Abstract
Aims: To evaluate the outcome of different types of polyethylene, bone cements and one design of uncemented fixation with porous and ceramic coating using radiostereometry, bone densitometry, conventional radiography and clinical parameters.
Materials and methods: Study 1: 201 patients were extracted from 5 prospective randomised studies to evaluate femoral head penetration at two years with radiostereometry in four basic designs, cemented Lubinus and Reflection cups, uncemented Trilogy and Reflection cups. Studies II and III. 60 patients (61 hips) were randomised to receive either highly cross-linked or conventional all PE cups. 32 patients with bilateral arthrosis received hybrid THA with highly crosslinked PE on one side and conventional on the contra lateral side. Femoral head penetration and the migration of the cups were evaluated with radiostereometry in the supine and standing positions. DEXA and conventional radiography were used to evaluate the bone mineral density and radiolucencies around the cemented acetabular component. Studies IV and V: 90 patients (97 and 96 hips respectively) were stratified depended on age, gender, diagnosis and preoperative BMD to create 3 main groups of socket fixation. In the first group fluoride containing cement was used, in the second group Palacos cum Gentamicin and in the third hybrid THA with porous coated HA/TCP cup. In the hybrid group the fixation of the femoral component was again randomised to either of the two cements. The results on femoral and acetabular sides are presented separately in studies IV and V, respectively.
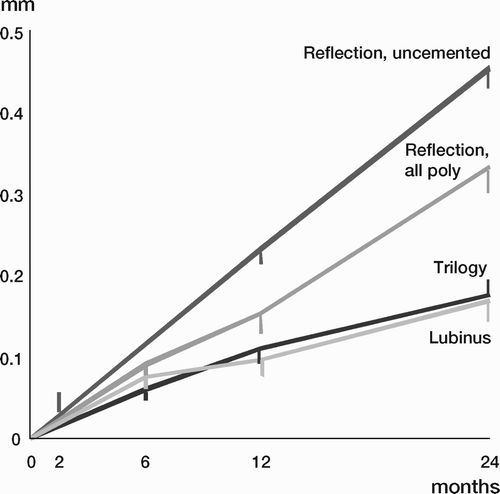
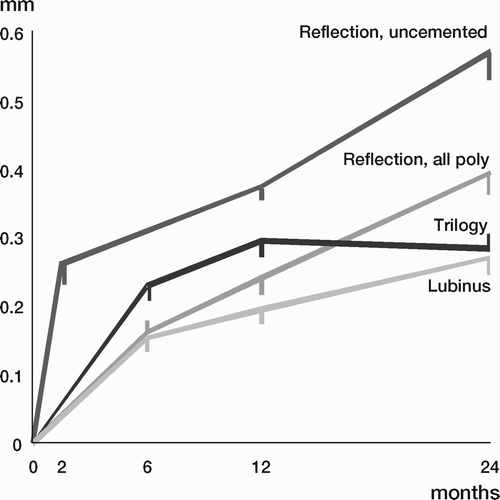
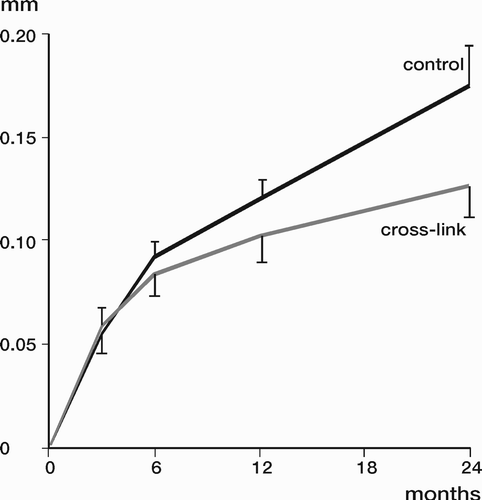
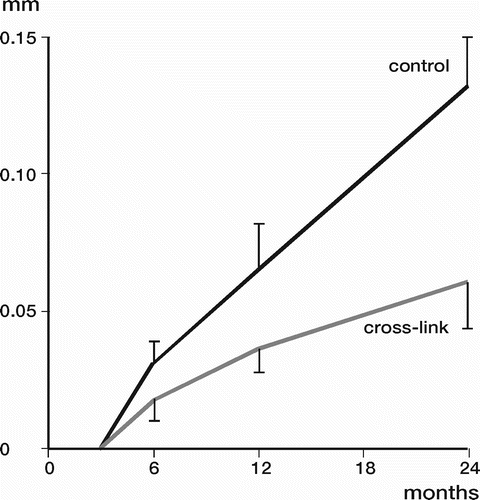
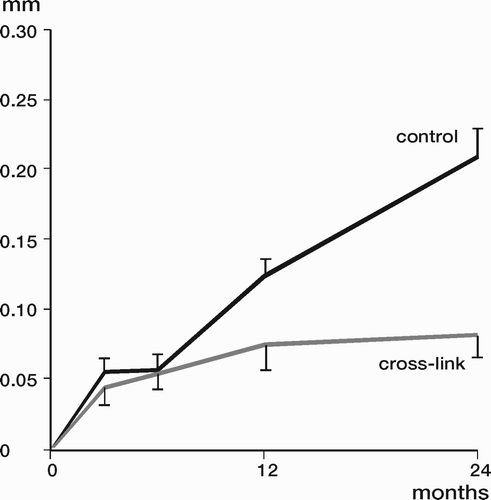
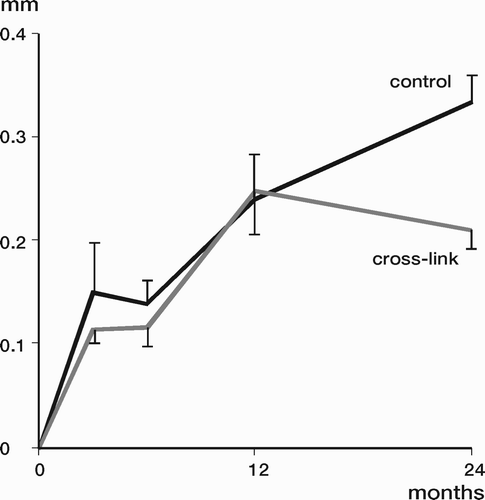
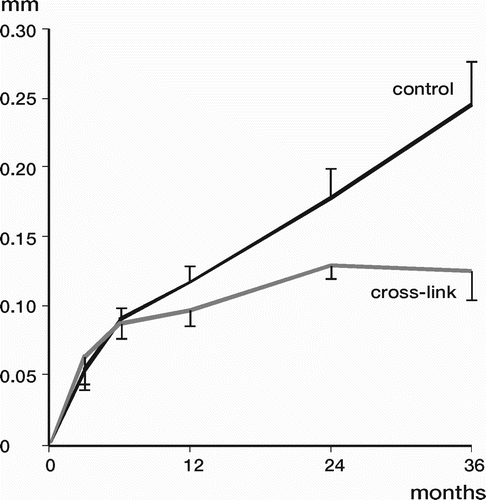
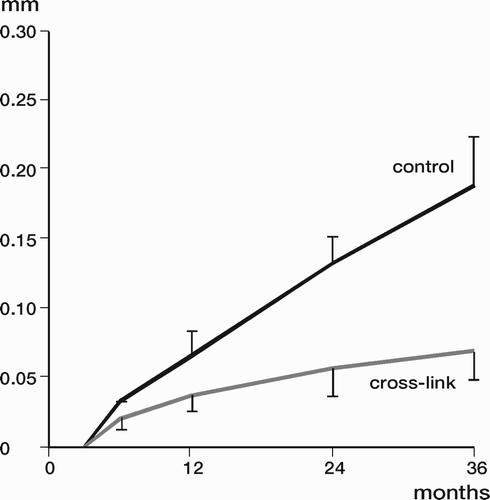
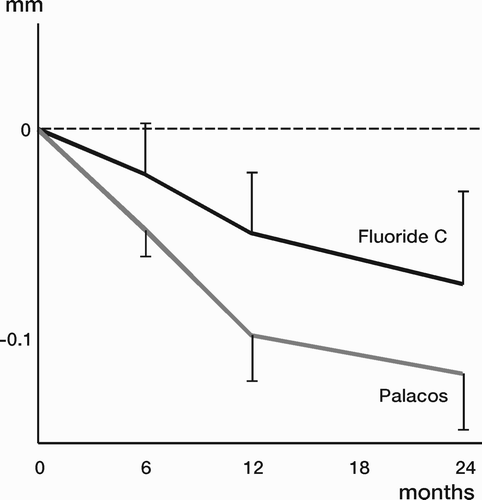
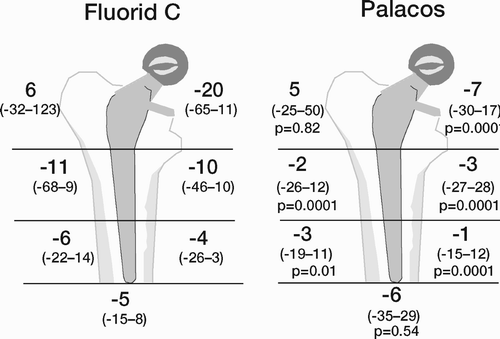
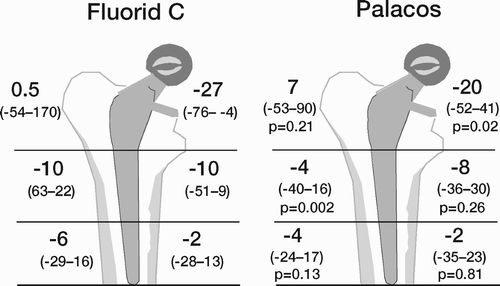
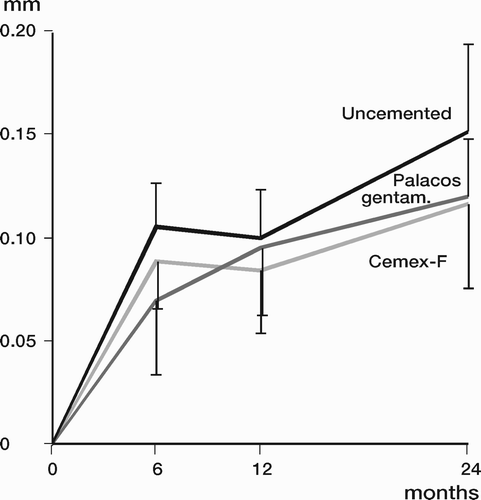
Results: Study I: Cups with polyethylene sterilized in EtO had almost twice the proximal and 3D penetration rates compared with gamma-sterilized polyethylene. Regression analysis showed that the type of sterilization, age and weight was the most important factors affecting the penetration rate. Studies II and III: In the cemented study the proximal penetration was lower in the study group independent of position at 3 years, while in the hybrid study the penetration was lower in the study group only in the supine position at 2 years. The migration of the cup did not differ between the plastics in both studies. At 2 years the periprosthetic radiolucency and BMD did not differ significantly between the 2 types of PE used in the cemented study. Study IV: The subsidence of the stem did not differ between the groups, but the periprosthetic BMD decreased more in fluoride cement group at 2 years. Conventional radiography revealed higher progression of radiolucent lines in the Palacos group, but only in one region. Study V: The proximal migration of the cup was almost similar in all three groups. The three dimensional migration was increased in patients with osteoporosis. Postoperative radiolucent lines tended to disappear with use of porous coating covered with HA/TCP.
Conclusions : Study I: EtO sterilized polyethylene increased the femoral head penetration. Age and weight were also important predictors of the penetration rate. Studies II and III. The highly cross-linked polyethylene decreased the penetration rate mainly after one year probably reflecting less wear. The different mechanical properties of the two types of PE studied did not affect the early fixation of the cemented cup. Study IV: There were no obvious advantage of addition of fluoride to acrylic bone cement when used to fixate the femoral component. Study V: Use of fluoride containing cement or uncemented fixation did not improve the early stability of the socket compared to Palacos with Gentamicin.
| Abbreviations | ||
| ap | = | anterior–posterior |
| BABC | = | bioactive bone cement |
| BMD | = | bone mineral density |
| CT | = | Computer Tomography |
| DEXA | = | Dual energy xray absorptiometry |
| EBRA | = | ein bild roentgen analyse |
| GUR | = | “Granular” UHMWPE and “Ruhrchemie” |
| HA | = | hydroxyapatite |
| HG | = | Harris–Galante |
| HMWPE | = | high molecular weight polyethylene |
| MMA | = | methylmethacrylate |
| PE | = | polyethylene |
| PMMA | = | polymethylmethacrylate |
| RLL | = | radiolucent lines |
| ROI | = | region of interest |
| RSA | = | radiostereometric analysis |
| SEM, SE | = | standard error of mean |
| Ti–6Al–4V | = | titanium–vanadium–aluminum alloy, “tivanium” |
| THA | = | total hip arthroplasty |
| TCP | = | tricalcium phosphate |
| UHMWPE | = | ultrahigh molecular weight polyethylene |
Introduction
The cemented total hip replacement (THR) was introduced in 1961. Since then total joint replacement has become widely recognized as one of the most cost-effective interventions in medicine. The effects of THR in terms of reduction of pain and improvement of function and health related quality of life are well documented in the literature. Worldwide more than 1,000,000 patients are treated with a hip replacement annually. In Sweden, the corresponding number is approximately 13,000. The results have improved over time, as reflected by a relative decrease of clinical failures requiring reoperation.
Survival rates of 90 to 95% after 10 years and 80 to 90% after 20 years are reported especially among elderly patients. In younger patients and those with compromised bone quality the results are inferior. Previous investigations have shown that the pathophysiology and incidence of implant failure show important differences between the two principal components of a total hip arthroplasty, the acetabular and the femoral components.
Aseptic loosening is the major complication and causes more than 70% of the revisions in hips in Sweden. The etiology of aseptic loosening is multifactorial, involving both mechanical and biological processes. Socket micromotion and wear are important factors that influence the long-term durability of THRs. The expected annual wear in PE cups or liners is approximately 0.1 mm. This means that billions of submicron-sized wear particles are released into the joint. These particles have been recognized as a major reason for osteolysis and failure.
During the past decade, high penetration rates have been related to variations in polyethylene quality and sterilization techniques. Different sterilization methods may have variable influence on the mechanical, chemical and wear properties of the polyethylene. Hip simulator studies have shown that highly irradiated polyethylene displays significantly improved wear resistance. This new polyethylene material has been extensively used in hip arthroplasty. However the clinical experience with such materials is limited.
Several studies have shown that micromovements of the implant and cement mantle open the interfaces, increase release of particles due to abrasive wear and may lead to asymmetrical loading of the cement mantle and subsequent debonding, cement fracture and loosening. Bioactive bone cements based on polymethylmethacrylate have been suggested as one way of improving implant fixation. So far these cements lack proper clinical documentation.
New implants must be evaluated before being introduced clinically on a large scale. Implant register studies are comparatively slow and blunt instruments. Conventional radiography adds important information about bone morphology, but suffers from limited reproducibility and resolution for measurements of migration and wear.
So far the method of choice to measure these parameters is radiostereometric analysis (RSA). With its high resolution this method can be used to detect small increases of migration and wear early during the postoperative years and in small patient populations. Such information has proved to be important in predicting later failures, which makes RSA a suitable method for the evaluation of new implants. Promising designs can be identified early and studied further. The patient population exposed to new implant technology can thus be limited. Implants more likely to cause complications can be identified early. The possibility of predicting failures before they cause clinical symptoms will facilitate their treatment, preventing the severe destruction of bone caused by loosening. RSA is therefore a valuable tool for the clinical study of new implants when preclinical tests have indicated that they might have superior performance compared to contemporary standards.
Conventional radiography also lacks resolution in measurements of skeletal bone mineral density. Therefore other methods such as dual energy Xray absoptiometry (DEXA) have evolved as alternatives for the study of bone remodelling around joint implants. Preferably the artificial joint should load the surrounding bone as physiologically as possible to maintain bone stock, reduce the risk of fatigue fracture and facilitate any future revision.
In this thesis RSA, DEXA, conventional radiography and clinical instruments were used to evaluate the efficacy of new polymer materials when used in total hip arthroplasty. Special emphasis was placed on their potential to reduce wear and improve fixation and bone remodelling around the prosthesis.
Evaluation of THR
The results of total hip replacement (THR) surgery have been studied using a range of outcome instruments which focus on pain, function and activity, quality of life, radiographic evaluation and need for revision of the implant. These instruments are important in evaluating the efficacy of hip arthroplasty and a firm basis for evidence-based health care.
Outcome measurements
There are two main types of outcome instruments or questionnaires:
Disease-specific questionnaires include questions about one specific disease ideally minimise the influence from associated diseases. Such instruments have a higher reliability than healthrelated questionnaires.
Different score systems have been used to describe the state of the patient in terms of pain, motion and walking ability (D'Aubigney and Postel Citation1954, Charnley Citation1979). To provide generally applicable systems for various hip disabilities and methods of treatment, Harris (Citation1969) introduced a rating scale, which included parameters such as pain, function, deformity and motion. The Harris Hip Score is the most frequently used disease-specific, but not self-administered, hip scoring system. In recent years self-administered scoring systems like the Westem Ontario and McMaster University Osteoarthritis Index (WOMAC) have become more popular (Bellamy et al. Citation1988). The WOMAC is a disease-specific, self-administered, health measure developed to study patients with osteoarthritis of the hip or knee. The original version contained 41 items in 5 domains: pain, stiffness, physical function, social function and emotional function. The final form of this index utilised the pain (5 questions), stiffness (2 questions) and physical function (17 questions) domains (Bellamy et al. Citation1988, Sun et al. Citation1997).
General or generic questionnaires include questions about health-related quality of life [HRQL), which facilitates comparison between the disabling effect of different medical conditions and their treatments. Two tests which are commonly used after THA are the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and the Nottingham Health Profile (NHP) with 36 and 45 questions respectively. The SF-36 is a general self-administered questionnaire developed for applications in psychometric theory. This test is the most frequently used health-status measure in Northern America and has been used after total joint replacement of the hip and knee (Martin et al. Citation1997, Ritter et al. Citation1995). The 36 questions are divided into eight domains: physical function, social function, role-emotional, role-physical, bodily pain, general health, mental health and vitality. The NHP is a self-administered general instrument, which consists of two parts with 45 yes-or-no answers. This test studies the quality of life after medical and/or surgical treatments such as total hip replacement (Wiklund and Romanus Citation1988). Like the SF-36 and WOMAC, the NHP has high validity and reliability (Hunt et al. Citation1981).
Conventional radiography
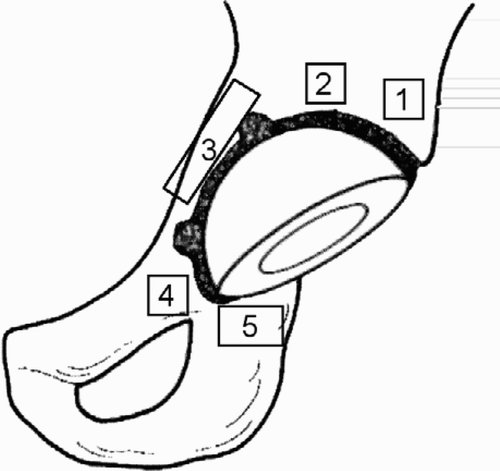
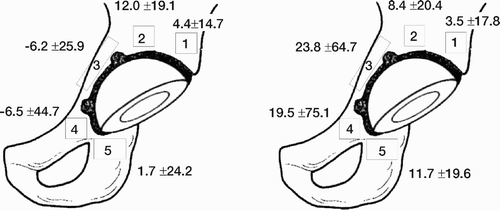
Because radiographic changes often precede clinical symptoms and more or less severe complications, conventional radiography is frequently used to evaluate total hip replacements. The aim is to evaluate implant stability, wear of the components and the response of the surrounding bone over time. The magnification, exposure parameters and patient position should be as consistent as possible between successive examinations to facilitate the evaluation. To obtain a systematic and standardised evaluation the interface around the cup is divided into three regions on the anteroposterior and the lateral view (DeLee and Charnley Citation1976). The femoral stem interface is analysed in 14 corresponding regions, 7 on the AP and 7 on the lateral view (Gruen et al. Citation1979, ).
Figure 1. The regions (zones) used in the analysis of cup and stem interfaces (AP and lateral projections).
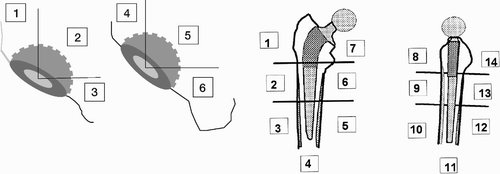
The presence of postoperative radiographic findings such as bone-remodelling, radiolucent lines (zones), erosions, granulomae or osteolytic lesions is defined and described in the different areas. Such changes have prognostic value for mechanical loosening. (Kobayashi et al. Citation1997). Computer Tomography (CT) scans can be used to visualise osteolytic lesions in the pelvis but normally have no place in the screening of hip implants. Barrack et al. (Citation1992) proposed a grading of the quality of the cement mantle surrounding the femoral component, which has become widely accepted. A similar grading has since than been used to assess the cement mantle around the acetabular component (Strömberg Citation1995).
Evaluation of stability
The simplest way to assess migration of the acetabular cup is to make direct measurements on radiographs using a ruler or preferably a slide-cali-per. With increasing use of digital radiography, new software packages have been introduced with varying possibilities to replace manual measurements. Various landmarks are used to construct reference lines in order to improve the accuracy of measurements. Sutherland et al. (Citation1982) used the teardrop line and Köhler’s line as references for measuring changes in cup position. Thoren and Sahlstedt (Citation1990) proposed the use of only one line: a tangent to the apertura pelvis. Nunn et al. (Citation1989) suggested use of the teardrop line and an additional perpendicular line through the teardrop. Wetherell et al. (Citation1989) proposed use of the “obturator brim line” and the “sacroiliac-symphysis line” as references.
