80.
- Miller B S, Harper W P, Hughes J S, Sonnabend D H, Walsh W R. Regional antibiotic prophylaxis in elbow surgery. J Shoulder Elb Surg 2004; 13(1): 57–9.
- Moher D, Liberati A, Tetzlaff J, Altman D G, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339(17): b2535.
- Moine P, Fish D N. Pharmacodynamic modelling of intravenous antibiotic prophylaxis in elective colorectal surgery. Int J Antimicrob Agents 2013; 41(2): 167–73.
- Mouton J W, Theuretzbacher U, Craig W A, Tulkens P M, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother 2008; 61(2): 235–7.
- Müller M, Dela Peña A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother 2004; 48(5): 1441–53.
- Parsons R L, Beavis J P, David J A, Paddock G M, Trounce J R. Plasma, bone, hip capsule, and drain fluid concentrations of cephazolin during total hip replacement. Br J Clin Pharmac 1978; 5: 331–6.
- Partlett C, Riley R D. Random effects meta-analysis: coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med 2017; 36(2): 301–17.
- Polk R, Hume A, Kline B J, Cardea J. Penetration of moxalactam and cefazolin into bone following simultaneous bolus or infusion. Clin Orthop Relat Res 1983; 177(7): 216–21.
- Sharareh B, Sutherland C, Pourmand D, Molina N, Nicolau D P, Schwarzkopf R. Effect of body weight on cefazolin and vancomycin trabecular bone concentrations in patients undergoing total joint arthroplasty. Surg Infect (Larchmt) 2016; 17(1): 71–7.
- Sørensen T S, Colding H, Schroeder E, Rosdahl V T. The penetration of cefazolin, erythromycin and methicillin into human bone tissue. Acta Orthop 1978; 49(6): 549–53.
- Voigt J, Mosier M, Darouiche R. Systematic review and meta-analysis of randomized controlled trials of antibiotics and antiseptics for preventing infection in people receiving primary total hip and knee prostheses. Antimicrob Agents Chemother 2015; 59(11): 6696–707.
- Wells G A, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst 2013; (3): 1–4.
- Wiewiorski M, Barg A, Hoerterer H, Voellmy T, Henninger H B, Valderrabano V. Risk factors for wound complications in patients after elective orthopedic foot and ankle surgery. Foot Ankle Int 2015; 36(5): 479–87.
- Williams D N, Gustilo R B, Beverly R, Kind A C. Bone and serum concentrations of five cephalosporin drugs: relevance to prophylaxis and treatment in orthopedic surgery. Clin Orthop Relat Res 1983; (179): 253–65.
- Yamada K, Matsumoto K, Tokimura F, Okazaki H, Tanaka S. Are bone and serum cefazolin concentrations adequate for antimicrobial prophylaxis? Clin Orthop Relat Res 2011; 469(12): 3486–94.
- Young S W, Zhang M, Freeman J T, Vince K G, Coleman B. Higher cefazolin concentrations with intraosseous regional prophylaxis in TKA knee. Clin Orthop Relat Res 2013; 471(1): 244–9.