Two-dimensional migration can be calculated from consecutive radiographs using reference points in the skeleton and implant landmarks. Some authors reported that vertical migration can be measured with a precision of 1–3 mm (Freeman and Plante-Bordeneuve Citation1994, Walker et al. Citation1995), but it is uncertain whether this can be achieved when anatomical landmarks are used in a prospective study. In a comparison between measurement on digitised radiographs and radiostereometry, Malchau et al. (Citation1995) reported an accuracy of vertical cup and stem migration between 4.4–6.6 mm and 3.9–12.3 mm, depending on the choice of landmarks. The precision increased when tantalum markers were used as reference points. Hardinge et al. (Citation1991) introduced automatic image analysis, the MAXIMA method. A personal computer equipped with a frame grabber card, high resolution video camera, copy stand and a light box were utilized. The radiograph was digitized by the camera. Poor quality radiographic images could be enhanced and standardised. The evaluation included migration and wear of the cup and subsidence and loosening of the stem. The reproducibility was reported to be high, but without any data documentation of accuracy.
The precision of measurements on conventional radiographs can be improved if measures are taken to compensate for errors caused by changes of projection and magnification between examinations. The “Ein Bild Rontgen Analyse,” EBRA-Digital measurement system (Russe Citation1988. Krismer et al. Citation1995) is a method for measuring 2-dimensional migration from digitized plain radiographs and consists of 2 software programs, EBRA-Cup and EBRA-Femoral Component Analysis (1998 version) which operate within an image analysis software shell (Optimas Corp, Washington,DC). The EBRA method differs from other measurement systems in that it contains an algorithm that excludes radiographs from a patient measurement series. This will happen if the patient position varies too much between 2 examinations. If the limits of position errors not are exceeded the software can reduce measurement errors caused by this factor (Krismer et al. Citation1995, Biedermann et al. Citation1999). The method has been found to be useful in measuring implant migration and for predicting cup and stem failure (Krismer et al. Citation1996, Citation1999, Hendrich et al. Citation1997). The precision (95% confidence interval) of this method in measuring migration has been shown to vary between ± 0.8 to 1 mm depending on type and direction of motion analysed (Phillips et al. Citation2002, Wilkinson et al. Citation2002).
Evaluation of wear
Penetration of the femoral head into the socket with time has been measured on plain radiographs using a ruler, caliper, or circular templates with or without specially designed magnifying glasses. The first to perform such measurements were Charnley and Cupic (Citation1973). They evaluated the femoral head penetration by measuring the difference between the shortest and greatest distance from the center of the femoral head to the metallic indicator on the equator of the cup. This method (uni-radiographic) evaluated the magnitude of wear, but did not consider its direction (Charnley and Halley Citation1975). To obtain more accurate measurements of the linear wear, a new method (duo-radiographic measuring) was introduced (Charnley and Halley Citation1975, Griffith et al. Citation1978). On an early postoperative and on the latest follow-up radiographs, the distance from the femoral head contour to the contrast wire of the Charnley cup was measured in the vertical and horizontal directions. The difference was interpreted as radiographic wear, after correcting for magnification. This method was later modified by Livermore et al. (Citation1990) and Bankston et al. (Citation1994). The thinnest part of the polyethylene layer on the latest follow-up radiograph was measured, corrected for magnification using the known femoral head diameter. On the first postoperative radiograph, the thickness of the polyethylene was measured in the same area and the difference between the two radiographs was considered as linear wear.
The EBRA method described above can also be utilised to measure wear with good resolution. (Ilchmann et al.Citation1995, Wilkinson Citation2002, Citation2003). Devane et al. (Citation1995) developed another computerassisted technique based on three dimensional reconstruction of the position of the cup. This is achieved by applying an edge detection algorithm to the contours of metal-backed cups on both the AP and lateral view. Measurements made using this technique include orientation of the acetabular component, two-and three-dimensional measurement of femoral head displacement over time, and calculation of the minimum volume of polyethylene debris. The authors reported an accuracy of ±0.15 millimeter on the basis of analysis of a phantom model with predetermined amounts of wear. Sychterz et al. (Citation1999) showed that the precision of three-dimensional measurements was greater than that of two-dimensional measurements in only 5% of the hips analyzed. Martel and Berdia (Citation1997) described a computer-assisted vector wear technique for the determination of polyethylene wear on digital radiographs. Two and three dimensional displacement of the femoral head with respect to the acetabular center was followed over time using the computer-assisted vector wear technique. The authors found the new technique to be at least ten times more repeatable than techniques with either callipers or a digitizing tablet. Although promising, this technique has so far not been evaluated against an accurate standard in a clinical study.
DEXA
Bone remodelling which is a continuing process is expected to change after the insertion of femoral prosthesis. This process will result in redistribution of bone adjacent to the prosthesis. The remodelling is most pronounced during the first two postoperative years, and then continues at a slower rate. Stress shielding has been thought to be responsible for most of the changes in bone density after THA, but other factors such as osteolysis, loosening and fracture are also associated with bone loss (Brown and Ring Citation1985, Cooke and Newman Citation1988). Standard radiographs, however, do not allow for adequate assessment of bone mineral density. Changes in density must be greater than 30% to be observed with certainty on plain radiographs and the precision and accuracy of such evaluations are poor (Vose Citation1966).
Dual energy X-ray absorptiometry, provides a noninvasive means of quantitative assessment of bone mineral content and density of the proximal femur in vivo. Two photon beams with low but different energies pass through the region of interest. Bone has different attenuation properties from soft tissue. The absorption pattern is related to the known absorption for Calcium and other substances. In this way metallic implants can be discriminated and excluded from the investigation. Bone has similar attenuation properties to cement and cannot be distinguished using conventional DEXA unless this is done manually.
DEXA provides the accuracy and precision which is necessary to detect and quantify the changes in bone that occur after THA. Kiratli et al. (Citation1992) determined the accuracy of bone mineral measurements in the proximal femur to be less than 1%. The precision varied between 2% and 4.5%. These errors were mainly caused by variable patient position and nonhomogeneous distribution of soft tissues. The rotational position of the femur is a critical factor: especially in cemented stems (Goh et al. Citation1995). The precision of bone mineral density determinations around the socket using 3- and 4-ROI models has been reported to be lower than 5%. (Wilkinson Citation2001).
The measurements are usually reported as the change in bone mineral content (BMC) or bone mineral density (BMD). BMD is the same as BMC divided by the area of the region of interest (ROI).
When DEXA is used, a difference in the distribution of adipose tissue may affect the bone mineral value since approximations must be made in order to use the relevant equations. TXA (Triple-energy X-ray absorptiometry) uses three photon energies and is advantageous, as no such approximations are required. TXA can be used to measure bone mineral in the presence of different amounts of homogenous and non-homogenous adipose tissues close to the measured bone (Swanpalmer et al. Citation1998 a, Citation1998 b).
RSA (Radio Stereometric Analysis)
Today most implants with clinical documentation exhibit small migration and wear. To document equivalent or even better performance of a new implant design requires methods with higher resolution than conventional radiography. Further on, more complex analyses including implant motions in three dimensions are of interest to obtain a better understanding of any failure mechanism. One method which fulfils these requirements is radiostereometric analysis (RSA). Because of its high precision this method has been used increasingly in orthopaedic research (Kärrholm Citation1989).
In Sweden, the method originates from Hallert who presented the basic principles for roentgen photogrammetry (Hallert Citation1970). In Citation1974 Göran Selvik modified and further developed the method, using the mathematical principles of rigid body fitting and calculation of three-dimensional motions. It was initially called roentgen stereophotogrammetric analysis (RSA). Subsequently the professor in orthopaedics in Lund, Göran Bauer, suggested a simpler name, radiostereometric analysis, which has become widely adopted.
This method relies on small markers implanted in the bone and the prosthesis. Certain regular geometries such as the femoral head, a circular wire or a metal-backed cup can also be used as landmarks. Recently, advanced mathematics based on contour fitting algoriths and CAD-CAM (Computer Assisted Design/Computer Assisted Manufacture), models have been introduced to reduce the need for implant marking (Valstar et al. Citation1997, Citation2001). So far, these methods have implied certain restrictions concerning applicability and resolution and lack sufficient documentation in clinical trial.
Spherical tantalum markers with a diameter of 0.8 or 1.0 mm are inserted in the skeleton and, also in the implant. These types of tantalum markers have been used as skeletal markers for almost 30 years without any adverse reactions. Because of its high density, tantalum can be easily detected on radiographs. Up to nine markers are often used in each segment to optimise marker spread and compensate for any instability of individual markers.
Polyethylene sockets and polyethylene inserts in cementless cups can be supplied with tantalum markets around the opening of the cup and into the dome. The markers may also be inserted into small titanium pegs attached on the outside of a metallic shell. Such marking might help to eliminate any uncertainty concerning motions between the liner and the shell. Preferably the manufacturer should implant the markers to optimise positioning and reduce the risk of damage.
Migration of the femoral component can be measured as translations of the femoral head centre. If the femoral component is supplied with tantalum markers a more complete analysis can be made, including femoral component rotations and translations of those parts of the stem supplied with markers (e.g. shoulder and tip).
Early in the history of radiostereometric evaluation of hip implants the examination were performed with the patient standing (Baldursson et al. Citation1979, Mogensen et al. Citation1982). These examinations were, however, abandoned in favour of studies in the supine position, because of problems with the quality of the images. Theoretically, examinations with the patients standing should be preferable in studies of wear, because the joint is more stable in this position and the load may be more reproducible ().
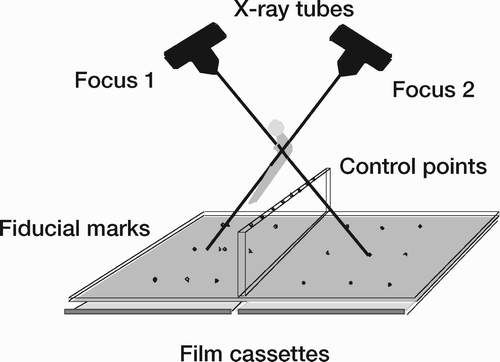
Routinely the patient is examined within a calibration cage. As an alternative a separate calibration examination including reference plates can be used (Kärrholm Citation1989), but this technique was not utilised in this thesis. The cage is supplied with markers, which define the laboratory coordinate system (fiducial markers) and markers used to calculate the position of the two roentgen foci (control points). Two radiographs are exposed simultaneously with the x-ray tubes angled 35 to 40° in relation to each other. The 2 central beams should preferably intersect at the level of the cup or stem ().
The two dimensional positions of the cage and patient markers are recorded. This information is used to compute the three-dimensional co-ordi-nates of the patient markers, based on their locations on the radiographs. The computer chooses the most probable coupling between markers on the 2 radiographs. All markers in the same segment (the bone, stem or cement) are modelled as rigid bodies.
To test the stability of these markers, the degree of deformation of each segment between two examinations is calculated and expressed as the “mean error of rigid body fitting” (Selvik 1989). The user can define the upper acceptable limit for this parameter. By exclusion of poorly defined or loose markers, the software will, if possible, identify a segment that maintains its stability between subsequent examinations.
The accuracy of the calculations also depends on the configuration or spread of markers. This property of a marker segment corresponding to tantalum balls placed in a prostheses or the surrounding bone is described mathematically by the “condition number” (Söderqvist and Wedin Citation1993). The condition number is low if the markers are well scattered and high if the markers are close to each other or situated along a line. The mean error of rigid body fitting and the condition number are crucial parameters in radiostereometric analysis and help the investigator to determine whether the measurements are reliable.
The relative motions of the implant are calculated using the corresponding bone markers as a fixed reference segment. If at least three well-spaced markers are identified in the implant and in the bone, both translations and rotations can be measured. Motion of an implant during a time period is named migration. This motion is described in relation to 3 body fixed axes; the transverse (medial-lateral axis, x-axis), the longitudinal (proximal-distal, y-axis) and the sagittal (anterior-posterior, z-axis).
The RSA method has been continuously developed to improve its applicability in different fields of orthopaedic research (Kärrholm Citation1989, Söderqvist and Wedin Citation1993, Nyström et al. Citation1994, Börlin Citation1997, Kärrhohn et al. 1997, and Vrooman et al. Citation1998). During the late twentieth century manual measurements of RSA radiographs were gradually replaced by digital measurements. This technique enabled further development of automated procedures and controls, a continuing process. Today the software used by us (UMRSA©, RSA Biomedical, Umeå) is partially automated, to facilitate detection and measurements of predefined positions and geometric images such as the cage markers and the femoral head. To define the centre of the marker, the software utilises all available information in the pixels included in the markers and fits mathematical models corresponding to the continuous change of grey-scales from the periphery to the centre of the marker (). Only a rough user-assisted approximation of the marker position needs to be given. An automated algorithm iterates the calculations of the centre to optimise its identification. Poorly defined markers are automatically discarded. Calculations of three-dimensional positions of cage and patient markers are performed in computer software, which includes several programs
Börlin et al. (2001) showed that the digital method was more reproducible than the manual one to identify the true marker centre and the edge of the femoral head. The mean error of rigid body fitting decreased and the precision increased. Bragdon et al. (Citation2002) used a phantom model to evaluate the accuracy and the precision of radiostereometric analysis in measuring wear. The accuracy defined as the closeness of agreement between a test result and an accepted reference value was best in the proximal direction (22μm) and worst for the posterior direction (86μm). The corresponding value for the precision defined as the closeness of agreement between repeated independent test results obtained under stipulated conditions was 5.5μm and 16μm. Önsten et al. (Citation2001) used two cadaveric models of hip arthroplasty to evaluate the accuracy and precision of RSA-based measurement of translation of the femoral component. The longitudinal axis had the highest accuracy and precision and the sagittal axis the poorest. The precision of RSA measurements in the clinical setting is poorer than in vitro because of greater variability in film quality, patient positioning and marker scatter. The precision error in clinical studies can be estimated by performing double examinations of each patient in a study group on at least one occasion. Calculation of the 99% confidence intervals of the errors is used to determine the limits for significant motions in individual cases.
Materials used in total hip arthroplasty
Polyethylene
History
Relatively few polymeric materials have been used in total joint replacements. These have included polyamide, polysulfone, polyetheretherketone, polytetrafluoroethylene, polyacetal, polyesters, and polyethylene (high-density polyethylene, ultra-high molecular weight polyethylene, and carbon-rein-forced ultra-high molecular weight polyethylene).
Charnley originally chose polytetrafluoroethylene of the Fluon type as bearing material on the basis of its general chemical inertness and its low coefficient of friction. The more familiar name, Teflon has been used widely for these polytetrafluoroethylene materials. Clinical failure with acetabular cups made of Fluon generally occurred within one to two years. These failures were attributed to the low creep resistance of this material and to its relatively poor abrasive-wear characteristics. Instead, Charnley turned to the use of high-molecular-weight polyethylene (Waugh Citation1990). The first hip prostheses made of this material were implanted in 1962.
In the 1970's and 1980's, efforts were made to find a material with still better wear properties than ultra-high molecular weight polyethylene. The two most notable trials were the use of polyacetal (polymethylene oxide) in the Christiansen hip prosthesis and of Poly II (carbon-fiber-reinforced ultra-high molecular weight polyethylene).
Polyacetal has the potential advantages of higher yield strength, higher crystallinity, and easier manufacturing than ultra-high molecular weight polyethylene. Polyacetal can be formed into parts rapidly by injection-moulding, which is faster and cheaper than machining from blocks of polyacetal. It was estimated that more than 10.000 devices containing this material were implanted between 1970 and 1986 (Mathiesen et al. Citation1986). However, by the mid-1980's, several groups of investigators had reported higher failure rates than for other contemporary prostheses and their use was discontinued (Alho et al. Citation1984, Mathiesen et al. Citation1986, Sudmann et al. Citation1983).
UHMWPE with carbon fibers within the matrix of polyethylene, known as Poly II, increased the modulus and ultimate tensile strength of the bearing. The creep was thus reduced and the longevity would increase according to laboratory studies. The compressive strength of Poly II with 15% carbon fiber was 25% higher than that of conventional UHMWPE. Thus Poly II was expected to be more resistant to the pitting and delamination often seen in knee arthroplasties. Unfortunately, the promising properties of Poly II observed in the laboratory could not be confirmed in the clinical setting. Shortly after implantation many patients presented with osteolysis and mechanical failure of their tibial bearing inserts (Wright et al. Citation1986, Wright et al. Citation1988a,Citationb, Busanelli et al. Citation1996). One possible explanation could be that the carbon fibers did not bond to the UHMWPE matrix and thus served as both stress and crack concentration sites (Connelly et al. Citation1984).
In 1991, a modified UHMWPE known as Hylamer was marketed by the DePuy-DuPont Orthopaedics joint venture (Newark, DE, USA). Hylamer is a hot isostatically pressed UHMWPE, leading to the formation of extended-chain crystallite morphology. Hylamer has a higher density and crystallinity than conventional UHMWPE. The most important difference occurs in the elastic modulus, which is nearly double for Hylamer that of conventional polyethylene. This material was also found to have substantially lower rates of fatigue crack propagation in the laboratory (Champion et al. Citation1994).
The clinical reports of the effectiveness of Hylamer are currently mixed, with results ranging from equal to greater incidence of excessive wear compared with conventional UHMWPE (Chmell et al. Citation1996, Livingston et al. Citation1997, Sychterz et al. Citation1998, Collier et al. Citation1998, Schmalzried et al. Citation1998a). Recently Norton et al. (Citation2002) reported catastrophic early failure of a cemented total hip replacement in which Hylamer and a Zirconia ceramic head had been used. After 5 years there was 68% failure rate. Wroblewski et al. (Citation2003) have prospectively studied the wear of Hylamer polyethylene in combination with a zirconia femoral head of 22.225 mm diameter. The mean rate of penetration of the cup was 0.22 mm/year after a mean of 6 years of follow-up. The authors decided to discontinue the use of Hylamer because of the high initial rates of penetration. After an average follow-up of 47 months, Iwase et al. (Citation2003) reported a penetration rate of 0.36 mm/y when Hylamer was used in Ogee sockets. These values are 2 to 4 times higher than expected and resulted in discontinued use of Hylamer.
Polyethylene standards
The mechanical properties of UHMWPE are linked to its chemical structure, molecular weight, crystalline organization, and thermal history. All of these factors affect the morphological, chemical, and mechanical processes, which may influence UHMWPE wear and performance after implantation.
Physically UHMWPE is a two-phase viscoelastic solid, consisting of crystalline domains embedded within an amorphous matrix. The crystalline phase of UHMWPE consists of folded rows of hydrocarbon molecules packed into lamellae with width 10–50 nm and length 10–50 μm. The surrounding amorphous phase consists of randomly oriented and entangled polymer chains traversed by tie molecules, which interconnect lamellae and provide resistance to mechanical deformation. UHMWPE is a complex composite material, which can evolve over time in response to its mechanical, chemical, and thermal history.
In the early 1960s, UHMWPE was classified as a form of high-density polyethylene (HDPE) in the polymer industry (Polyethylene resins. In: Modern plastics encyclopaedia for 1962). Thus, Charnley's earlier references to UHMWPE as HDPE are technically accurate for his time (Charaley Citation1963, Charnley Citation1968). Today, HDPE is classified as polyethylene with a density greater than 0.940 g/ cm3and molecular weight typically below 200.000 g/mol, whereas UHMWPE has a molecular weight of greater than 3.1 million g/mol (Coughlan and Hug Citation1986). The term UHMWPE designates that the average molecular weight is greater than 1 million g/mol.
Since 1998, the nomenclature was based on the availability of four grades for the worldwide orthopaedic market GUR 1150, 1050, 1120, and 1020 resins. The first digit of the grade name was originally the loose bulk density of the resin, i.e. the weight measurement of a fixed volume of loose, unconsolidated powder; the 1 corresponded to a bulk density of over 100 g/1 for standard grades. The second digit indicates the presence (1) or absence (O) of calcium stearate while the third digit codifies the average molecular weight of the resin. The fourth digit is an internal code designation (). The acronym GUR stands for ‘Granular’, ‘UHMWPE’ and ‘Ruhrchemie’ (Kurtz et al. Citation1999). Calcium stearate has been used since the late 1960s. This additive acts as a scavenger for residual catalyst components that can potentially corrode conversion equipment. It also acts as a lubricant and a release agent for consolidation (Eyerer et al. Citation1990). Several investigators have observed an association between the presence of calcium stearate and reduced ultimate tensile strength, reduced elongation to failure, and an increased number of fusion defects (Hamilton et al. Citation1996, Schmidt and Hamilton Citation1996). Recently all orthopaedic manufacturers have switched to UHMWPE resins without calcium stearate content (e.g., GUR 1020 and 1050).
Table 1. Nomenclature of UHMWPE resins
Implant manufacture
All polyethylene begins as a bulk powder, produced from a synthesis process called polymerization where individual molecular units of ethylene are linked together to form long chains. Inconsistencies in this process can create significantly different polyethylenes. Inherent differences in the properties of the bulk powder include molecular weight, density, and percent crystallinity. These properties are important because they affect the ultimate performance of the fabricated polyethylene implant.
Three fabricating methods are currently used in manufacturing orthopaedic implants from powder, ram extrusion, compression moulding and net shape compression moulding.
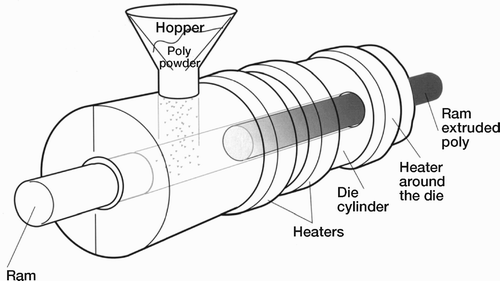
In ram extrusion, the polyethylene powder is extruded into cylindrical bar stock ranging from five to fifteen centimeters in diameter. A measured quantity of polyethylene powder is introduced into a chamber. The powder is then pushed into a heated cylindrical barrel by a ram. As the ram retracts, the chamber is refilled with powder. As the process continues, each stroke of the ram advances the polyethylene through the heated barrel where the powder is consolidated into a continuous round bar. The implant is then machined from the bar stock ().
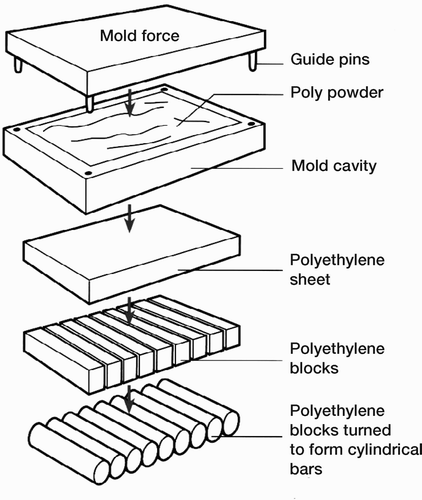
In compression moulding, the raw polyethylene powder is moulded into large sheets. The powder is introduced into a cavity which is then heated as a platen covers the entire cavity and applies extreme pressure to the material for a specified time. The resulting sheets are cut into smaller blocks or sectioned and turned on a lathe to form cylindrical bars from which the implants are machined (). In net shape moulding, also a compression-mould-ing process, the polyethylene powder is placed into a mould, the cavity of which is the final or nearfinal shape of the implant. A nitrogen atmosphere is introduced as the powder is compressed. Heat is then applied under pressure over a period of time to consolidate the material and form the part. In all three processes, the polyethylene is heated to a temperature above its melting point.
In retrospective studies of shelf-stored and/or retrieved implants, components fabricated from block moulded or net-shaped moulded polyethylene, gamma-sterilized, and stored in air have undergone substantially lower levels of oxidation than similarly treated machined or ram-extruded components. It has been suggested that the moulded polyethylene was more completely fused than typically extruded material (Gillis et al. Citation1998, Currier et al. Citation2000). Manufacturers no longer irradiate and store the polyethylene in air. Therefore the marked differences in oxidation levels that occurred between moulded and ram-extruded components in the past may no longer occur (McKellop Citation2001). However, Rasquinha et al. Citation(2002) reported a lower mean wear rate for machined polyethylene liners, manufactured by moulded instead of the ram extruded technique. All the liners were sterilized by gamma-irradiation in argon gas.
The resins GUR 1020/1120 have long been available from converters to device manufacturers as bulk compression moulded slabs while the resins GUR 1050/1150 generally have been made available to manufacturers only in the form of extruded rods. More recently converters have begun supplying GUR 1050 in compression moulded sheet form as well. Studies that examine issues such as compression moulding versus ram extrusion of implants often ignore the confounding variable of resin grade since the compression moulded stock will most likely be GUR 1020 and the extruded stock GUR 1050.
Sterilization
Gamma irradiation with 25 to 40 kGy in an air environment has been the industry standard sterilization technique since 1968. Gamma radiation breaks polymer chains, creating reactive free radical sites. The oxygen present in the polyethylene during radiation or that diffuses into the material during shelf storage and/or during in vivo use reacts with residual free radicals generated by the radiation (Eyerer and Ke Citation1984, i and Burstein Citation1994, Rimnac et al. Citation1994, Premnath et al. Citation1996, Yeom et al. Citation1998, ). The resultant chain scission reduces the molecular weight and leads to increased density, stiffness and brittleness (Roe et al. Citation1981, Eyerer and Ke Citation1984, Sutula et al. Citation1995, Collier et al. Citation1996, Gillis et al. Citation1998). These changes reduce the fracture strength and the resistance to wear (Shen and Dumbleton Citation1974, Rose et al. Citation1980). Since 1995 almost all the manufacturers have changed to alternative sterilizing techniques either by substitution of radiation with ethylene oxide or gas plasma sterilization or by gamma radiation in a reduced oxygen environment.
Methods that do not use radiation such as ethylene oxide and gas plasma avoid the creation of free radicals. These surface sterilization techniques do not break bonds within the material but leave it essentially unchanged from its virgin state. The highly toxic ethylene oxide gas neutralizes bacteria, spores and viruses by reacting with DNA through displacement of a hydrogen atom resulting in deactivation of the molecule (Dempsey and Thirucote Citation1989, Schneider Citation1994). Gas plasma is a dry low pressure, low temperature technique performed under partial vacuum. A plasma is ionized gas created by a magnetic field. The plasma flows into a chamber and sterilizes the material surface by oxidizing bacteria cellular components (Booth Citation1995, Parisi and Young Citation1991). The sterilization cycle time for the EtO is 41 h (Ries et al. Citation1996) and for gas plasma 75 min to 4 h (Kyi et al. Citation1995, Feldman and Hui Citation1997).
Other manufacturers continue to sterilize their implants with gamma radiation but do so with the polyethylene components sealed in some type of low oxygen atmosphere, including vacuum (Greer et al. Citation1998), inert gas (Streicher Citation1988), or with an oxygen scavenger (Bapst et al. Citation1997). When little oxygen is present, the free radicals generated during irradiation can form carbon-carbon cross-links between adjacent polyethylene molecules (). Several laboratory tests have shown that, in an appropriate amount, such cross-link-ing improves the wear resistance of the polymer (Dumbleton et al. Citation1974, Grobbelaar et al. Citation1978, Rose et al. Citation1982 Oonishi et al. Citation1992). This sterilization method can markedly reduce the oxidative mechanical degradation, but the remaining free radicals from radiation process may induce some oxidation during long-term use in vivo.
Sterilizing an UHMWPE acetabular cup without radiation avoids immediate and long-term oxidative degradation of the implant, but does not improve the inherent wear resistance of the polyethylene. The trade-off is between preventing oxidation and sacrificing the potential benefit of radiation-induced cross-linking. Sterilizing with gamma irradiation in reduced oxygen environment impedes the oxidation until the package is open. After implantation the long term oxidation can not be eliminated. Ideally, cross-linking with gamma irradiation to reduce wear should be done in a manner that avoids both immediate and longterm oxidation.
Highly cross-linked UHMWPE
Clinical problems related to wear of UHMWPE have stimulated the development of more wear resistant polyethylene materials. Three long-term retrospective radiographic studies (Grobbelaar et al. Citation1999, Wroblewski et al. Citation1999, Oonishi and Kadoya Citation2000) including 145 cases followed 10-22 years have shown that increasing cross-linking of polyethylene above the amount achieved with conventional radiation doses improves its wear resistance. Gamma radiation (1000 kGy) in air (Oonishi and Kadoya Citation2000), gamma radiation (100 kGy) in the presence of acetylene (Grobbelaar et al. Citation1999), and silane chemical crosslinking (Wroblewski et al. Citation1999) have been utilized with seemingly the same positive clinical effect.
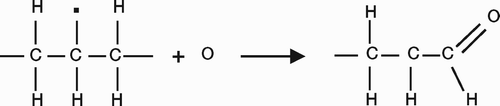
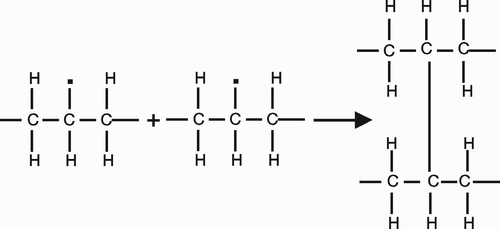
Cross-links between the molecular chains are achieved by reaction with free radicals (). These are generated by ionizing radiation or by using peroxide or silane chemistries. Today, radiation is the preferred method of crosslinking and neither peroxide nor silane chemistry is used.
Radiation generates free radicals, most of which are incorporated into the molecule as crosslinks. However, some free radicals remain trapped in the crystalline regions and may initiate a cascade of events that cause oxidation and subsequent embrittlement of the polyethylene. Since these radicals are present in crystalline domains, the most effective method of eliminating them is to melt the plastic after the radiation. Melting liberates the trapped radicals from the crystalline domains, allowing them to react chemically and further increase the crosslinking and reduce the risk of subsequent oxidation (Sun et al. Citation1998, McKellop et al. Citation1999, Muratoglu et al. Citation1999, Citation2001). After the cross-linking and melting process, the polyethylene is typically sterilized by another method (such as ethylene oxide or gas plasma) to prevent formation of new free radicals that would lead to polyethylene oxidation.
There are several commercially available variations of the method to improve the wear and oxidation resistance of polyethylene used in total hip arthroplasty (). Muratoglu et al. (Citation2001a) showed that highly cross-linked PE produced by heating and electron beam irradiation had no detectable wear after 20 million cycles in the hip simulator, which has been approximated to 20 years of normal walking. The electron-beam cross-linked UHMWPE liners showed no detectable change in weight while the conventional UHMWPE acetabular liners lost weight steadily because of wear. In conditions of third body abrasive wear with aluminium oxide and bone cement particles, this material has significantly superior wear performance compared with conventional UHMWPE (Bragdon et al. Citation2003). Hip simulator tests showed that the wear rate of this type of polyethylene is not influenced by the size of the head within the range of 22 and 46 mm in diameter (Muratoglu et al. Citation2001b).
Table 2. Currently available highly crosslink polyethylene liners
Although cross-linking improves the wear resistance, it can also reduce the mechanical properties of UHMWPE, including a decrease in toughness, ultimate tensile strength, yield strength, elongation at break, elastic modulus, and hardness (Baker et al. Citation1999, Muratoglu et al. Citation2001a). There is a reported decrease in the resistance to fracture when cross-linked polyethylene is subjected to constant or cyclic loads (Pruitt et al. Citation1995). The clinical implications of this effect of cross-linking are not known, but can still be regarded as a concern against unrestricted use.
Bone cement
History
Bone cement is a polymer principally composed of polymethyl methacrylate (PMMA). This material has been widely known since 1930 and is also known by its commercial names of Plexiglass or Perspex. Originally, the material was utilised above all for producing safety glass, but in the Thirties, both an English company called ICI (Drury et al. Citation1935) and a German researcher, Otto Roehm, proposed another use: the creation of dental prostheses. In 1940, Schnebel (1940) and the German company Degussa and Kulzer (Citation1943) prepared a record of the cold polymerisation of PMMA using tertiary amines as accelerators for the reaction. This discovery marked the beginning of the modem era of PMMA, because the polymer could be made in operating rooms and could be adapted to the geometries most suitable to each specific surgical application. Judet and Judet (Citation1956) were the first to introduce an arthroplastic surgical method, but the PMMA prosthesis used did not achieve sufficient fixation for biological and mechanical reasons. In 1958, Sir John Charnley first succeeded in anchoring a femoral head prosthesis in the femur with auto-polymerizing PMMA (Charnley Citation1960). Charnley called the material used “bone cement on acrylic basis”.
Basic principles
Bone cement consists of a powder component including beads of polymethylmethacrylate with varying diameters ranging from less than 1μm to more than 100 μm. A radio-opacifier (about 10 per cent of barium sulphate or zirconium oxide) and an initiator (benzoyl-peroxide) are added. The liquid phase consists of the monomer methylmethacrylate. Small amounts of additives (hydroquinone, dimethylparatoluidine) protect the monomer from polymerization by itself, or are involved in the initiation of the polymerization process when the two components are mixed together. A colour is sometimes added to the powder (). In most bone cements marketed in Sweden antibiotics are included, i.e. gentamicin or tobramicin. Addition of these substances reduces the strength of the cement but the benefits of antibiotic additives are assumed to be greater even if data from randomised studies are lacking (Malchau et al. Citation2000).
Table 3. Composition of commercial bone cements
Before insertion of the implant, the prepolymerised methylmethacrylate powder and liquid monomer are mixed, usually in a specifically dedicated system, which enables the ambient pressure to be reduced, thereby reducing the number of voids.
Modern cementing technique (brushing, distal plugging of the femoral canal, high-pressure lavage, tamponades soaked in adrenaline solution, retrograde injection of cement into the femur, cement pressurization) has significantly reduced the mechanical failure rates (Herberts and Malchau Citation1997).
Release of heat during curing of the cement may raise the interface temperatures to 40–70° C (Mjöberg Citation1986, Toksvig-Larsen et al. Citation1991, Wykman Citation1992). During one-minute exposure, the threshold temperature for impairing bone regeneration has been determined to 44–47° (Eriksson and Albrektsson Citation1983). The production of heat is proportional to the amount of monomer used (Debrunner et al. Citation1974). Not only heat (Feith Citation1975) but also the monomer itself can be toxic to the bone cells (Willert et al. Citation1974, Linder Citation1976). Cell injury and necrosis initiate inflammatory reactions. The fibrous membranes commonly seen between the bone and the cured cement (Charnley Citation1970, Freeman et al. Citation1982, Linder and Carlsson Citation1986, Goodman et al. Citation1988) have been believed to be the result of injuries caused by heat (Mjöberg Citation1986) or monomer toxicity (Pedersen et al. Citation1983, Stürup et al. Citation1994). Remodeling of the bone starts 4-6 weeks after cement administration (Morberg Citation1991). Necrosis and delayed remodelling affect the bony anchorage.
Different solutions to decrease the release of heat during curing have been tried. Mjöberg (Citation1986) used RSA to study a low monomer containing cement. The author showed improved initial fixation compared to conventional cement but the follow-up was short, 4 months and no further follow-up has been presented. Bone cement with the same properties is today commercially available (Cemex®, Tecres, Italy). Previously, Nivbrant et al. (Citation2001) compared Palacos with a low temperature curing cement (Cemex) using RSA to measure the migration of the Lubinus SP2 prosthesis. In this randomised study the cup and stem migration did not differ between the 2 groups up to 5 years after the operation.
Mechanical properties of the bone cement
On the femoral side of a THR the metallic stem is subjected to the highest levels of stress (Weightman Citation1990), but the surrounding cement is the weak link because of its inferior mechanical characteristics. The state of stress in the cement depends on many factors, including the thickness of the cement mantle (Lee et al. Citation1994, Fisher et al. Citation1997), the structure of the cement (distribution and dimension of the pores) (Harrigan and Harris Citation1991), the shape and the type of alloy used to manufacture the prosthesis (Mann et al. Citation1995, Fisher et al. Citation1997) and the friction and bonding conditions between the shaft and the cement (Harris Citation1992, Ivarsson et al. Citation1994).
The fixation of the cement to the bone is only accomplished by mechanical interlock between the two surfaces. It has been demonstrated (Cameron Citation1994) that in the presence of fragments in the canal the strength of the bone–cement interface may be reduced by as much as 50%. The typical values of maximum tensile stress of bone cement are between 29 MPa and 49 MPa (Lewis Citation1997). The maximum traction stress observed in the cement mantle is about 10 MPa (Harrigan and Harris Citation1991, Mann Citation1995), which can change as a function of the type of bond created between the shaft and the cement (Mann Citation1995) and the thickness of the mantle (Lee et al. Citation1994). Even though many researchers have studied the load transfer in cement, the mechanisms which lead to debonding of the stem and breakage of the mantle are not completely understood.
A certain period after prosthesis implantation, a stable situation is often reached when a fibrous layer of varying thickness may be formed between the bone and the cement. Such a fibrous layer or membrane has been suggested to play an important role in the transfer of loads between materials with different elastic moduli, such as the bone and the metallic shaft. Its presence does not necessarily compromise the stability of the prosthesis. It appears, in fact, to reduce the state of stress on the interface (Yetkinler and Litsky Citation1998), and it has further been estimated that when its thickness is less than 1 mm, it can prevent loss of mechanical stability (Pietrabissa Citation1996). However, histological studies of human retrieval samples (Charnley Citation1979, Linder and Hanson Citation1983) and of animal experiments (Draenert Citation1981) have demonstrated that long-lasting close contact between cement and bone may be achieved without signs of tissue irritation.
Cement porosity due to air entrapped by mixing and other phenomena during mixing and curing of the cement influence its mechanical properties. The pores can constitute points of stress concentration and facilitate the development and propagation of cracks. However, pores may also have the opposite function. When a cement fracture reaches the pore it can stop and fade out (Topoleski et al. Citation1993, Lewis Citation1997). Despite this finding the current opinion is to reduce the porosity as much as possible (Lewis Citation1997).
Creep
Fatigue failure and creep are two critical factors for the endurance of bone cements. The bone cement creeps under dynamic and static loading conditions. As a result, stems which are debonded from the cement may gradually subside, depending on their shape and surface roughness, causing expansion of the cement mantle around the shaft. This phenomenon causses a redistribution of the stresses in the cement, which may have favourable or damaging effects on the entire prosthetic system. According to Verdonschot and Huiskes (1997) the amount of stem subsidence which can be explained by creep is only around 0.05 mm. Kärrholm et al. Citation(2000) showed that stems which subside less than 0.1 mm during the first two years have a low revision rate in the Swedish National Hip Arthroplasty Registry. Lee et al. (1978) claimed that the cement can tolerate a considerable amount of deformation if subjected to continuous pressure at body temperature over weeks or months.
Two phenomena have been described which are correlated to creep in the cement. The first is the debonding of the shaft from the cement, which can induce locally increased stress resulting in fractures inside the mantle. The other is plastic flow of the cement. It has been shown that creep can have positive effects when occurring in association with smooth, tapered shafts without collars. The subsidence of the shaft results in stabilisation of the implant and a consequent reduction of the stress in the cement (Lewis Citation1997). The exact consequences of creep are, however, still unknown especially concerning its relation to aseptic failure and its effects when used with polished versus matte or rough surface finishes.
Modifications of bone cement
Modification of the components of the cement were explored to address some of its problems such as poor adhesion to bone, poor biocompatibility, low mechanical strength and loosening.
Weightman et al. (Citation1987) evaluated a bone cement with a different polymer (butylmethacrylate) to obtain a lower modulus of elasticity and decrease the risk of cement fracture. This cement was never used in a large-scale clinical trial, probably because of its inferior mechanical properties. Sturup et al. (Citation1994) studied another type of coldcuring cement also containing butylmethacrylate. The preclinical evaluation appeared to be satisfactory and this cement was used in several thousands of patients. However, Thanner et al. (Citation1995) demonstrated inferior mechanical and clinical properties when this cement was compared with Palacos and it turned out to be a large-scale clinical failure (Furnes et al. Citation1997).
Reduction of the viscosity of cement was another Modification believed to improve implant fixation by increasing the penetration of the cement into the cancellous bone (Krause et al. Citation1982). Prospective randomized evaluations (Mjöberg Citation1986; Carlsson et al. Citation1993) and information obtained from the Swedish and Norwegian Total Hip Registers have shown that low-viscosity cement provides an inferior or the same fixation compared with highviscosity cements (Malchau et al. Citation1993; Havelin et al. Citation1995). Reinforcement of the cement with metal wires and other different types of fibers has also been investigated (Saha and Pal Citation1984), but has not come into clinical use.
Bioactive bone cements
To improve the mechanical properties of PMMA bone cement, bioactive glass-ceramic particles have been added (Hennig et al. Citation1979). Matsuda et al. (Citation1997) reported that bone cement consisting of bioactive glass powder showed higher bond strength than PMMA bone cement for up to 6 months after surgery in a canine total hip arthroplasty (THA). Fujita et al. (Citation2000) evaluated a bioactive bone cement (BABC) consisting of silane-treated apatite-and wollastonite-contain-ing glass-ceramic (AW glass-ceramic) in a canine THA model using conventional PMMA cement as controls. The mechanical properties of the BABC were better than those of PMMA bone cement, but femoral bone resorption and cement fractures were seen on the acetabular side after 2 years in the BABC group. Weak bonding between the BABC and the acetabular component made of UHMWPE, relatively high elastic characteristics of BABC and weakness of the calcium phosphorous layer formed on the surface of this cement seemed to be responsible for these failures.
Downes et al. (Citation1990) showed that human growth hormone (hGH) released from hormone-loaded poly(methylmethacrylate) (PMMA) cement stimulated osteoid formation in a rabbit model but no further clinical studies have been performed.
Fluoride additives to low-temperature curing cements are another example of BABC.
Histological studies in the rabbit comparing low-temperature curing cement with and without addition of fluoride have shown activation of osteoblasts and increased volume of bone matrix (Magnan et al. Citation1994, Sundfeldt et al. Citation2002).
Metal used as femoral head
The metals used in conjunction with polyethylene principally have included stainless steel, titanium alloy and cobalt-chromium alloy.
Stainless steel
Improved stainless steels are still used in current THR designs as load-bearing joint components. These steel materials are iron-based alloys containing chromium, nickel, and molybdenum. Stainless steel is usually annealed, cold-worked, or cold-forged to improve alloy strength. A potential problem mainly applicable to femoral stems is the relatively high modulus of elasticity of stainless steel, which is about 200 GPa. This value is about 10 times higher than that of bone. The wear rate of polyethylene against stainless steel has been shown to be comparable to that against cobaltchrome alloy in laboratory tests (McKellop et al. Citation1981, Lancaster et al. Citation1997) and in clinical studies (Wroblewski Citation1997, Devane and Horne Citation1999). The variability of wear rate is wide; from 0.07 to 0.66 mm/year (Livermore et al. Citation1990, Wroblewski et al. Citation1992, Bankston et al. Citation1995, Calaghan et al. 1995).
Titanium
Ti6Al4V is composed of approximately 90% titanium, 6% of aluminum and 4% of vanadium. Under ideal circumstances the wear rate of polyethylene against titanium alloy appears to be comparable to that with the other metals. The literature demonstrates wide variability in the average wear rates for polyethylene against titanium alloy in vivo, ranging from 0.08 to 0.25 mm/year (Cates et al. Citation1993, Hernandez et al Citation1994, Nashed et al. Citation1995).
The titanium alloy has greater vulnerability to abrasion by entrapped third-body particles, which can cause severe wear (Lombardi et al. Citation1989, McKellop and Rostlund Citation1990). Hardening of the surface of the titanium alloy by techniques such as gas nitriding, solution nitriding, or ion implanting can markedly improve its resistance to abrasive wear. Good clinical results have been reported for titanim nitride-hardened TiAlNb alloy (Semlitsch and Willert Citation1997). Nevertheless, if a hardened surface eventually is penetrated, accelerated wear can be expected (Mishra et al. Citation1996, Barouk et al. Citation2004).
Cobalt-chromium
Cobalt-chromium alloys are usually composed of 30–60% cobalt, 20–30% chromium, and 7–10% molybdenum, and various amounts of nickel (Gibbons Citation1982). These alloys are strong, hard and corrosion resistant. These properties of cobalt-based alloy gives them a high wear resistance and makes them ideally suited for articulating surface applications. The presence of carbides on the surface also helps their wear resistance by providing a dispersion of very hard carbides embedded in a matrix of metal alloy, upon which surface contact is made. A downside of the high work hardening rate of this material is the difficulty in machining and deformation processing it. These cobalt-based alloys also have very good corrosion resistance in chloride-containing solutions (Kuhn Citation1981). Heat and pressure improve their strength. The wear of cobalt-chromium alloys is substantially less than with Ti6A14V and stainless steel. Femoral heads made of CoCrMo alloy and head diameters 32–42 mm were used in the late 1960s and early 1970s, by McKee and Farrar in the metal-on-metal design. Although cobalt-chromium alloys are hard and tough, there is constant metal release from prosthetic articulations (Saikko et al. Citation1998).
Ceramics
Ceramics are a complex group of materials consisting of metallic oxides and other compounds manufactured at high temperatures. Ceramic materials are extremely stiff and strong in compression, but weak in tension, owing to their extreme brittleness (Jarcho Citation1981). Examples of ceramic biomaterials are calcium phosphates, glass ceramics, aluminum oxide, titanium oxide, zirconium and carbon (Black Citation1988). Ceramics in THR are classified into materials that are bioinert (alumina and zirconia) or bioactive (calcium phosphates and glass ceramics). The bioinert ceramics are suitable as femoral head materials and calcium phosphates as a coating material in cementless prosthesis.
Ceramics as coating
Calcium phosphate ceramics are biocompatible and can become attached to bone with chemical bonds (Jarcho Citation1981). There are numerous calcium phosphates, but only tricalciumphosphate and hydroxyapatite (HA) have received extensive in vitro and in vivo testing (Jaffe and Scott Citation1996). These materials can be differentiated from each other by their calcium-to-phosphate ratio, which is 1.5 for tricalcium phosphate and 1.67 for HA.
Calcium phosphates cannot be used as an implant because of their brittleness. However, these ceramics can be applied to metallic surfaces allowing a combination of the mechanical properties of the substrate with the biologic properties of the calcium phosphate. The most common method used today is the plasma spraying technique, developed in the 1980s (deGroot et al. Citation1987, Lacefield Citation1988, Lemons Citation1988). Calcium phosphates are composed of the same ions as in natural bone mineral and participate in the equilibrium of calcium phosphate ions at the interface. Shortly after implantation a thin layer of newly formed apatite can be observed attached to the coating.
These coating materials are bioresorbable, but the rate and degree of resorption vary between different calcium phosphates. The degree of resorption is also related to the surface area. Tricalcium phosphate is more bioresorbable than hydroxyapatite and is therefore more suitable for stimulating early ingrowth of bone into porous surfaces.
The quality of the coating depends on several factors including crystallinity, purity, density and thickness. Dense coatings with high crystallinity are less resorbable than porous structures with less crystallinity. Theoretically, they have less ability to stimulate bone ingrowth. Increasing the amorphous phase of the coating has been proposed to improve bone attachment. Coatings composed of 70% HA and 30% tricalcium phosphate are believed to provide both primary attachment and long-term fixation. Such a coating is available on hip prostheses with a fibre metal surface. The negative effect may be an increased rate of resorption, which may be compensated for by ingrowth into the porous metal substrate. Decreased thickness of the ceramic layer improves its fatigue strength. Thin coatings are also necessary to preserve a porous surface of an implant. A coating thickness of about 50–75 μm was previously recommended for clinical use (Geesink Citation1987).
Animal studies suggest that the bioactive surface encourages bone to bridge gaps up to 2 mm in both normal and osteoporotic bone (Soballe Citation1993a), whereas porous metals need closer contact to avoid fibrous fixation. HA appears to also have the ability to convert a fibrotic to an osseous fixation in animal models with loaded implants (Mouzin et al. Citation2001) both in stable and unstable implant conditions (Soballe Citation1993b). In two animal models Rahbek et al. (Citation2000, Citation2001) showed that an HA coating can inhibit migration of PE particles along the interface by creating a seal of tightly-bonded bone on the surface of the implant.
Retrieval studies have also shown increased ingrowth of bone into porous surfaces with HA coating, confirming the osteoconductive properties of this material (Bauer et al. Citation1991, Bloebaum et al. Citation1991, Citation1993, Tonino et al. Citation1999).
Applications of HA coatings in total hip arthroplasty are now well-documented and clinical and radiological results have been promising (Kroon and Freeman Citation1992, Kärrholm et al. Citation1994, Geesink and Hoefnagels Citation1995, Moilanen et al. Citation1996, D'Antonio et al. Citation1996, Capello et al. Citation1998, Thanner et al. Citation1999, Eckardt et al. Citation2003, Skinner et al. Citation2003). However several authors have been concerned about excessive wear and osteolysis, probably related to the disintegration of HA coatings (Bloebaum and Dupont Citation1993, Bloebaum et al. Citation1994, Litner et al. Citation1994, Rokkum and Reigstad Citation1998, Rokkum et al. Citation2002, Havelin et al. Citation2002, Reikeras and Gunderson Citation2002). However, it is not clear whether these observations are related to the use of hydroxyapatite or other problems related to implant design, such as thin polyethylene or insufficient liner locking.
Ceramics as bearing surfaces
Ceramic femoral heads are made of either aluminium oxide or zirconium oxide. In 1970, Boutin was the first to report the use of an alumina ceramic bearing for total joint arthroplasty (Boutin Citation1972). At about the same time, Mittelmeier also developed a ceramic-on-ceramic bearing for total hip arthroplasty consisting of a threaded noncemented cup and a press-fit femoral stem (Mittelmeier and Heisel Citation1992).
Theoretically ceramics are superior to metal when used as femoral heads because their surface can be made smoother. The coefficient of friction will be reduced and lubrication properties are better, factors important for optimum wear characteristics. Ceramic heads are harder than those of metal and less susceptible to third-body wear and scratching of the surface. These materials are chemically more stable than metallic heads, which means that they maintain their surface finish without evidence of ion release. Fracture toughness and wear are directly related to the properties of the material. The ceramic should have high purity and density and low porosity and grain size, (2 to 3 μm ± 1) (Pizzoferrato et al. Citation1992, Clarke and Willmann Citation1994).
The initial enthusiasm engendered from low wear rates in the laboratory (Jacobs et al. Citation1994) was dampened by early failures, arising from poor implant design and use of low-quality ceramics. Factors associated with early failure of the initial ceramic hip bearings included improper positioning of the acetabular component and small femoral head sizes. Vertical cup placement, which increased contact stresses at the rim of the cup, resulted in localized fragmentation and third-body wear (Dalla Citation1996, Bader et al. Citation2002). In some instances the use of small ceramic femoral heads (<28 mm) contributed to this problem. Poorly designed tapers in some designs caused stress concentration and increased risk of fracture (Cuckler et al. Citation1995). Multiple reports of fracture of ceramic components exist in the literature. (Michaud and Rashad Citation1995, Fritsch and Gleitz Citation1996, Simon et al. Citation1998, Hasegawa et al. Citation2003, Allain et al. Citation2003, Hannouche et al. Citation2003). During the last two decades substantial improvements have been made, including prosthesis design, implantation technique, and most importantly, the quality of the alumina (Walter Citation1992, Ueno et al. Citation1999). These improvements reduced the fracture risk from 1% to 0.5‰ for a 10-year period (Toni et al. Citation1995, Fritsch et al. Citation1996, Sedel Citation2000). Damage of the ceramic caused by impingement and probably also micro- or macroseparation remains as a potential problem with aluminum ceramics.
Zirconium oxide ceramics were introduced in 1985 to prevent femoral head fractures observed with alumina ceramics (Piconi and Maccauro Citation1999). Zirconia can exist in three metamorphous (phases) termed cubic, monoclinic, and tetragonal. The majority of zirconia heads are made of yttriumstabilized tetragonal polycrystal ceramic called YTZP. The ability of zirconium oxide ceramics to withstand fracture is at least twice that of alumina ceramics. Zirconium oxide may therefore be used in heads with smaller diameter, which will reduce the friction further (Hamada et al. Citation1999, Cales Citation2000). Like alumina, zirconia has high biocompatibility and high resistance to scratching. These characteristics indicated a potential to improve the longevity of a THA. However, recently published studies of THR using zirconia/polyethylene articulations have not been encouraging (Allain et al. Citation1999, Kim et al. Citation2001, Norton et al. Citation2002). These studies describe problems with radioactivity and in stability. Aging of zirconium oxide ceramics induces a transformation of the tetragonal phase into the monoclinic phase, with an associated reduction in its ability to withstand fracture (Piconi and Maccauro Citation1999, Cales Citation2000). Blaise et al. (Citation2000) showed that the phase transformation did not affect the mechanical- and wear- performance of zirconia femoral heads, while Haraguchi et al. (Citation2001) reported two cases of surface deterioration of zirconia femoral heads associated with phase transformation after THR. Recently, Hernigou and Bahrami (Citation2003) compared zirconia with alumina heads after 12 years of follow-up. After 8 years the authors observed a higher polyethylene wear rate in the former group, which was associated with changes in roughness and roundness of the femoral head. The authors interpreted these changes as an effect of zirconia degradation.
Recently, new surface coatings of ceramic materials or diamond have been studied in the laboratory. These preclinical tests have revealed promising results, but the efficacy of such surfaces in the clinical setting remains to be studied (Lappalainen et al. Citation2003, Santavirta Citation2003).
Wear debris from bearing surfaces
Polyethylene particles
Polyethylene particles are the most investigated particles and several authors have discussed their role in the aseptic loosening process. The UHMWPE particles released from the joint have been estimated to be 0.070–6.3 μm in size, median 0.30 μm (Clarke Citation2002). Howie et al. (Citation1988) presented an animal study in which polyethylene particles alone could cause bone resorption in the absence of motion and infection. Later studies have, however, not been able to confirm these results (Howie et al. Citation1993, Van Der Vis et al. Citation1997).
Polyethylene particles are phagocytosed by macrophages that act in two major ways in the bone remodelling process. First they release cytokines involved in bone remodelling such as Prostaglandin E2 (PGE2), Interleukin 1 alfa and beta (IL 1α, IL 1β), Interleukin 6 (IL 6) and Tumor necrosis factor alfa (TNFα). These cytokines modulate osteoblast and osteoclast activity, which in turn increases the osteolysis (Murray and Rushton Citation1990, Howie et al. Citation1993, Kim et al. Citation1998, Ferrier et al. Citation2000, Hirashima et al. Citation2001). Secondly macrophages may differentiate into osteoclasts after stimulation of PE-particles (Quinn et al. Citation1998, Sabokbar et al. Citation1998).
Polyethylene particles produced in artificial hip and knee joints are different in size and shape (Landy and Walker Citation1988) but the effect on the macrophages and bone remodelling is caused by the submicron sized particles 0.3–1 μm (Green et al. Citation1998).
Several animal studies have addressed the question of whether PE-particles alone are responsible for bone resorption around implants. However, the amounts and size of particles varied between them. The time for introduction of the particles and design of the studies also differed greatly and it is difficult to find any clear connection between PE-particles and bone resorption based on animal studies (Dowd et al. Citation1995, Allen et al. Citation1996, Van Der Vis et al. Citation1997, Aspenberg and Van der Vis Citation1998, Lalor et al. Citation1999, Brooks et al. Citation2000, Sundfeldt et al. Citation2002a,Citation2002b, Zysk et al. Citation2003).
Dowd et al. (Citation2000) proposed that PE wear is linear in hip arthroplasty and also found a significant correlation between osteolysis and PE wear in a human study. When investigating Charnley arthroplasties, Sochart (Citation1999) found that wear rates below 0.1 mm a year resulted in more than 90 percent survival of the implant whereas annual wear greater than 0.2 mm resulted in failure of all implants within 25 years. These two studies imply that loosening of the implant may be affected by the wear rate and thus particles appear to be involved in the loosening process.
Probably there is an inter-individual variation in reaction to wear debris (Matthews et al. Citation2000a, Citation2000b), and the effects of wear particles could vary with time. Polyethylene debris produced initially in vivo is probably smaller and more bioactive than particles produced at later stages (Aspenberg and Herbertsson Citation1996). Third body wear can also generate particles with yet another biological significance. Thus, it appears difficult to draw any conclusions concerning the relationship between time in situ and the bio-effects of particles. There might be a common initial pathway for the loosening process in all hip implants. The response to particles may only explain why some prostheses loosen early and some late.
Few studies have evaluated the biological response to highly cross-linked polyethylene debris. Illgren et al. (Citation2003) compared the in vitro macrophage inflammatory response to highly cross-linked and conventional polyethylene particles. The levels of inflammatory cytokines released in response to these two kinds of particles were not significantly different at low concentrations (0.1-1X SAR) (Shanbhag et al. Citation1997). At the highest concentration (3X SAR) the levels of cytokines were 2-3 fold higher for highly cross-linked particles. Ingram (Citation2003) showed that cross-linking increases the biological activity of the debris when articulating on slightly scratched surfaces.
Any increase in the specific biological activity of particles from highly cross-linked polyethylene needs to be considered together with the reduction in wear volume when predicting the long term osteolytic potential.
Metallic particles
The CoCr particles released are extremely small; estimated at 20–80 nm (0.020–0.080 microns), some 250 times smaller than a red blood cell (Clarke Citation2002).
Doorn et al. (Citation1996) evaluated wear debris from metal-on-polyethylene and metal-on-metal articulations. Although the volumetric wear from metal-on-metal articulations was low, the calculated number of metal wear particles was higher than for the same volume of polyethylene wear.
At low, nonlethal doses, metal particles can stimulate macrophages to release various intercellular mediators, proinflammatory and bone resorbing cytokines, initiating a cascade of reactions which can lead to osteolysis and aseptic loosening (Shanbhag et al. Citation1995, Lee et al. Citation1997, Haynes et al. Citation1998, Nakashima et al. Citation1999).
In peripheral blood monocyte cultures, metallic particles (CoCr and Ti) were found to be more stimulatory for PGE2, IL-lα, IL-1β and TNF-α than polyethylene particles (Shanbhag et al. Citation1995, Blaine et al. Citation1997). Haynes et al. (Citation1998) studied their ability to stimulate rat peritoneal macrophages to release inflammatory mediators. While Co-Cr-alloy particles were very toxic and caused cell death, their ability to stimulate the release of mediators was quite limited or even inhibited.
Tissue explant studies have also been conducted to investigate the role of particulate debris in implant failure. Kadoya et al. (Citation1997) studied the histology of interface tissues and noted that osteoclastic bone resorption was significantly more extensive in the presence of metal than polyethylene particles. Although polyethylene particles led to macrophage recruitment and activation, metallic particles mediated bone loss through stimulation of osteoclastic activities. These findings are not in harmony with the findings of Doorn et al. (Citation1998), who studied cobalt-chromium particles retrieved from metal-on-metal total hip arthroplasties and reported less local activity than PE particles. Since particles from metal wear are small, every macrophage can store more particles resulting in fewer macrophages required to store the total amount and fewer activated. Alternatively, metal particles corrode and disappear and since they are so small, they are more easily excreted from the body.
The very small size of metallic debris released by metal-on-metal bearings (Doorn et al. Citation1997) combined with the fact that the bioavailability of metal is thought to be a function of the total surface area of the released debris (Shanbhag et al. Citation1994) have increased the concerns about the biologic response to metal-on-metal designs.
Ceramic particles
Light microscopy have revealed the presence of particles with a size between 0.4 and 7 μm around ceramic-on-ceramic total hip replacements (Henssge et al. Citation1994, Lerouge et al. Citation1997). The tissues with these particles have been reported to display a mixed pathology with granulomatous necrotic and necrobiotic tissue (Hatton et al. Citation2002). In a rabbit model, Kubo et al. (Citation1999) found marked histiocytic responses around particles of ultra high molecular weight polyethylene, stainless steel and cobaltchromium alloy. The histiocytic response was less intense in the vicinity of alumina particles. The inflammatory response in terms of TNFα release has been found to be less pronounced in the presence of ceramic than polyethylene particles. In an animal model, Warashina et al. (Citation2003) found that the inflammatory response and bone resorption induced by ceramic particles were much smaller than those induced by polyethylene and titanium particles. Ito et al. (Citation1993) found that wear products from zirconia ceramic on polyethylene combinations were more cytotoxic than debris from titanium on polyethylene combinations. Ceramic particles released are essentially insoluble, which means that their biological effects are caused by the particles as such, rather than soluble substances.
There are some reports of osteolysis in association with ceramic-on-ceramic hip prostheses due to severe wear (Borssén et al. 1991, Wirganowicz and Thomas Citation1997, Nevelos et al. Citation1999, Nevelos et al. Citation2001). Yoon et al. (Citation1998) reported on 103 uncemented Mittelmeier Biolox prosthesis and suggested that 23 femoral components and 49 acetabular components had radiological signs of osteolysis at a mean implantation time of 92 months. They observed abundant ceramic particles (mean size of 0.71 μm) in the interfacial tissues and concluded that ceramic wear particles could stimulate a foreign body response leading to osteolysis. Hatton et al. (Citation2003) studied alumina ceramic wear particles generated under microseparation conditions. Such wear particles could induce osteolytic cytokine production. However, the volumetric concentration of the particles needed to generate this response was very high. Given the low wear rates of ceramic-on-ceramic bearings, even in the presence of frequent microseparation, the authors found it unlikely that sufficiently high concentrations would occur in vivo. In conclusion, it remains unclear to what extent, if any, ceramic wear debris plays a role in the loosening process of ceramic-on-ceramic hip prosthesis.
Toxicity of the wear debris
One prevalent concern with regard to metal-on-metal articulations is the potential for long term adverse effects mediated by elevated serum metal ion levels and remote site deposition of metal particles. In a prospective randomised study with 2 years follow-up MacDonald et al. (Citation2003) evaluated polyethylene versus metal bearings. Patients receiving a metal-on-metal articulation had significantly elevated erythrocyte and urine metal ions compared with patients receiving a polyethylene insert. Several other studies of modern metal-on-metal articulating total hip arthroplasties have shown elevation of cobalt and/or chromium in the serum and urine (Jacobs et at. Citation1996, Brodner et al. Citation1997, Citation2003, Schaffer et al. Citation1999, Maezawa et al. Citation2002).
The smaller size of metal particles can lead to greater transport of particles from the joint region. Shea et al. (Citation1997) demonstrated metal alloy particles in the pelvic and axillary node chains of seven of 23 patients who had lymph node dissections after primary total hip arthroplasty. Urban et al. (Citation2000) identified dissemination of metallic wear particles to the liver, spleen, and abdominal lymph nodes in patients with a previously failed implant and in those with a primary hip or knee joint replacement.
One concern regarding the lymphoreticular dissemination of metal alloy particles and elevated serum metal levels is the potential carcinogenic effect. Chromium is an essential trace metal (Versieck and Cornelis Citation1989) and its depletion in the body consistently results in specific changes, while repletion reverses these abnormalities. Hexavalent chromium, a possible implant degradation product, has been listed as a class-I human carcinogen. Increased levels of cobalt can induce polycythemia and testicular toxicity and can interfere with DNA repair (Angle Citation1995, Woods Citation1995). Reports in the literature about clinical evidence for an increased risk of implant site tumors after total hip replacement remain controversial (Jacobs et al. Citation1992, Gillespie et al. Citation1996, Langkamer et al.Citation1997, Rock Citation1998). The relationship between cancer risk and the release of metal debris into body fluids needs to be elucidated (Nyren et al. Citation1995).
Immune response to metallic particulates remains a matter of controversy. Evans suggested that an immune response may contribute to aseptic loosening and found that nine of 14 patients with loose metal-on-metal implants had cutaneous sensitivity to one or more of the components of the alloys (Evans Citation1974). In subsequent studies, metal hypersensitivity was not found to be associated with loosening (Brown et al. Citation1977, Rooker and Wilkinson Citation1980). Wooley et al. (Citation1997) found an elevated proliferative cellular response to polymethylmethacrylate and metallic particles in patients undergoing revision surgery. The existing literature is conflicting regarding the role of specific immune responses in aseptic loosening, osteolysis, and remote tissue effects.
Ceramics and polyethylene are generally considered to be biologically inert in bulk form. The literature demonstrates that, in particulate form, the biological response is similar to that of metal particles (Ito et al. Citation1993, Catelas et al. Citation1999, Kubo et al. Citation1999). The systemic effects and dissemination properties of ceramic and polyethylene particles are unknown. Because ceramics and polyethylene are insoluble in biological media, the concerns of potential systemic effects relate only to wear debris.
Creep and wear mechanisms
Wear in hip arthroplasties is mainly caused by 3 basic mechanism's: adhesion, fatigue and abrasion (McKellop Citation2001). Adhesive wear occurs if the bond strength between the polyethylene and the metal exceeds the inherent strength of either material. Typically, polyethylene is pulled off from the surface, forming fibrils and/or small pits. When adhesive wear occurs on a micron or submicron scale, the bearing surface can still appear highly polished to the eye (Jasty et al. Citation1994, McKellop et al. Citation1995). Fatigue wear is cracking, pitting and/or delamination caused by cyclic stresses applied to the bearing surface. This is the main wear mechanism in knee arthroplasty. Abrasive wear occurs when damaged areas on the hard femoral head cut scratches on the opposing softer plastic surface during sliding. Such damages may be the edge of an existing scratch, embedded third-body particle, or even original contaminants (inclusions) exposed by the wear process itself. This type of wear is analogous to sanding a piece of wood with very fine sandpaper. Cooper identified two types of abrasive wear in UHMWPE: one involving ‘macroscopic’ and the other ‘microscopic’ asperity contact between the sliding surfaces (Cooper et al. Citation1993). Furthermore, seemingly smooth surfaces on total hip components have undulating peaks and valleys corresponding to a certain surface roughness (Ra). UHMWPE components vary in surface Rfrom 0.28 to 0.89 μm. The polished metal counterfaces of orthopaedic bearings, in contrast, vary from 0.01 to 0.05 μm (Essner et al. Citation1998, Cooper et al. Citation1993).
When two surfaces first slide against each other under load, many of these asperities are removed, producing a high initial wear rate referred to as the “wearing in period”. As the femoral head adapts to a polyethylene acetabular component the contact area increases. This plastic deformation has also been described as creep. This process results in better conformity, lower contact stresses and lower rate of wear (Bartel et al. Citation1986, Wroblewski et al. Citation1996). The creep declines with time and becomes negligible by 12–18 months (Schmalzried and Callaghan Citation1999). Because of the initial difference in surface roughness at the UHMWPE and metal counterfaces, the initial wear rate involves the removal of the larger ‘macroscopic’ asperities on the UHMWPE surface, whereas the long-term wear rate is governed by the ‘microscopic’ asperity size of the metal counterface (Cooper et al. Citation1993). Thus changes of the surface roughness of UHMWPE components may be expected to affect the initial wear rate, whereas reduced surface roughness of the metal component will also affect the long-term wear behaviour. Several authors (Hall et al. Citation1997, Kusaba and Kuroki Citation1997, Essner et al. Citation1998) have observed a correlation between the clinical wear rate and the arithmetic mean surface roughness (Ra) of explanted femoral heads, whereas others found no or only a small association (Hall et al. Citation1996, Wang et al. Citation1998, Elfick et al. Citation1999). Because the femoral head may be damaged by several mechanisms after implantation (e.g., three-body wear), the relationship between manufactured surface finish and clinical wear rate remains unclear.
Wear particles may not only be generated from the articulating surfaces, but also from other interfaces such as modular junctions and implant/ cement or implant/bone interfaces. Such particles are likely to migrate and act as third bodies in the articulation.
Alternative bearing surfaces
Metal on conventional polyethylene
The “traditional” bearing surface, a metal ball and a polyethylene socket, has been widely used since 1962. Polyethylene has several advantageous properties, including low friction and good shock absorption. A well-designed hip replacement with a metal-on-polyethylene bearing demonstrates low wear rate and good long-term clinical results (Callaghan Citation2000, Malchau et al. Citation2000, Keener Citation2003a, Keener Citation2003b).
The average wear rate is usually 0.1–0.2 mm/ year (50–100 mm3/year) (Griffith et al.Citation1978, Gomez-Barrena et al. Citation1998, Schmalzried et al. Citation1998b, Sochart Citation1999, Yamaguchi et al. Citation1999) corresponding to hundreds of millions of wear debris particles released into the surrounding tissues (McKellop et al. Citation1995) which (especially in cementless implants) may result in osteolysis as early as 5–7 years after implantation (Schmalzried et al. Citation1992, Amstutz et al. Citation1992).
Today total hip arthroplasties are implanted in younger and more active patients than 1-2 decades ago. One important reason for this change is the improved knowledge about optimum cementing technique and the introduction of new surface treatments of uncemented implants. In addition the demand from patients to maintain a high quality of life despite disabling hip disease at an early age has increased. Against this background, reduction of the wear problem has become increasingly important. This has been addressed by the development of more wear resistant polyethylenes (highly crosslinked), or by use of alternative bearing surfaces such as ceramic-on-polyethylene, metal-on-metal, and ceramic-on-ceramic.
Metal on highly cross-linked PE
In the 1970s Oonishi and associates induced very high levels of cross-linking by exposing finished cups to 1000 kGy gamma radiation in air (Oonishi et al. Citation1992, Citation1996, Citation1997). Between 1971 and 1978, 62 patients underwent total hip replacement using this material in the socket. Twenty eight hips could be evaluated 6 to 17.3 years postoperative. The wear rate was 0.05mm/yearr for cross-linked and 0.28mm/yearr for non irradiated cups (Oonishi and Kadoya Citation2000). Grobbelaar et al. (Citation1999) reported two clinical follow-up series with acetabular components gamma irradiated at 100 kGy in the presence of acetylene gas. The first study had 1422 years follow-up. Fifty-six of 64 hips displayed no measurable wear. The average calculated wear rate of this entire series was 0.011 mm/year. The second series included 39 hips followed for 16 years. Thirty hips displayed no measurable wear and 9 a total linear penetration of 0.7–1.5 mm. Wroblewski et al. (Citation1999) studied the wear of silane cross-linked polyethylene in hips with a mean clinical follow-up of 10 years. After an initial “bedding-in” penetration of 0.2–0.4 mm/year, the average penetration rate decreased by an order of magnitude to 0.02 mm/year.
The highly cross-linked polyethylenes mentioned above were not commercially available and were manufactured by the respective group of investigators. Today there are several commercially available contemporary approaches to producing highly cross-linked polyethylene ().
Several hip simulator studies has shown improved wear resistance of highly cross-linked compared with conventional polyethylene (Hamilton et al. Citation1997, McKellop et al. Citation1998, Edidin et al. Citation1999, McKellop et al. Citation1999, Muratoglu et al. Citation1999, Citation2001a, Citation2001b, Citation2002, Bragdon et al. Citation2003, D'Lima et al. Citation2003).
There are few clinical studies comparing the highly cross-linked and conventional polyethylenes. Hopper et al. (2003) evaluated polyethylene liners, cross-linked with 50 kGy of gamma irradiation in a clinical study with 2–3 years follow-up. The mean wear rate was 0.08 mm/year. Martel et al. (2003) reported a prospective randomised study in which CrossfireTM highly cross-linked polyethylene was compared with conventional polyethylene. A significant reduction in 2- and 3dimensional linear wear rates (42% and 50%) was found in the highly cross-linked group. Rothman et al. (Citation2004) compared the wear performance of highly cross-linked (105 kGy) with conventional polyethylene in bilateral THR. The wear rates for the two polyethylenes were 0.075 mm/year and 0.14 mm/year respectively after 2 to 3 years follow-up. Manning at al (Citation2004) recently presented the four year results of 109 hips in which WIAM highly cross-linked polyethylene had been used. The steady state wear rate was 11 microns/year, while in an age matched population of patients with traditional polyethylene the wear rate was 143 microns/year. The wear reduction observed in the groups with cross-linked polyethylene in these studies is more modest than predicted by in vitro studies.
Further clinical studies with longer follow-up are needed to substantiate these low wear rates, to evaluate the occurrence of osteolysis associated with these implants and to ensure that these new materials are not associated with any other unexpected adverse events.
Ceramic-on-polyethylene
Alumina and zirconia femoral balls have been used widely as bearing surfaces against polyethylene cups. Most clinical studies have shown wear rates ranging from 0.025 to 0.075 mm/year with use of aluminium heads, which is substantially lower than observed with the use of a corresponding metal/ polyethylene articulation (Clarke and Willmann Citation1994, Sauer et al. Anthony Citation1998). Wear of about the same magnitude as for alumina/polyethylene articulations has been reported with zirconia heads (Willmann Citation2000). In a clinical follow-up, Urban et al. (Citation2001) studied alumina heads articulating against cemented all-polyethylene sockets. After 20 years and despite using non-contemporary cement technique outstanding results were reported with mean linear wear rate of 0.034 mm/year and 79% prosthetic survival based on revision as end point.
All studies have, however, not been able to confirm reduced wear rate with ceramic heads. In a hip simulator study McKellop et al. (Citation1992) recorded slightly greater polyethylene wear with alumina but less with zirconia when compared with heads made of cobalt-chromium alloy. In a study with 13 years follow-up the mean linear wear rate of polyethylene cups articulating against alumina heads was the same as reported for many metal on polyethylene bearings (0.10 mm/year, Hasegawa Citation2001). In another evaluation little difference was reported between the use of alumina and metallic heads (Devane and Horne Citation1999). Unacceptable high wear rates, osteolysis, and loosening with the use of an early type of Zirconia ball have also been observed (Piconi et al. Citation1998).
The reasons for this disagreement among the studies are not clear. Change of material quality over time, influence of third-body particles from some stem/cup combinations and poor study design could be some explanations. Future prospective and randomised studies are necessary to clarify this issue.
Metal-on-metal
The first true metal-on-metal THR was the prototype developed by Philip Wiles in 1938 (Wiles Citation1957). These were unsuccessful because of the low quality of the stainless steel used, poor manufacturing and lack of adequate fixation. In the early 1950s, McKee (Citation1951) did a small series of THRs using a metal artificial joint made of stainless steel. It relied on cementless fixation, but lacked the properties necessary to become integrated to bone. With the introduction of cement in the 1960s and early 1970s, the metal-on-metal design by McKee and Farrar became popular. These prostheses were made of CoCrMo alloy and had head diameters of 32–42 mm. The early versions of these prostheses had a poor fit between the head and socket, while the quality clearly improved with time. Hip simulators indicated that loosening was likely to be caused by mechanical forces and not biological processes initiated by wear. Disregarding the early failures, the long-term survivorship of the early metal-on-metal designs has been comparable to that of the metal-on-polyethylene Charnley (Jacobsson et al. Citation1990, Amstutz and Grigoris Citation1996, Schmidt et al. Citation1996, Schmalzried et al. Citation1996a). The steady-state wear rates were found to be a few micrometers per year (Willert et al. Citation1996, Kothari et al. Citation1996, McKellop et al. Citation1996). Recently, Brown et al. (Citation2002) reported excellent long-term results of the McKeeFarrar prosthesis with an implant survival of 74% after 28 years.
Nonetheless, the first generation of metal-on-metal prostheses were gradually abandoned in the 1970s because the overall results achieved by the Charnley type low-friction arthroplasties appeared to be better. Further on, continuous release of metal from the metal-on-metal articulation has remained a cause of concern.
In view of the growing awareness of the problem of extensive osteolysis caused by polyethylene wear debris, a number of second-generation metal-on-metal implants have been developed including surface replacements (Schmalzried et al. Citation1996b, McMinn et al. Citation1996). Weber developed the Metasul prosthesis, which has been widely used clinically since 1988. The designs include cemented and uncemented versions. The metal socket liner is usually polyethylene-backed in order to reduce the difference between the elasticity of metal and bone. The damping effect of polyethylene may be a reason for the good results of more recent metal-on-metal bearings, but some all-metallic sockets seem to do equally well (Amstutz et al. Citation2004, McMinn et al. Citation1996).
It is also apparent that metal-on-metal implants have the ability to “self-heal”, that is, to polish-out isolated surface scratches caused by third-body particles or subluxation damage (McKellop et al. Citation1996).
Clinical and laboratory wear studies have indicated that metal-on-metal implants often exhibit 10 to 20 times greater wear-in during the initial 1 to 2 years of clinical use, or one to two million cycles in a hip simulator (Weber Citation1996, Medley et al. Citation1996, Chan et al. Citation1999). During the steady-wear phase after the first year of use, the linear wear continues to be less than 5 μm for several years, which corresponds to a volumetric wear rate lower than 0.2mm3/year (Medley et al. Citation1996, Chan et al. Citation1999, Sochart Citation1999, Rieker et al. Citation1999). Recently St John et al. (Citation2004) compared in a hip simulator study metal-on-polyethylene with metal-on metal articulation. The wear rates after wearing-in period were about 12-20 times greater for polyethylene in terms of weight loss and about 110-180 times greater in terms of volume of material removed from the bearing surfaces. These results are consistent with those reported by Chan et al. (Citation1997) whose results suggested that the wear rates for metal-on-metal devices were a 20–100-fold decrease over metal-on-polyethylene devices.
Dorr et al. (Citation2000) reported medium-term (5.2 years follow-up) results of 70 patients with Metasul articulation. One patient had revision of a loose cup while no hip displayed radiographic evidence of acetabular osteolysis. The clinical results were similar to those of total hip replacements with a metal-on-polyethylene articulation. The authors regarded their results as encouraging. In another study 266 patients, received third-generation Zweymueller-SL total hip arthroplasties with metal-on-metal articulation. The mean follow-up was 52 months. No aseptic loosening or osteolysis were observed. The authors concluded that metal-on-metal articulations were just as satisfactory as those of a conventional polyethylene on ceramic articulation, while the metal-on-metal articulation does not seem to give rise to new problems or complications (Korovesis et al. 2003). In a randomised study, Lombardi et al. (Citation2004) evaluated 46 hips with metal-on-polyethylene and 53 with metal-on-metal articulation after a mean followup of 5.7 years. Three patients in the polyethylene group and none in the metal group had evidence of acetabular radiolucencies. No acetabular revisions or device-related complications were observed in neither group. The authors concluded that a metal-on-metal articulation may represent a viable alternative for total hip arthroplasty in the young high demand and active patients.
Metal-on-metal articulation may avoid polyethylene wear debris, but as previously mentioned there remain concerns about the actual extent of metal wear and the long-term effects of local and systemic exposure to metal ions and particles.
Ceramic-on-ceramic
In early years, so-called monobloc conicallythreaded or spherical press-fit cups of alumina ceramic were used with varying success. Although several authors reported acceptable mid- and longterm results (Nizard et al. Citation1992, Sedel et al. Citation1994), these earlier designs had fixation problems partly because the alumina surface did not achieve biological fixation to bone and perhaps partly because of the difference in modulus of elasticity between the bone and the implant. To reduce the rigidity of the ceramic-on-ceramic bearing, newer designs have been supplied with an outer lining of polyethylene, whereas others avoid the different elasticity between the implant and the bone by using ceramic liners inserted into a metallic shell designed for bony fixation (Dalla Pria Citation1996, D'Antonio et al. Citation2002, Bierbaum et al. Citation2002).
Boehler et al. (Citation2000) examined hemispheric monolithic alumina ceramic sockets and modular titanium sockets with alumina ceramic inlays. The mean annual linear wear rate 7 years postoperatively was about 39 and 27 μm respectively. Prudhommeaux et al. (Citation1998) examined the in vivo wear of high quality alumina.The overall wear calculated by the weight of debris generated was approximately 1000 times less than a metal-on-polyethylene and 40 times less than a metal-on-metal joint. Other hip simulator studies and clinical retrievals have indicated that the steady-state wear rate of alumina-on-alumina bearings can be as low as 1–2 μm/year (Willmann Citation1998, Oonishi et al. Citation1999).
In a multicenter, prospective and randomized study, an alumina-on-alumina ceramic bearing was compared with cobalt chrome-on-polyethylene bearings. After 4 years the clinical performance was equal between the groups (Bierbaum et al. Citation2002). Modern alumina-on-alumina bearings are, however, not immune to high wear (Winter et al. Citation1992, Nevelos et al. Citation1999).
Wear-factor related to the patient and surgical technique
Polyethylene wear in vivo is multifactorial with a complex interaction of variables. It is therefore not surprising that rates of polyethylene wear are highly variable (Schmalzried Citation1998b). Patientrelated variables, such as age and gender have some relation to the activity of the patient, but the type and intensity of activity performed vary individually and partly independent from other demographic variables. There are variables related to the hip prosthesis, which include all aspects of the acetabular and femoral implants (not just the polyethylene). There are also variables related to the surgical procedure such as implant handling, contamination and prosthetic positioning. Generation of particles from modularity and implant instability or migration are also important. Loosening of the implant can adversely affect wear, and vice versa. These variables are important as they can affect the loads on and the motions of the bearing and the degree of three-body-wear mechanisms. There is also variability caused by the resolution of the technique used to measure wear.
Cemented sockets and aseptic loosening
In cemented acetabular components, the path of least resistance for joint fluid and wear particles is at the cement-bone interface. Schmalzried and associates, (1992b) has demonstrated in autopsy studies that after the implantation of a cemented socket, the subchondral bone reconstitutes and acts as a partial barrier that prevents joint fluid and particles to gain access to the trabecular bone. The soft-tissue membrane created by the biologic reaction to wear particles dissects along the cementbone interface, leading to disruption of this interface. As the interfacial disruption progresses to the acetabular dome, fixation is lost. Radiographically, the osteolytic pattern is linear, and the radiolucency occurs at the cement-bone junction. These lesions occurred as an increase in extension from the periphery to the central region of the interface (Hultmark et al. Citation2003). The component tends to migrate into the radiolucent areas in the superior aspect of the acetabulum. Although cystic lesions are uncommon, continuous bone loss may result in massive enlargement of the acetabulum especially if the disease is longstanding and untreated. Radiographically, the hallmarks of cemented socket loosening are circumferential radiolucency, fracture in cement mantle, or component migration.
Cementless sockets and aseptic loosening
The pattern of osteolysis around cementless sockets depends of whether bone ingrowth has occurred or not. If the socket is stable and ingrown, the path of least resistance may be remaining gaps, regions with less dense bone or local osteoporosis and screw holes, which allow particles to migrate into the trabecular bone surrounding the implant. This results in two patterns of osteolysis depending of the local particle concentration. High particle loads may be more likely to result in more or less rapidly growing lesions with indistinct margins. Loosening does not result until the bone loss is extensive, and failure is usually acute and catastrophic. The patient may remain clinically without symptoms until the component loosens. The second pattern of osteolysis is a more slowly growing lesion with sclerotic margins. Sclerotic bone often forms at the implantbone interface, probably as a result of micromotion. The pattern of osteolysis in this case is quite similar to that seen with cemented sockets. Linear osteolysis occurs with the implant migrating into the radiolucent area. In addition, if late migration of the component is noted in the absence of previous radiolucency, then fibrous fixation with progressive osteolysis should be assumed to have developed. The consequences of osteolysis in this case are progressive bone loss and clinical loosening.
The radiographic determination of whether a socket has bone ingrowth is quite difficult. A cementless socket that is radiographically stable is often presumed to be ingrown, although that may not be the case. Cementless sockets in which bone-ingrowth does not occur are predisposed to late migration.
Aims of the thesis
The outcome of different types of polyethylene, bone cements and one design of uncemented fixation with porous and ceramic coating were studied after 2–3 years follow-up in one cohort study (I) and 4 prospective and randomised studies (II–V).
Specific aims:
Study
– to evaluate factors which might influence femoral head penetration in all polyethylene sockets and polyethylene liners. Two cemented and 2 uncemented cup designs were studied.
Studies II and III
– to evaluate the clinical performance of electron beam irradiated highly cross-linked polyethylene including femoral head penetration, cup fixation, bone remodelling (cemented design), radiographic appearance (cemented design) and early clinical results. One cemented and one uncemented cup design were studied. Sockets or liners made of conventional polyethylene sterilized with gamma irradiation in reduced oxygen environment constituted controls.
Study IV
– to evaluate if addition of fluoride to a low temperature curing cement (Cemex) improves the stability of Spectron stems, the quality of the bone, the cement/bone interface and the early clinical results. Palacos with Gentamicin cement was used in the control group.
Study V
– to evaluate if porous coating with an outer ceramic layer or fluoride containing cement improved the early stability of the acetabular cup, the development of postoperative radiolucent lines and the early clinical results. Palacos with Gentamicin cement was used in the control group.
Material
Patients
Study I. 201 patients were extracted from 5 prospective randomised studies (). These studies evaluated socket fixation and femoral head penetration using radiostereometry. Four basic designs; cemented Lubinus (Link, Germany) and Reflection (Smith & Nephew, USA) cups, uncemented Trilogy (Zimmer, USA) and Reflection (Smith & Nephew, USA) cups were included (). In the cemented patients only cups fixed with Palacos cement (Schering-Plough, Belgium) were studied. In total 237 hips (231 patients) were available. Thirty of these were excluded because too few tantalum markers were visible on the postoperative or 2 year follow-up examination. Six patients had undergone bilateral operations, but only the first operated hip was included. In total, 201 total hip arthroplasties in 201 patients (117 women) remained for study I (). 132 patients had been operated at the Sahlgrenska University Hospital in Göteborg, 55 at the Northern University Hospital in Umeå and 14 at the Akademiska University Hospital in Uppsala.
Figure 9. Acetabular implants from left to right. Reflection, all-poly (Smith & Nephew, USA), Lubinus, eccentric (Waldemar Link, Germany), Reflection, ± HA, ± screws or pegs (Smith & Nephew, USA), and Trilogy with 70% HA and 30% TCP ± screws (Zimmer Inc., Warsaw, USA).

Table 4. Patient distribution in Paper I–V
Table 5. Study I: Demographic data and cup specifications
Table 6. Study I: Patient-related data for the uncemented pressfit Reflection group
Study II. Sixty patients (61 hips) with a median age and weight of 55 (35–70) years and 82 (47–120) kg on the waiting list for THR at Sahlgrenska University Hospital participated (). The patients were randomized using closed envelopes to receive either highly cross-linked or conventional all PE cups (Sulene™, Centerpulse, Zurich, Switzerland). One patient who had undergone bilateral surgeries, received different PE on each side. The results of 52 patients (53 hips) with 2 years follow-up were included.
Table 7. Study II: Demographic data
Study II and III. In study III, 32 patients with bilateral primary or secondary arthrosis of the hip received bilateral hybrid THA. Surgery started on the most painful side, which was randomised to either highly cross-linked or standard PE liner (Zimmer Warsaw, Indiana) using closed envelopes. The type of PE not used on the first side was inserted on the opposite side. Twenty-seven patients (54 hips) were followed up for 2 years (). The results of 49 patients from Study II followed 3 years postoperatively were included ().
Table 8. Hybrid study (Study III): Demographic data
Table 9. Cemented study (Study III): Demographic data
Studies IV and V. Ninety patients (97 hips and 96 hips respectively in each study) median age 70 (31–81) years and weight 69 (38–108) kg on our waiting list for THR accepted to participate (, ). All types of preoperative diagnoses were included. The choice of fixation was stratified and randomised based on age (≤55 / <55 years), gender, diagnosis (primary arthrosis, inflammatory arthritis/longterm cortisone treatment, sequele after femoral neck fracture) and bone quality according to preoperative DEXA measurements (less or equal vs. higher BMD than age matched controls). The last variable was excluded from the stratification protocol in patients with osteo-main groups (two cemented groups and one hybrid synthesis in their hip. A dedicated software was group). In the first group fluoride-containing used for stratification and randomization (Pocock cement (Cemex-F, Tecres S.p.A. Italy) and in the 1983). The stratification was designed to create 3 second group, Palacos with Gentamycin (Schering Plough Germany) was used (). In both cemented groups, the same types of cement were used to fixate both components. In the third group an uncemented cup and cemented stem were used. In this group the fixation of the femoral component was again randomised to either of the two cements. The results on femoral and acetabular sides are presented separately in papers IV and V, respectively. Patients operated bilaterally were included with both sides provided that the two sides had been stratified to different type of fixation.
Table 10. Study IV: Demographic data
Table 11. Study V: Demographic data
Table 12. Composition of the bone cements
A modified Hardinge approach and third generation cementing technique were used in all studies.
Implants
Stems
Studies I–V. All patients in studies II, III, IV, V and 117 hips in study I received Specton EF stem (Smith & Nephew, Memphis, TN, USA). This stem is made of cobalt chromium alloy. Its proximal third is grit blasted (average surface roughness, 2.8μm). The distal part is smoother (average surface roughness, 0.7 μm) and a centraliser is attached to the tip of the stem. The femoral stems had been supplied by the manufacturer with 3 titanium towers each with a tantalum marker attached to its tip. Femoral heads of cobalt-chrome alloy with a diameter of 28 mm were used in all hips except 55 patients in study I and 3 patients in studies IV and V were zirconium heads were used. Thirty patients in study I received cemented Lubinus stems, 17 cemented Anatomic-Option stems and 37 uncemented stems (6 Epoch, 17 Anatomic, 3 Cone, and 11 Bimetric, ).
Table 13. Different stems used in paper I
Cups
Study. Four cups with different sterilization method were used (, ). The Lubinus cups and the liners to the Trilogy cups had been sterilized with gamma irradiation (25 kGy). The Lubinus cup (n=30) is manufactured by compres-sion-molded technique and sterilized with gamma irradiation in reduced oxygen environment. The Trilogy liners (n=65) were also manufactured with compression-molded technique. Sterilization had been done in an inert nitrogen environment. The metallic shell of this design is made of titanium-aluminum-vanadium alloy (Tivanium, Zimmer, Inc, Warsaw, IN) with a fiber mesh of pure titanium attached to it. The mesh is plasma-sprayed with 40-μm coating consisting of 70% hydroxyapatite and 30% tricalcium phosphate. Twenty-one cups had no holes for screws. In the remaining 44 cups 1–3 screws were inserted in the 3 screw holes available.
The 2 remaining groups had polyethylene sterilized with ethylene oxide (EtO). Thirty-seven were cemented all-polyethylene (Reflection all-poly) and 69 were uncemented porous coated press-fit cups (Reflection). Both designs were manufactured using ram-extrusion technique (). The uncemented Reflection cup is made of tita-nium-aluminum-vanadium alloy. It has porous bead coating made of commercially pure titanium. Based on a randomization protocol designed for this subpopulation, 15 of these cups had additional screw fixation and 19 were supplied with pegs. Thirty-five had no additional fixation, but 18 of those 35 were coated with a 45 μm thin layer of pure hydroxyapatite (). The thickness of the Trilogy liners varied between 6.3 and 11.3 mm and the Reflection liners between 7 and 12 mm. The Lubinus, Trilogy and cemented Reflection cups were inserted with femoral heads made of cobaltchromium alloy. Fifty-five of the 69 uncemented Reflection cups articulated against a ceramic head made of zirconium oxide. The remaining 14 patients had femoral heads made of cobalt-chro-mium alloy.
Study II. All patients received a flanged all-PE cup of the same design (Weber, Centerpulse Orthopedics Ltd, ). The Durasul™variation of this cup is made from cylindrical disc-shaped samples machined from compression molded GUR 1050 UHMWPE bars. The dics-shaped samples were irradiated with a process called warm irradiation with adiabatic heating, and subsequent melting (Muratoglu et al. Citation2001a). The Durasul™cups were machined from irradiated cross-linked PE and the Sulene™cups from conventional compression-molded sheets of UHMWPE. The Durasul™cups were sterilized using EtO gas and the controls were sterilized using gamma irradiation (25–40 kGy) in inert nitrogen atmosphere.
Study III. All patients with hybrid hips received Trilogy cups with three cluster holes. The liners were machined from highly cross-linked PE (Longevity Zimmer Warsaw, Indiana USA,) or conventional compression-molded sheets of UHMWPE (GUR 1050). The Longevity liner was sterilized using gas plasma and the controls were sterilized with gamma irradiation (25–37 kGy) in nitrogen atmosphere.
Study V. Reflection all poly cups (59) were used in both cemented groups. All hips in the uncemented group operated with press-fit Trilogy cup (n=37, ). Additional fixation with one screw was done in one hip, 28 cups had two and the remaining 8 hips had three screws inserted in the three screw holes available. Twenty-eight mm femoral heads made of cobalt chromium were used in all but 3 hips, which received heads made of zirconium (2 uncem. 1 Cem-F). None of the bilaterally operated patients received the same method of fixation on both sides.
Methods
Clinical evaluation
In studies II, III, IV and V, the patients were followed prospectively using a standardised form including Harris hip score. In study I, the activity level was evaluated using a questionnaire, which was sent to the patients 2–7 years (median 3 years and 9 months) after the operation. Each question was rated with an increasing number of points (3–5/question) corresponding to the increasing activity level of the patient. The same questionnaire was used in studies II and III at the 2 years control. This questionnaire included 9 questions in study I and 10 in studies II and III. One question in study I had different answer alternatives at Sahlgrenska and Umeå Hospital and had to be excluded. The repeatability of the questionnaire was studied in 20 patients in study I who agreed to answer a second time at an interval of 15 days ( in Appendix).
Radiography
Conventional radiographic examinations including AP, lateral, and pelvic views centered on the symphysis were exposed within 3–4 days postoperatively and after 2 years.
The cementing of the cup in studies II and V was graded according to a Modification of the classification of Barrack (Hultmark et al. Citation2003). In Study IV the cementing of the stem was graded according to the original Barrack classification (Barrack et al. Citation1992). Radiolucent lines wider than 0.3 mm were recorded based on the observations of Hultmark et al. (Citation2003). The extent of such lines were classified into 4 grades (no lucency, <50%, 50–99% and 100%) in the different Gruen regions (Gruen et al. Citation1979). On the acetabular side a Modification of the DeLee and Charnley (Citation1976) regions was used because the cup was divided in three equal parts on frontal and lateral views (). The maximum width of each line was also measured. Radiolucent lines were defined as linear lucent areas parallel to the femoral or acetabular component with a maximum width up to 2 mm. Radiolucencies with a cystic or linear appearance greater than two millimetres in width were recorded as osteolytic lesion. Heterotopic bone formation was classified according to Brooker et al. (Citation1973). The inclination of the cup was measured on the postoperative radiographs of the pelvis in studies I–III and V.
Radiostereometry
Radiostereometry (RSA) (Selvik 1989, Kärrholm Citation1997) was used to study wear and cup migration in studies I, II, III and V. RSA was even used to study stem translation and rotation in study IV. The postoperative examination was performed 3 to 4 days after surgery. In studies I, IV and V, the following examinations were performed at 6, 12 and 24 months, while in studies II and III, RSA examinations were also performed at 3 months. In study I, the first RSA recording after the postoperative one took place after 2 months in the uncemented Reflection group and not after 6 months as indicated in paper 1. In all examinations the patients were in the supine position. In studies II and III examinations were also done with the patients standing. These examinations started at 3 months. Postoperative examinations with the patient standing were tried also within the postoperative week but these examinations were subsequently excluded because the patients experienced too much discomfort.
Translations of the femoral head centre using the cup markers as a fixed reference segment represented the penetration of the femoral head into the polyethylene cup or insert. This motion, called femoral head penetration, is the combined effect of deformation of the polyethylene (creep) and wear. The migration of the cup was measured as rotations about the three cardinal axes and translations of the cup centre.
Proximal/distal translation of the gravitational centre of the segment defined by the stem markers and the centre of the femoral head and the corresponding translations of the centre of the cement markers using the bone markers as reference segment are presented in study IV. Rotations of the stems about the three cardinal axes were also measured.
To maintain the precision of the measurements the RSA was only performed if at least 3 well-defined markers could be identified. These markers should have an acceptable configuration (condition number < 120, Söderkvist Citation1993) and stability (mean error of rigid body fitting < 0.32, Selvik 1989).
The precision of the measurements was calculated by repeated examination of the patient with an interval of 10–20 minutes (double examinations). Based on previous studies (Kärrholm and Snorrason 1992) we assumed that the error was normally distributed. The 99% confidence interval was calculated as the mean difference + 2.7 times the standard deviation ().
Table 14. Precision based on double examination
Hips missing from the RSA evaluation in each study are shown in the .
Table 15. Hips missing from RSA evaluation in each study
DEXA
Periprosthetic BMD was measured using a Lunar DPX-L or Lunar DPX-IQ densitometer (Lunar Corporation, Madison, WI) in studies II and IV. Fifteen patients with hip arthroplasty were scanned one time with each type of densitometer to establish a cross calibration between the instruments. In each ROI, a linear correlation (mean, r = 0.96 range, 0.90–0.98) was observed. The mean difference ± 1 SD between the two densitometers was 0.9 +/-6.4%. The mean difference was used to adjust all values from the DPX-L device in study II and from DPX-IQ in study IV. These DEXA measurements were taken one week postoperatively, and after 12 and 24 months. During scanning, the patient was placed in the supine position. A foot brace and knee supports were used to obtain standardized positions. The pelvic scan was centred on the acetabular component. It was 15 cm wide, started at the lower border of the inferior pubic ramus and continued proximally to the lower limit of the ipsilateral sacroiliac joint ().
The paint option of the orthopaedic software (V 4.6 Lunar Corporation, Madison, WI, USA) was used to evaluate the BMD in five ROIs around the acetabular component trying to exclude the cement mantle (study II, ).
Two methods were used to analyse the femoral scans in study IV. In the first one the software (V 4.6 Lunar Corporation, Madison, WI, USA;) automatically divided the proximal femur into 7 Gruen regions based on the implant length excluding the tip of the greater trochanter. We expanded this region manually to also include this part of the trochanter. In the second method all visible cement was manually excluded from the scan field using the software facility ‘paint’, but leaving the position of the ROIs unchanged from the first analysis set. The ROI 4 was excluded from the manual analysis ().
Figure 14. Classification of periprosthetic BMD on femoral side in 7 ROIs according to Gruen for automatic (left) and manual (right) DEXA analysis.
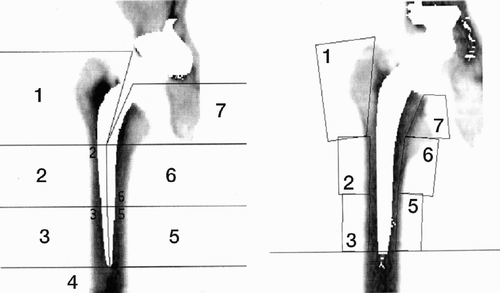
In both studies (II and IV) 20 patients were examined twice using the DPX-IQ with a median 6 and 8 days respectively between the two examinations. The precision was expressed as the coefficient of variation (CV) according to the formula: CV% = 100 X ((δ/√2) /μ) for each ROI, where δ represents the standard deviation of the difference between the paired BMD measurements, and μ is the overall mean of all the BMD measurements for each individual ROI (Wilkinson et al. Citation2001). In study II the precision varied between 5% and 11% while in study IV the precision varied between 1.7% and 4.6% for the different ROIs in the automatic DEXA analysis and between 3.6% and 6.2% when the cement mantle was excluded from the analysis. In study IV the intra and interobserver error were also evaluated.
Statistical analysis
Kruskal-Wallis test and Mann-Whitney test were used to evaluate any difference between the groups at a given time interval in all the studies. Wilcox-on's matched pairs signed ranks test was used to evaluate changes between various observations over time. Probability values less than 0.05 represented a significant difference. Stepwise linear regression analysis was used to determine whether various factors affected the penetration rate in study I. Kendall's tau test was used to evaluate the repeatability of the questionnaire in study I. Pearson test was used to evaluate the correlation between different factors in study I.
Ethics
Each of the studies (I–V) had been conformed with the Helsinki declaration and was approved by the local ethical committee(s).
Results
Study I. Increase in early polyethylene wear after sterilization with ethylene oxide—radiostereometric analyses of 201 total hips
Femoral head penetration
The proximal penetration at 2 years was less than 0.2 mm in gamma sterilized designs (Lubinus and Trilogy and more than 0.3 and 0.4 mm in cemented and uncemented Reflection cups respectively (p=0.0005, Kruskal-Wallis test, , ). The mean medial/lateral and anterior/posterior penetration were less than 0.1 mm in all 4 designs without significant difference (p <0.1, ). The mean total or three-dimensional penetration at 2 years was almost equal in patients with gamma sterilized polyethylene (Lubinus/Trilogy = 0.27/0.29 mm), but significantly higher in patients with EtO sterilized polyethylene (Reflection cemented/uncemented = 0.40/0.57 mm, p=0.0005, , ).
Table 16. Penetration at the 2-year follow-up (signed values, mm).
In study I the first radiostereometric recording after the postoperative one took place after 2 months in the uncemented Reflection group and not after 6 months as stated in Paper 1. This error has been corrected in and .
Regression analysis
Stepwise linear regression analysis showed that the proximal penetration increased with use of EtO sterilized cups (p=0.0005), younger age at operation (p=0.0005), male gender (p=0.003), increasing activity score (p=0.007), and proximal migration of the cup (p=0.05, adjusted r2using these variables = 0.52) The total or three dimensional penetration increased with use of EtO sterilized polyethylene (p=0.0005) high weight (p=0.0005) and lower age (p=0.004, adjusted r2for these variables = 0.28). The remaining variables including in regression analysis (diagnosis, socket size, cement-no cement, inclination of the cup, side, medial/lateral, anterior/posterior and three dimensional migration of the cup) had no influence on penetration rate. There was weak correlation between activity score and age (r=0.18, p=0.05, Pearson correlation).
Analysis of subgroups
In the uncemented Reflection group, fixation with pressfit and HA had the highest three-dimensional penetration rate (0.76 mm), while pressfit with screws had the lowest 0.47 mm (p=0.007). The proximal penetration did not differ significantly between the four subgroups (p=0.07).
Uncemented Reflection cups articulating against femoral head made of zirconium oxide had significantly lower three-dimensional penetration than femoral heads made of cobalt-chromium alloy (0.50 and 0.62 mm, respectively p=0.02). The penetration rate in the three directions did not differ between the 2 subgroups (p<0.2).
Forty-four Trilogy cups had an additional screw fixation. In the remaining 21, only press-fit fixation was used. The mean proximal/three-dimensional penetration rates were 0.17/0.26 mm 0.20/0.34 mm in the groups with and without screws, respectively (p=0.2 and p=0.03).
The activity level did not differ significantly between the groups (). The repeatability of the activity questionnaire varied between fairly good to full agreement between the answers given on the two occasions (Kendall's tau=0.7-1.0, in Appendix).
Conclusion
The ethylene oxide-sterilized polyethylene doubled the femoral head penetration rate, as compared with gamma sterilization in a reduced oxygen environment.
Study II. Highly cross-linked polyethylene in cemented THA—randomized study of 61 hips
Radiostereometry
Supine position
When evaluated supine the proximal penetration tended to become smaller in the study group at the end of follow-up (study/control: 0.13/0.18 mm, p=0.08, ). The mean mediolateral, AP, and three-dimensional penetration in supine position did not differ significantly between the groups (p<0.1).
Standing position
Between 3 and 24 months the mean proximal femoral head penetration with the patients in standing position was 0.06 mm in the study group and 0.13 mm in control group (p=0.03, , ). The three-dimensional penetration at 2 years was equal in both groups (0.20 mm). The mean mediolateral and AP penetration were smaller than 0.06 mm (p<0.1).
Table 17. Penetration between 3 and 24 months follow-up. The examination was performed with the patients standing.
Migration:
The migration of the cup at 2 years was lower than 0.1 mm and the rotation lower than 0.3° in both groups and in all three directions (p <0.3 and p <0.4 respectively).
Bone mineral density
A comparison between the immediate postoperative and the 2-year examination revealed an increase in region 2 in the study group and in region 5 in the control group (p=0.01 and p=0.02). Comparison of the relative change of BMD between the study and control groups at the 2 year follow-up revealed no statistically significant differences (p <0.09, ).
Conventional radiography
Postoperative radiolucencies were more extended in the study group in region 3 (p=0.01). At two years the distribution of lines were not significantly different between the groups (p <0.08). The maximum width of these lines was almost equal at the two occasions (p <0.10) and none of the lines exceeded 2mm.
Clinical examination
The hip and pain scores and the change in these scores between immediately postoperative and the 2 years follow-up were almost similar in the two groups. At this time the median value of the activity score was equal in the two groups of patients (study/control: 20/20, p=0.4, )
Conclusion
Highly cross-linked PE showed increased resistance to penetration. Different mechanical properties of the two types of PE studied did not affect the fixation, periprosthetic bone loss, and radiographic appearance of the cup.
Study III. Highly cross-linked polyethylene in total hip arthroplasty—random-ised evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis
Radiostereometry Hybrid study
Supine position
The mean proximal penetration was almost identical in the two groups up to 6 months. At 2 years the proximal penetration reached 0.08mm in the study group and 0.21 mm in the control group (p <0.001, , ). The mean three dimensional penetration was 0.22 mm and 0.32 mm respectively (p=0.006, , ). The penetration in mediolateral and anterior/posterior direction through out the follow-up period was lower than 0.08 mm in both groups without significant difference (p ≥0.5, ).
Table 18. Hybrid study, supine examinations 0–2 years
Standing position:
Medial penetration was recorded in the study group (0.06 mm), whereas minimal lateral penetration was observed in the control group (0.02 mm, p=0.05). The proximal, three dimensional and anterior/posterior penetration did not differ significantly (p <0.4).
Migration:
The mean proximal migration of the uncemented cup was about the same (study/ controls: 0.09/0.06 mm, p=1.0). The anterior/ posterior, and mediolateral migration was lower than 0.13 mm (p<0.3). Small rotations of the liner were recorded (absolute mean values < 0.19°; p<0.2).
Cemented study
Supine position:
Up to 6 months the proximal penetration was identical in both groups as in the hybrid study. At 3 years the mean proximal penetration was doubled in the control group (study/controls: 0.13/0.25 mm, p=0.002, , ). The mean total or three-dimensional penetration at the latest follow-up reached 0.23 and 0.30 mm (study/controls: p=0.2, ).
Table 19. Cemented study, supine examinations 0–3 years
Standing position:
At 3 years higher proximal penetration had occurred in the controls (study/ controls: 0.07/0.19 mm, p=0.007, ). The three dimensional penetration during the same follow-up period was 0.24 and 0.28 mm respectively (study/controls: p=0.6).
The mean mediolateral and AP head penetration at 3 years was 0.09 mm or less in both groups of patients and in both positions (p <0.1).
Migration:
The migration of cemented cups in the three directions was 0.11 mm or less in both groups without significant difference (p <0.4). The rotation of the socket was small (absolute mean value <0.28° p <0.3).
Clinical examination
The hip and pain scores and the changes in these scores between immediately postoperative and 2 years follow-up did not differ significantly in the two groups of patients in hybrid study (p <0.6, ). In the cemented study, the median hip pain score was the same at 3 years (median, range 44, 30–44; 44 40–44, p=0.02). The median activity scores at 2 years were almost the same (study/ controls 21/22, p=0.5, ).
Conclusion
Both the cemented cups and the liners made of highly cross-link polyethylene showed less early proximal wear according to the results of the supine examinations. The cemented cups made of the new polyethylene demonstrated less proximal wear also in the standing examinations.
Study IV. Fluoride containing acrylic bone cement in total hip arthroplasty—randomised evaluation of 97 stems using radiostereometry and DEXA
Radiostereometry
The subsidence of the stem at 2 years was numerically higher in Palacos group but this difference did not reach significance (C-F/P = 0.07/0.12mm, p=0.3, , ). The mean rotation of the stem was lower than 0.8° in both groups (p< 0.2). In 79 cases, where subsidence between stem and cement could be studied, the corresponding distal migration of the stems at two years was 0.15 mm in fluoride cement and 0.09 mm in Palacos group (p=0.6) indicating that subsidence occurred inside the cement mantle.
Table 20. Stem migration in relation to bone at the 2 years follow-up
In 29 of 32 patients with rheumatoid arthritis or with continuous treatment with cortisone in whom subsidence could be evaluated at two years the mean value in the fluoride cement and Palacos groups were 0.16 and 0.13 mm, respectively (p=0.7). These values did not differ compared with the remaining patients with other diagnoses or without cortisone treatment (p <0.8).
Bone mineral density
The absolute overall changes in BMD at two years were more pronounced proximally (region 1, 2, 6 and 7) than distally (regions 3, 4 and 5, ). The most pronounced loss occurred in region seven, where the BMD decreased in the manual analysis with 27 and 20% (C-F and P), respectively (). Inclusion of the cement mantle in the analysis, increased the BMD in different regions up to 49% in Palacos group and up to 26% in fluoride cement group. In 5 of the 7 regions, there was more pronounced reduction of BMD at 2 years when Cemex-F had been used (automated analysis, p≤0.01, regions 2, 3, 5-7, ). When the cement mantle was excluded the differences decreased and became only significant for regions 2 and 7 (p≤0.02, ). The changes of BMD in the subpopulation of patients with rheumatoid arthritis and/or on long term cortisone treatment showed a similar pattern.
Conventional radiography
Conventional radiographs at 2 years showed wider and more extensive lines in region 4 and 12 when Palacos cement had been used (p ≤0.04). Comparing the progression of radiolucencies up to 2 years examination between the two groups revealed significant difference only in one region (region 12; C-F vs. P: 0 vs. 2 years p=0.02 for the extension and p=0.01 for the width of lines, and in Appendix). Osteolysis and ectopic bone formation did not differ between the groups.
Clinical results
The hip and pain scores and the change in these scores between the preoperative and 2-years follow-up examination were similar in the 2 groups ().
Conclusion
There is no obvious early advantage of addition of fluoride to acrylic bone cement when used to fixate the femoral component in THA.
Study V. Bioactive cement or ceramic/ porous coating vs. conventional cement to obtain early stability of the acetabular cup. Randomised study of 96 hips followed with radiostereometry
Radiostereometry
At 2 years we observed medial translation of the uncemented cups (0.12 mm) while the cemented cups showed lateral translation (C-F/P = 0.01/0.09mm; p=0.05, Kruskal-Wallis test). The proximal migration at 2 years was 0.15 mm for uncemented cups and 0.12 mm in both cemented groups (p=0.8, , ). Small mean anterior-posterior translations (< 0.06 mm) and rotations (< 0.23°) were observed throughout the period of observation (p <0.1, ).
Table 21. Cup migration at 2 years follow-up. All hips
Forty of 42 patients with diagnosis suggesting osteoporosis (rheumatoid arthritis, failed femoral neck fracture or on cortisone treatment) were analysed separately. The three-dimensional migration in those patients was higher compared to the remaining patients (0.44 mm/0.32 mm respectively p=0.03, ). Evaluation of migration within each group with different fixation (C-F, Uncemented, Palacos) revealed significantly higher three-dimensional migration in osteoporotic patients only in the Palacos group (P: osteoporosis/ non osteoporosis = 0.46/0.24 mm, p=0.04, ).
Figure 28. Migration of the socket in patients with and without osteoporosis at 2 years. Mean, 95% CI.
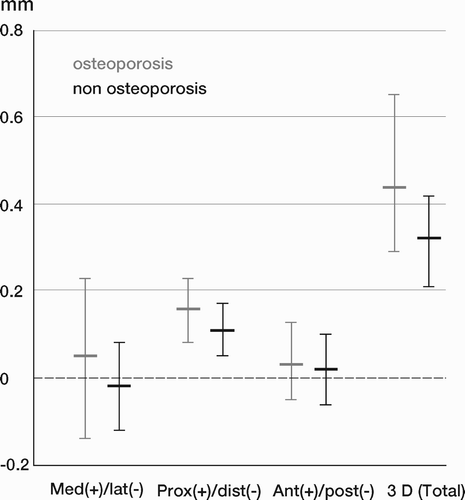
Figure 29. Three-dimensional (total) migration of the socket in each group of fixation at 2 years. Mean, 95% CI.
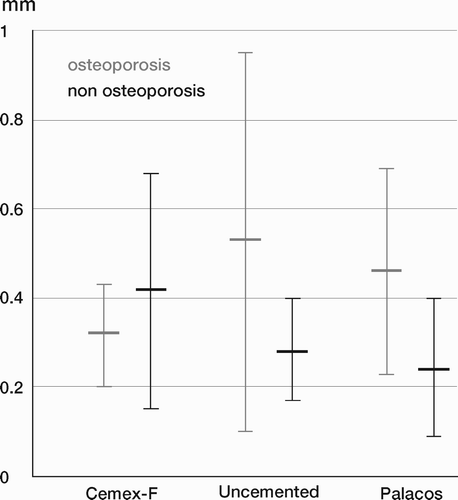
The mean medial/lateral and anterior/posterior penetration was lower than 0.06 mm without any significant difference between the groups (p<0.1). The proximal penetration at the same follow-up occasion in the three groups (C-F, Uncemented, Palacos) was 0.32, 0.12 and 0.30 mm respectively (p <0.001). The three dimensional penetration at 2 years in the three groups was 0.42, 0.21 and 0.45 mm (p <0.001, Kruskal-Wallis test).
Conventional radiography
The extension and maximum width of radiolucent lines at 2 years control did not differ between the 2 cemented groups (p <0.3). Comparison of the radiolucencies in uncemented and Palacos group on the postoperative radiographs did not differ (p <0.2). In the Palacos group, these lines became wider and more extended during the follow-up period. At 2 years control the radiolucent lines in Palacos group were wider and more extended in four regions (p <0.004, Tables in Appendix 4 and 5). In 17 cases in the uncemented group the width of radiolucent lines decreased during the followup period in any of the regions. In these cases the mean proximal migration of the cup was numerically higher (0.21 and 0.09 mm respectively, p=0.7).
Clinical results
The hip and pain scores at the 2 years control and the changes in these scores between the preoperative and 2 years follow-up examination were not significant different between the groups (p <0.14, ).
Conclusion
Fluoride containing cement or uncemented fixation did not improve the early postoperative stability of the socket.
Discussion
Study I
During the past decade, high penetration rates have been related to variations in polyethylene quality and sterilization techniques (Sutula et al. Citation1995, Collier et al. Citation1996b, Ries et al. Citation1996b, Bargmann et al. Citation1999). Sterilization by gamma radiation in air increases the potential for oxidative ageing and degradation of the mechanical properties of the polyethylene (Sutula et al.Citation1995, Collier et al. Citation1996b, Bargmann et al. Citation1999). McKellop et al. (Citation2000) tested in a hip simulator artificially aged polyethylene sterilized by gamma irradiation in air, gamma irradiation in reduced oxygen environment and gas plasma. They suggested that for at least ten years of clinical use, the in vivo wear of cups sterilized with gamma irradiation in reduced oxygen environment will be substantially lower than that of cup sterilized without irradiation. Similar results were reported by Greer et al. (Citation1998) in a comparison between artificially aged polyethylene sterilized with either EtO or gamma irradiation in vacuum foil package.
In our clinical study we found significantly higher proximal and three dimensional femoral head penetration in polyethylene sterilized with EtO compared with polyethylene sterilized with gamma irradiation in reduced oxygen environment. Our study only embraces the postoperative two years. With time, the polyethylene may change due to oxidation. In addition third bodies in terms of degraded cement and metal particles may enter the joint. Fixation failure of the liner cannot be excluded, and this would result in increased production of particles and increased penetration with time.
Orishimo et al. (Citation2003) studied retrospectively the wear rate over time of two porous coated acetabular cups sterilized with either gamma irradiation in air or EtO. The wear rate evaluated by the Martel method did not show significant increase in wear rate up to 14 years in both groups. There was a tendency for the EtO sterilized liners to wear at a higher rate than gamma sterilized cups at all follow-up intervals. The mean shelf life time for gamma irradiated cups was 3 months. In another retrospective study by Hopper et al. (Citation2003b) polyethylene liners that had been sterilized with gamma irradiation in air had significantly lower wear rate than gas-plasma-sterilized liners (0.1 compared with 0.2 mm/year; <0.001) when these patients was followed 5.2 and 3.9 years respectively. The mean shelf life of the liners sterilized with gamma radiation was 1 year. Puolakka et al. (Citation2003) analyzed 20 retrieved gamma irradiated in air sterilized polyethylene liners. There was a correlation between shelf life time and volumetric wear rate. Liners with a shelf time of 3 years or more had a significantly higher volumetric wear rate than those with a shelf life time less than 3 years. Based on these studies, it is probable that gamma sterilized in air polyethylene with shelf life less than 3 years performs equal as polyethylene, which has been gamma sterilized in reduced oxygen environment. In our study, we did not attempt to obtain shelf storage data since none of the cups was sterilized in air.
Many previous studies exclude at least the first postoperative examination and do not include the initial deformation (creeping) of the polyethylene (Hopper et al. Citation2003b, Orishimo et al. Citation2003). The wear rate in our study (including creeping) was 0.1 mm/year for gamma irradiated cups and over 0.17 mm/year for EtO sterilized polyethylene. Only the uncemented Reflection cups exceeded the suggested threshold for osteolysis 0.2 mm/year. The long term consequences of these findings are unclear, but several studies have demonstrated an increased disposition for osteolysis with higher wear rates (Sochart Citation1999, Dowd et al. Citation2000).
Not only the number of polyethylene particles but also the biological response to these particles determines the ultimate effect of polyethylene wear. According to Green et al. (Citation1998) small particles in the phagocytosable range (0.3-1 microns) appear to be the most biologically active. Experimental studies have shown that particle diameter, surface area and volume are higher for EtO sterilized components (Scott et al. Citation2001). The biological response of macrophages to polyethylene particles varies depending on these factors (Green et al. Citation1998). It has also been shown that oxidized polyethylene particles are more efficiently phagocytised than non-oxidized polyethylene resulting in higher release of Interleukin-1 (Kamikawa et al. Citation1998). The biologic response to wear particles from gamma irradiated or EtO sterilized polyethylene is therefore difficult to predict.
In the present study EtO sterilized polyethylene resulted in more than doubled penetration rate compared with gamma sterilization in reduced oxygen environment, but due to the complex interaction of polyethylene oxidation and particle quality the longterm effects of this observation on the clinical results is not quite clear. Other effects of increased removal rate of polyethylene such as joint instability, material failure and head against shell wear due to liner dissociation or severe wear are more obvious reasons for concern and prompts for regular follow-up especially in patients with high activity level.
Study II and III
Problems associated with UHMWPE have stimulated development of more wear resistant polyethylene materials to minimise the osteolysis believed to origin from wear debris. Radiation, peroxide, or silane treatments have been used to increase cross-linking of the polyethylene (Kurtz et al. Citation1999a). Each of these methods has been reported to improve the wear characteristics relative to non cross-linked or nominally cross-linked polyethylene (McKellop et al. Citation1999, Muratoglu et al. Citation2001a, Wroblewski et al. Citation1999). Today, radiation is the preferred method of cross-linking.
Cross-link of the PE decreases the mobility of the polymeric chains (Muratoglu et al.Citation1999) resulting in reduced surface deformation, which is the starting process for wear particle generation (Kurtz et al. Citation1999b, Bragdon et al. Citation1996, Jasty et al. Citation1997).
There are five types of highly cross-link polyethylene used in USA and further 1 in Japan (). Two of these types of polyethylene, both electron beam radiated (95 -100 kGy) and melted was used in studies II and III. Hip simulator test have shown increased resistance to wear for this kind of highly cross-linked polyethylene (Muratoglu et al. Citation2001a,Citationb Bragdon et al. Citation2003).
In both studies we found relatively high and equal penetration during the first 6 months regardless of position and polyethylene quality used. Thereafter, the penetration rate decelerated in the highly crosslinked groups, whereas the control groups showed linear increase in both studies. In the hybrid group this difference was more obvious when the patients were studied supine. In standing position the difference disappeared, probably because of drop-out of cases in which both hips could not be evaluated. In the cemented cup study significant differences between the two types of polyethylene were detected only for proximal penetration in both positions. Different elastic modulus and creeping behaviour of the two types of highly cross-linked polyethylene studied and thinner polyethylene in the liners could explain this discrepancy. Measurements of the three-dimensional penetration should always be regarded cautiously. This parameter corresponds to a vector, which per definition can not be negative. This means that even if the penetration is zero the measurement error of the method used will be added to the recordings. In the cemented cup study more patients or longer follow-up might be needed to detect any difference in three-dimen-sional penetration.
Previous RSA studies assessing 22, 26 and 28 mm femoral head penetration into uncemented acetabular cups showed proximal penetration about 0.1 mm while the three dimensional penetration was between 0.1 and 0.2 mm (Önsten et al. Citation1996, Citation1998, Thanner et al. Citation2000). In our study the liners in the control group showed wear rates of approximately the same magnitude (0.10 and 0.16 mm, respectively). RSA studies of cemented conventional polyethylene cups have shown mean annual proximal and three dimensional wear rates between 0.05 to 0.2 mm and 0.1 to 0.2 mm respectively using 28 or 32 mm femoral heads of cobalt chromium (Kärrholm et al. Citation1997, Mjöberg et al. Citation1990, Nivbrant et al. Citation2001, Önsten et al. Citation1998). Our cemented control group showed wear rates in the lower range of these limits (0.08 and 0.10 mm).
Radiostereometric evaluations of femoral head penetration have been based on examinations done with the patients supine. We did not find any difference when comparing the proximal penetration between the two positions. One problem with examinations performed with the patients standing is inferior radiographic quality especially in obese patients. The voluminous soft tissues tend to displace distally and cover the hip region which will make the outline of the femoral head less distinct on the radiographs. This problem and difficulties to visualise liner markers were the main reason why several of the standing examinations not could be evaluated.
Despite that adhesive/abrasive wear in UHMWPE will decrease with increasing crosslinking, the subsequent removal of free radicals by melting will imply reduction of its fatigue strength by decreasing the PE crystallinity (Muratoglu et al. Citation2001a). The manufacturer of one type of highly cross-link polyethylene, “Crossfire” prefers annealing to remelting of the material on the grounds that this process induces less change in material morphology and properties than does melting. However, because trapped free radicals in crystalline regions remains in the polyethylene unless it is heated above the melt temperature, annealing is probably not as effective as melting in extinguishing these radicals. Hip simulator test of artificial ageing and retrieved components with less than 3 years in vivo use of this type of highly cross-linked polyethylene show increased levels of crystallinity and oxidation (Muratoglu et al. Citation2000, Bhattacharya et al. 2004a). In order to minimize the oxidative degradation the manufacturer of “Crossfire” implants recommends that they be shelf-stored in the nitrogen filled package, and only for a limited time period prior to implantation (less than 5 years). In view of this, the developers argue that any oxidative degradation of “Crossfire” implants will be far less than historically occurred with polyethylene components that were irradiated and stored in air. Recently, mechanical elimination of free radicals by plastic deformation of the polyethylene and blending of UHMWPE powder with a potent antioxidant, α-Tocotherol to increase oxidation resistance without melting has been tested experimentally with promising results (Bhattacharya et al. 2004b, Oral et al. Citation2004a,Citationb).
We did not observe any significant difference of the periprosthetic radiolucency or BMD at the two years follow-up in the cemented study between the groups. The cup migration at three years in cemented study and at two years in hybrid study were almost equal between the two types of PE used in each study, indicating that the different mechanical properties of the plastics used had no influence. So far our observation period is, however, short and none of our cups were inserted in extreme ante- or retroversion or with excessive inclination.
The two types of electron beam highly crosslinked polyethylene showed decreased penetration rate mainly after the one year follow-up most probably reflecting less removal of polyethylene material (wear). If this reduced wear rate persists the expected implant longevity will increase. Further follow-up is needed to evaluate if this also means reduced occurrence of osteolysis and to ensure that these new materials not are associated with any other unexpected adverse events.
Studies IV and V
Bioactive bone cements with fluoride mixed with polymethylmethacrylate have been proposed to improve the quality of the bone cement interface. Thereby the fixation might improve. There could also be a potential for reduced loss of bone mineral in the proximal femur during the postoperative period (Magnan et al. Citation1994, Sundfeldt et al. Citation2002b). The mechanical properties of the low curing temperature cement (Cemex Rx) tested in our studies have shown no or small changes by the addition of 6% sodium fluoride (Minari et al. Citation2001). According to Magnan et al. (Citation1994) the release rate of fluoride from Cemex-F (6% fluoride) is within the therapeutic range. The control cement Palacos cum Gentamicin used in our studies has extensive documentation (Malchau et al. Citation2000, Nivbrant et al. Citation2001). In a preclinical pilot study preceding our clinical trial the magnitude of subsidence and rotation of Spectron stems did not differ between Palacos and Cemex cement in the laboratory (Lidén et al. Citation1998).
We found numerically (but not significantly) lower stem subsidence in Cemex-F group. The subsidence of the stems occurred mainly inside the mantle. This motion may be an effect of cement creep. Different chemical composition and contrast media included in the two cements may be the reason for their different creeping behaviour (Cigada et al. Citation2001). The subsidence of Spectron stems in study IV was similar to previous reports (Kärrholm et al. Citation2000) suggesting that the design of this stem has an important influence on the pattern of migration.
In patients with supposed compromised bone quality (rheumatoid and cortisone treated patients), addition of fluoride cement did not improve the bone/cement interface stability. Stem subsidence did not differ between patients with osteoarthritis and those with compromised bone quality in neither group. This is in accordance with previous observation by Önsten et al. (Citation1995), who found about equal migration of Charnley stem in patients operated due to osteoarthritis and rheumatoid arthritis.
Socket fixation with Palacos cement has been the standard method in Sweden at least in elderly population. In patients with compromised bone quality inferior results and especially concern about the fixation of the cup have been reported (Carlsson et al. Citation1986). Porous coated components were introduced in the mid-1980s to improve the fixation and longevity of hip arthroplasties. Ceramic coating has been reported to increase the early stability suggesting that this mode of fixation could be an alternative in this patient population (Thanner et al. Citation1999).
In the total material we found almost no difference in proximal migration of the socket between the three groups. In the control group with standard cement we found more pronounced three dimensional migration in cases classified as osteoporotic (rheumatoid arthritis, failed femoral neck fracture and patients on cortisone treatment), while in the uncemented group this difference did not reach significance. This is in agreement with previous RSA studies were Charnley and Lubinus cups were compared between patients with compromised bone quality and osteoarthritis (Önsten et al. Citation1993, Snorrason et al. Citation1993). In the fluoride cement group the mean migration was numerically lower (but nor significantly different) in patients with osteoporosis. It might be that the lower curing temperature of Cemex-F cement and less release of monomer may produce less acetabular necrosis, or that fluoride cement does reduce postoperative bone resorption and thereby improves the early implant stability mainly in osteoporotic patients. If so this effect is small and not sufficient to result in any significant differences, based on the study population available. Overall the small variations observed between patients with osteoarthritis and those with supposed inferior bone quality must be interpreted cautiously due to low number of patients and short follow-up.
The cemented cups in our study tended to migrate laterally while the uncemented translated medially. Thien (personal communication, 2004) observed the same tendency in a randomised comparison between Lubinus cups and uncemented hydroxyapatite coated ABG sockets after 2-3 years follow-up. This variability in direction of migration between cemented and uncemented designs could be related to increased elasticity of the whole polyethylene designs. It could also be caused by the presence of screws or pegs, which may change the lever arm for forces transmitted to the cup from the femoral head.
In the present study the penetration rate in uncemented socket was lower, probably due to the different sterilization method used as shown in study I. No difference was noted between the two cemented groups. After 5 years follow-up Nivbrant et al. (Citation2001) found, contrast to our findings, higher proximal penetration of Lubinus sockets fixed with Palacos cement than of those fixed with Cemex cement. The authors speculated that particles of barium sulphate released from the Cemex cement caused less third body wear than particles of zirconium oxide from the Palacos cement. Shorter follow-up and use of another stem design with metallic head in our study may be the reason for the different results.
Conventional radiography showed no difference between the two cemented groups on the acetabular side while on the femoral side, we observed accelerated progression of radiolucent lines only in one region in the Palacos group. Different chemical composition or differences in the long-term interaction between the bone tissue and the two types of cements are possible explanations. This difference could be documented only in one region and only on the femoral side. Therefore it should be viewed cautiously. Half of the uncemented cups showed tendency to disappearance of the postoperative radiolucent lines. Contrary to the findings by Thanner et al. (Citation1999) those hips were associated with slightly more proximal migration. The findings from these two studies leave the question open if “gap filling” is an effect of bi-directional calcification or socket migration.
During the first year, the DEXA analysis of the stem revealed more loss of periprosthetic BMD than during the second year. This early bone loss is primarily a consequence of stress shielding (Huiskes et al. Citation1992). At two years the bone loss was especially pronounced in the calcar area.
More loss of bone mineral density was observed in the Cemex-F group. There might be several explanations. Inclusion of the cement in DEXA analysis causes an artefact because of seemingly increased bone mineral density. The low density of Cemex-F cement implies smaller “radiation shielding effect” on bone mineral, suggesting that a larger volume of bone tissue will be analysed. Another explanation could be that the fluoride and barium-sulphate concentrations are too high and toxic resulting in a true bone resorption. The equal and stable fixation of the cement mantle in the two groups according to RSA does, not support this presumption. The substitution of hydroxide (OH-) with fluoride (F-) converts hydroxyapatite to fluorapatite crystals, which are more resistant to osteoclastic resorption of bone (Earnes and Reddi Citation1979). Low rate of bone remodeling may be associated with increased fragility or brittleness of the skeleton by retarding repair of microdamage (Parfitt et al. Citation1987). The two effects of fluoride on the skeleton (increased volume, decreased remodelling) may have opposing effects on the mechanical characteristics of the skeleton. If that hypothesis is correct, the decreased BMD as observed with DEXA for the fluoride cement could mirror the presence of less mineralised hypertrophic bone tissue, which is more resistant to resorption.
Addition of fluoride to acrylic bone cement has no obvious advantage on the early stability of the femoral component in THA when compared with Palacos cement. The reason for increased loss of BMD could not be completely addressed due to different cement density. Neither the uncemented fixation nor the fluoride cement improved the early stability of the socket. The uncemented cups did, however, display less radiolucent lines after 2 years, which might be of importance in terms of interface access of joint fluid and particles. We therefore still think that cemented fixation based on well-documented designs and types of cement should be used as standard implant provided that the surgeon is familiar with its use.
Conclusions
Study I
Polyethylene cups sterilized in EtO had almost twice the proximal and 3D penetration rates compared with gamma-sterilized polyethylene cups. The penetration rates did not differ between the gamma-irradiated designs. The type of sterilization, age and weight were the most important predictors of the penetration.
Studies II and III
In both studies, highly cross-linked polyethylene showed decreased penetration rates mainly after the one year follow-up probably reflecting less wear. The different mechanical properties of highly cross-linked and conventional PE did not alter the performance of the cups in terms of fixation and early clinical results. In the cemented design, the periprosthetic bone loss and radiographic appearance did not differ.
Study IV
The choice of cement did not influence the subsidence or rotation of the stem. Fluoride cement had no influence on stem fixation in patients with compromised bone quality. DEXA analysis revealed more loss of periprosthetic BMD in the fluoride cement group. Formation of stable fluorapatite crystals, toxic effects of the fluoride or lower radio-opacity of the fluoride cement might explain this finding.
Study V
Choice of fixation did not influence the migration or rotation of the cup. Patients with suspected suboptimal primary fixation due to osteoporosis showed increased three-dimensional migration of the cup. Postoperative radiolucent lines (gaps) tended to disappear with use of porous coating covered with a ceramic layer. Proximal and 3D penetration rates were increased in the cemented compared with uncemented cups probably due to the different sterilization methods used.
The future
PMMA cement has several inherent problems such as non-bone-bonding character, relatively low mechanical strength, and high heat generation during its polymerization. Several attempts have been made to improve the mechanical and biological properties of the bone cement such as addition of fluoride, apatite-and wollastonite-glass-ceramic, hydroxyapatite, β− tricalcium phosphate and human growth hormone. Although promising in laboratory none of these cements have shown improved implant fixation when used clinically.
The physical, chemical and bioactive properties of the cement need to be improved for certain indications such as revisions and use in patients with poor bone quality and low age. A new cement with lower toxicity, lower exothermic reaction during curing and with more strength in shear and tension than PMMA cement would be desirable. Improved biocompatibility and addition of bioactive properties to improve periprosthetic bone mineral density and preferably better bonding to bone would minimise the risk for revision arthroplasty. Unfortunately the optimum characteristics of a bone cement e.g. in terms of elasticity and creep remains unknown and is probably related to the mechanical properties, surface structure and shape of the implant used. Probably the site of implantation, bone quality and relative local distribution of trabecular and cortical bone present are important, which makes this problem still more complex.
Another important aspect of new cements is that their handling properties often differ from those cements in contemporary use. The behaviour of any new cement must therefore be learnt by practise and during conditions similar to those present in the operating theatre before it is used in patients. Use of cement also often implies increased time for the surgical procedure, which has to be considered in any comparison of costs and benefits between cemented and uncemented fixation.
Traditional polyethylene oxidizes, wears and generates particles over time, which most probably contributes to increased risk of periprosthetic osteolysis. Even contemporary sterilization methods such as radiation and package in oxygen reduced or oxygen substituted environment do not eliminate oxidation over time. Thus, there is a need for alternative bearing in total hip replacement surgery and especially in patients with high activity and long life expectancy.
All three major alternate bearings, ceramic-on-ceramic, metal-on-metal and highly cross-linked polyethylene produce major reductions in volumetric wear. The electron beam, melted highly cross-linked polyethylene has an in vivo penetration rate after the bedding in period, which is less than 8 microns per year. This is not substantially different from ceramic on ceramic or metal on metal. Therefore, the inherent risk of periprosthetic osteolysis with these alternate bearings is probably smaller than observed with conventional polyethylene.
In the competition between different articulations highly cross-linked polyethylene has some advantages. The polyethylene is more adaptable than the hard bearing surfaces. This means that extended lip liners, offset liners, constrained liners and further special designs may be used. These options are not possible with any of the hard bearings. Another advantage with polyethylene is forgiveness. Impingement in hard-on-hard bearings may lead to serious complications such as chipping of the ceramics or metallosis in a metal on metal articulation. Impingement should also be avoided with use of polyethylene, but if it occurs, the consequences are often more benign at least in the short term perspective. There is no striped wear, as occurs with hard-on-hard bearings. Microseparation results in less material damage with use of polyethylene than with the 2 other types of articulations. A few degrees of additional abduction above the geometrical limits for a particular socket is far less harmful if it is made of polyethylene compared to the situation in ceramic-on-ceramic or metal-on-metal bearings. Polyethylene is also more familiar to the majority of orthopaedic surgeons. In the operating room the cross-linked polyethylene is identical to those types of polyethylene, which have been used fore 3 to 4 decades. Finally the cost is a major factor. The hard-on-hard bearings are substantially more expensive. The fracture incidence of ceramics components has decreased with improved manufacturing technology, but the risk of polyethylene fracture appears to be still smaller (Harris Citation2004).
On the other hand using highly cross-link polyethylene carries some risks. Particles generated from this new material are smaller with higher inflammatory response. Compared with joints including conventionally sterilised polyethylene the total particle production is, however, reduced with more than 85%, which has implications for the magnitude of the inflammatory response.
The significance and importance of the irradiation and melting induced changes of the mechanical properties of the polyethylene is not known. Long term follow-up is needed to evaluate this issue.
Charnley preferred small head sizes in total hip replacement because they resulted in transmittance of low frictional torque to the acetabular implant. Mueller advocated larger head sizes with improved joint stability and lower contact pressure. Large heads do, however, imply increased volumetric wear. Therefore, 32 mm heads were abandoned in the early 90tiesin favour of 28 mm heads. Another consequence of using larger heads is that polyethylene liners are relatively thin. The highly crosslink polyethylene and the hard bearings can be used with bigger femoral heads, which increases the range of motion and the hip joint stability.
Amorphous diamond coatings has been studied as an alternative bearing surface in the laboratory (Santavirta Citation2003). Such coatings may provide wear rates 104to 105times lower than conventional THR articulations, because of their extremely hard surface and low coefficient of friction without any corrosion paths (Santavirta et al. Citation1999 Lappalainen et al. Citation2003).
Oxidized Zirconium (OxZr) is another material, which has similar advantages. Oxinium materials are the results of a process that allows thermally–driven oxygen to diffuse and transform the metallic zirconium alloy surface into a durable low-friction oxide. The Oxinium material is harder than commonly used cobalt chrome, and with only the surface changing during the manufacturing process, the rest of the implant remains metal to maintain its overall strength. OxZr provides superior abrasion resistance without the risk of brittle fracture, thereby combining the benefits of metal and ceramics. Knee simulator tests have shown that OxZr can reduce polyethylene wear substantially (Ries et al. Citation2002). Although promising, these two coatings still lack clinical documentation.
During the last decade it has become evident that many designs of total hip arthroplasty can in patients with normal bone quality be fixed to the bone with a high degree of reproducibility. This has had the effect that younger patients have been operated on in increasing numbers. Wear and periprosthetic bone loss have remained a serious and comparatively frequent complications. The introduction of more wear resistant articulations has the potential to solve these problems making the procedure safer also among these patients. So far there is no or very scarce evidence that these articulations can be used safely during decades without complications causing progressive and often silent bone destruction resulting in difficult revisions with high morbidity. In the case of metal on metal articulations release and accumulation of ions remains a long term concern and especially if the patients will suffer from a temporary or permanent disease associated with impaired renal function.
Because evidence of long term superiority of these new articulations is lacking it is of utmost importance that these new implants and materials are introduced into clinical practice in a controlled way. Careful surveillance of preclinical and gradually enlarged randomised studies followed by multicenter trials is necessary to avoid disastrous mistakes so common in the past.
Acknowledgements
I want to express my sincere gratitude to:
Professor Johan Kärrholm, my tutor and supervisor for outstanding support, guidance, encouragement, patience, enthusiasm all these years and invaluable help with writing the summary.
My co-tutos, Jonas Thanner, Peter Herberts and Henrik Malchau, for support, valuable criticism and co-operation.
Professor Jon Karlsson, for providing scientific conditions to perform this work and criticism of the summary.
My co-authors Bo Nivbrant, Stephan Röhrl, Hans Ström, for co-operation in study I and Christian Anderberg in studies IV and V.
My colleagues in the Joint Replacement Unit for support and friendship; Tuuli Saari, Lars Regnér, Lars Carlsson, Göran Garellick, Kent Olofsson, Sverrir Hilmarsson and Truike Thien.
All my other colleagues at the Department of Orthopaedics at Sahlgrenska University Hospital for friendly attitude.
Göran Garellick, for help with illustrations.
Eva Fermén, Karin Davidsson and Katarina Wolf, for outstanding secretarial help.
Birgitta Runze, Bita Sharegi Atefeh Harir and Elinor Gustavsson, for helping with the radiostereometric examinations.
Erna Nilsson, for helping with DEXA examinations, for illustrations and finding the radiographs for studies IV and V.
Linda Johansson, for helping me in the academic chaos.
Ramin Namitabar, for IT support.
William H. Harris, for making studies II and III possible.
Warren Macdonald, for language correction.
All the patients included in the studies.
My parents for support.
Above all my wife and daughter Maria and Fani-Theodora; without their support this thesis would never be finished.
References
- Allain J, Le Mouel S, Goutallier D, Voisin M C. Poor eightyear survival of cemented zirconia-polyethylene total hip replacements. J Bone Joint Surg (Br) 1999; 81(5)835–42
- Allain J, Roudot-Thoraval F, Delecrin J, Anract P, Migaud H, Goutallier D. Revision total hip arthroplasty performed after fracture of a ceramic femoral head. A multicenter survivorship study. J Bone Joint Surg (Am) 2003; 85(5)825–30
- Allen M, Brett F, Millett P, Rushton N. The effects of particulate polyethylene at a weight-bearing bone-implant interface. A study in rats. J Bone Joint Surg (Br) 1996; 78(1)32–7
- Alho A, Soreide O, Bjersand A J. Mechanical factors in loosening of Christiansen and Charnley arthroplasties. Acta Orthop Scand 1984; 55(3)261–6
- Amstutz H C, Campbell P, Kossovsky N, Clarke I C. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop 1992, 276: 7–18
- Amstutz H C, Grigoris P. Metal on metal bearings in hip arthroplasty. Clin Orthop 1996; 329(Suppl)S11–34
- Amstutz H C, Beaule P E, Dorey F J, Le Duff M J, Campbell P A, Gruen T A. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg (Am) 2004; 86(1)28–39
- Angle C R. Organ-specific therapeutic intervention. Metal Toxicology, R A Goyer, C D Klaassen, M P Waalkes. Academic Press, San Diego 1995
- Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg (Br) 1996; 78(4)641–6
- Aspenberg P, van der Vis H. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand 1998; 69(1)1–4
- Bader R, Steinhauser E, Gradinger R, Willmann G, Mittelmeier W. Computer-based motion simulation of total hip prostheses with ceramic-on-ceramic wear couple. Analysis of implant design and orientation as influence parameters. Z Orthop Ihre Grenzgeb 2002; 140(3)310–6
- Baker D A, Hastings R S, Pruitt L. Study of fatigue resistance of chemical and radiation crosslinked medical grade ultrahigh molecular weight polyethylene. J Biomed Mater Res 1999; 46(4)573–81
- Baldursson H, Egund N, Hansson L I, Selvik G. Instability and wear of total hip prostheses determined with roentgen stereophotogrammetry. Arch Orthop Trauma Surg 1979; 95(4)257–63
- Bankston A B, Ritter M A, Keating E M, Faris P M. Measurement of polyethylene thickness in total hip arthroplasty. A technique analysis. J Arthroplasty 1994; 9(5)533–8
- Bankston A B, Cates H, Ritter M A, Keating E M, Faris P M. Polyethylene wear in total hip arthroplasty. Clin Orthop 1995; 317: 7–13
- Bapst J.M, Valentine R H, Vasquez R. Wear simulation testing of direct compression molded UHMWPE irradiated in oxygenless packaging. Society for Biomaterials, R E Merchant, 1997; 72, Transactions: Twenty-Third Annual Meting of the Society for Biomaterials
- Bargmann L S, Bargmann B C, Collier J P, Currier B H, Mayor M B. Current sterilization and packaging methods for polyethylene. Clin Orthop 1999; 369: 49–58
- Barrack R L, Mulroy R D, Jr, Harris W H. Improved cementing techniques and femoral component loosening in young patients with hip arthroplasty. J Bone Joint Surg (Br) 1992; 74(3)385–9
- Bartel D L, Bicknell V L, Wright T M. The effect of conformity, thickness and material on stresses in ultra-high molecular weight components for total joint replacement. J Bone Joint Surg (Am) 1986; 68(7)1041–1051
- Barouk P, Maynou C, Hildebrand H F, Aubertin F, Breme J, Cassagnaud X, Mestdagh H. High incidence of total hip arthroplasty aseptic loosening with ion-coated titanium femoral heads. Rev Chir Orthop Reparatrice Appar Mot 2004; 90(1)26–32
- Bauer T W, Geesink R C, Zimmerman R, McMahon J T. Hydroxyapatite-coated femoral stems. Histological analysis of components retrieved at autopsy. J Bone Joint Surg (Am) 1991; 73(10)1439–52
- Bellamy N, Buchanan W W, Goldsmith C H, Campbell J, Stitt L W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15(12)1833–40
- Bhattacharyya S, Doherty A M, Wannomae K K, Oral E, Freiberg A A, Harris W H, Muratoglu O K. Severe in vivo oxidation in a limited series of retrieved hihhly crosslinked UHMWPE acetabular components with residual free radicals. 50th Trans Orthop Res Soc 2004a; 276
- Bhattacharyya S, Matrisciano L, Speigelberg S H, Harris W H, Muratoglu O K. Mechanical elimination of residual free radicals in a irradiated UHMWPE rod: Advantages over melting. 50th Trans Orthop Res Soc 2004b; 1474
- Biedermann R, Krismer M, Stockl B, Mayrhofer P, Ornstein E, Franzen H. Accuracy of EBRA-FCA in the measurement of migration of femoral components of total hip replacement. Einzel-Bild-Rontgen-Analyse-femoral component analysis. J Bone Joint Surg (Br) 1999; 81(2)266–72
- Bierbaum B E, Nairus J, Kuesis D, Morrison J C, Ward D. Ceramic-on-ceramic bearings in total hip arthroplasty. Clin Orthop 2002; 405: 158–63
- Black J. Orthopaedic biomaterials. In research and practice. Churchill Livingstone, New York, Edinburgh, London, Melbourne 1988
- Blaine T A, Pollice P F, Rosier R N, Reynolds P R, Puzas J E, O'Keefe R J. Modulation of the production of cytokines in titanium-stimulated human peripheral blood monocytes by pharmacological agents. The role of cAMP-mediated signaling mechanisms. J Bone Joint Surg (Am) 1997; 79(10)1519–28
- Blaise L, Villermaux F, Cales B. Ageing of zirconia: Everything you always wanted to know. Bioceramics 2000; 13: 553–6
- Bloebaum R D, Merrell M, Gustke K, Simmons M. Retrieval analysis of a hydroxyapatite-coated hip prosthesis. Clin Orthop 1991; 267: 97–102
- Bloebaum R D, Bachus K N, Rubman M H, Dorr L D. Postmortem comparative analysis of titanium and hydroxyapatite porous-coated femoral implants retrieved from the same patient. J Arthroplasty 1993; 8(2)203–11, A case study
- Bloebaum R D, Du Point J A. Osteolysis from a pressfit hydroxyapatite-coated implant. J Arthroplasty 1993; 8: 195–202, A case study
- Bloebaum R D, Beeks D, Dorr L D, Savory C G, DuPont J A, Hofmann A A. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop 1994; 298: 19–26
- Boehler M, Plenk H, Jr, Salzer M. Alumina ceramic bearings for hip endoprostheses: the Austrian experiences. Clin Orthop 2000; 379: 85–93
- Booth A F. Industrial sterilization technologies. New and old trends shape manufacturer choices. Med Device Diagn Ind 1995; 17: 64–72
- Borssen B, Kärrholm J, Snorrason F. Osteolysis after ceramic-on-ceramic hip arthroplasty. Acta Orthop Scand 1991; 62(1)73–5, A case report
- Boutin P. Total athroplasty of the hip by fritted aluminum prosthesis. Experimental study and 1st clinical applications. Rev Chir Orthop Reparatrice Appar Mot 1972; 58: 229–246
- Bragdon C R, O'Connor D O, Lowenstein J D, Jasty M, Syniuta W D. The importance of multidirectional motion on the wear of polyethylene. Proc Inst Mech Eng (H) 1996; 210: 157–165
- Bragdon C R, Malchau H, Yuan X, Perinchief R, Karrholm J, Borlin N, Estok D M, Harris W H. Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model. J Orthop Res 2002; 20(4)688–95
- Bragdon C R, Jasty M, Muratoglu O K, O'Connor D O, Harris W H. Third-body wear of highly cross-linked polyethylene in a hip simulator. J Arthroplasty 2003; 18: 553–61
- Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Elevated serum cobalt with metal-on-metal articulating surfaces. J Bone Joint Surg (Br) 1997; 79(2)316–21
- Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg (Am) 2003; 85(11)2168–73
- Brooker A F, Bowerman J W, Robinson R A, Riley L H. Ectopic ossification following total hip replacement. Incidence and method of classification. J Bone Joint Surg (Am) 1973; 55(8)1629–32
- Brooks R A, Sharpe J R, Wimhurst J A, Myer B J, Dawes E N, Rushton N. The effects of the concentration of high-den-sity polyethylene particles on the bone-implant interface. J Bone Joint Surg (Br) 2000; 82(4)595–600
- Brown G C, Lockshin M D, Salvati E A, Bullough P G. Sensitivity to metal as a possible cause of sterile loosening after cobalt-chromium total hip replacement aithroplasty. J Bone joint Surg (Am) 1977; 59(2)l64–8
- Brown I W, Ring P A. Osteolytic changes in the upper femoral shaft following porous-coated hip replacement. J Bone Joint Surg (Br) 1985; 67(2)218–21
- Brown S R, Davies W A, DeHeer D H, Swanson A B. Longterm survival of McKee-Farrar total hip prostheses. Clin Orthop 2002; 402: 157–63
- Busanelli L, Squarzoni S, Brizio L, Tigani D, Sudanese A. Wear in carbon fiber-reinforced polyethylene (poly-two) knee prostheses. Chir Organi Mov 1996; 81: 263–7
- Börlin N. High precision measurements in digital radiographs. Umeå University, UmeåSweden 1997, Licentitiate thesis
- Börlin N, Thien T, Karrholm J. The precision of radiostereometric measurements. Manual vs. digital measurements. J Biomech 2002; 35(1)69–79
- Cales B. Zirconia as a sliding material: histologic, laboratory, and clinical data. Clin Orthop 2000; 379: 94–112
- Callaghan J J, Pedersen D R, Olejniczak J P, Goetz D D, Johnston R C. Radiographic measurement of wear in 5 cohorts of patients observed for 5 to 22 years. Clin Orthop 1995; 317: 14–8
- Callaghan J J, Albright J C, Goetz D D, Olejniczak J P, Johnston R C. Charnley total hip arthroplasty with cement. Minimum twenty-five-year follow-up. J Bone Joint Surg (Am) 2000; 82(4)487–97
- Cameron H U. Cemented acetabulum - Part 2: Implant-Bone Interface in Bone Implant Interface. Nosby, a cura di George Stamathis, 1994; 263–271
- Capello W N, D'Antonio J A, Manley M T, Feinberg J R. Hydroxyapatite in total hip arthroplasty. Clinical results and critical issues. Clin Orthop 1998; 355: 200–11
- Carlsson A S, Gentz C F, Sanzen L. Socket loosening after hip arthroplasty. Radiographic observations in 241 cases up to 15 years. Acta Orthop Scand 1986; 57(2)97–100
- Carlsson A S, Nilsson J A, Blomgren G, Josefsson G, Lindberg L T, Onnerfalt R. Low- vs high-viscosity cement in hip arthroplasty. No radiographic difference in 226 arthrosis cases followed for 5 years. Acta Orthop Scand 1993; 64(3)257–62
- Catelas I, Petit A, Zukor D J, Marchand R, Yahia L, Huk O L. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials 1999; 20: 625–630
- Cates H E, Faris P M, Keating E M, Ritter M A. Polyethylene wear in cemented metal-backed acetabular cups. J Bone Joint Surg (Br) 1993; 75(2)249–53
- Champion A R, Li S, Saum K, Howard E, Simmons W. The effect of crystallinity on the physical properties of UHMWPE. 40th Trans Orthop Res Soc 1994; 19: 585
- Chan F W, Bobyn J D, Medley J B, Krygier J J, Yue S, Tanzer M. Wear performance of metal-metal hip implants. Arch Am Acad Orthop Surg 1997; 1: 57–60
- Chan F W, Bobyn J D, Medley J B, Krygier J J, Tanzer M. The Otto Aufranc Award. Wear and lubrication of metal-on-metal hip implants. Clin Orthop 1999; 369: 10–24
- Charnley J. Anchorage of the femoral head prosthesis of the shaft of the femur. J Bone Joint Surg (Br) 1960; 42: 28–30
- Charnley J. Tissue reaction to the polytetraflouroethyene. Lancet 1963; II: 1379
- Charnley J. Total prosthetic replacement of the hip. Triangle 1968; 8: 211–6
- Charnley J. The reaction of bone to self-curing acrylic cement. A long-term histological study in man. J Bone Joint Surg (Br) 1970; 52(2)340–53
- Charnley J, Cupic Z. The nine and ten year results of the low-friction arthroplasty of the hip. Clin Orthop 1973; 95: 9–25
- Charnley J, Halley D K. Rate of wear in total hip replacement. Clin Orthop 1975; 112: 170–9
- Charnley J. Low fricrion arthroplasty of the hip. Theory and practice. Berlin 1979; 25–40, Springer
- Chmell M J, Poss R, Thomas W H, Sledge C B. Early failure of Hylamer acetabular inserts due to eccentric wear. J Arthroplasty 1996; 11(3)351–3
- Cigada A, Brunella M F, Chiesa R. Biomechanical aspects of bone cement the effect of dynamic stress. In Pipino F: Bone cement and cemented fixation of implants: Genova. 2001; 48
- Clarke I C, Willmann G. Structural Ceramics in Orthopedics. Bone Implant Interface, Cameron. St Louis, Mosby. 1994; 203–252
- Clarke I C. THR Simulator Wear: Debris Generation, Characterization, and Limitation. 5th annual symposium on Alternative Bearing Surfaces in Total Joint Replacemen, New York, Sept, 2002
- Collier J P, Sperling D K, Currier J H, Sutula L C, Saum K A, Mayor M B. Impact of gamma sterilization on clinical performance of polyethylene in the knee. J Arthroplasty 1996; 11(4)377–89
- Collier J P, Bargmann L S, Currier B H, Mayor M B, Currier J H, Bargmann B C. An analysis of hylamer and polyethylene bearings from retrieved acetabular components. Orthopedics 1998; 21(8)865–71
- Connelly G M, Rimnac C M, Wright T M, Hertzberg R W, Manson J A. Fatigue crack propagation behavior of ultrahigh molecular weight polyethylene. J Orthop Res 1984; 2: 119–25
- Cooke P H, Newman J H. Fractures of the femur in relation to cemented hip prostheses. J Bone Joint Surg (Br) 1988; 70(3)386–9
- Cooper J R, Dowson D, Fisher J. Macroscopic and micro scopic wear mechanisms in ultra-high molecular weight polyethylene. Wear 1993; 162–164, 378–84
- Coughlan J J, Hug D P. Ultrahigh molecular weight polyethylene. Encydopaedia of polymer science and engineering, H F Mark, N M Bikales, C G Overberger, G Menges, J Kroschwitz. Wiley, New York 1986
- Cuckler J M, Bearcroft J, Asgian C M. Femoral head technologies to reduce polyethylene wear in total hip arthroplasty. Clin Orthop 1995; 317: 57–63
- Currier B H, Currier J H, Collier J P, Mayor M B. Effect of fabrication method and resin type on performance of tibial bearings. J Biomed Mater Res 2000; 53(2)143–51
- Dalla Pria P. Recent innovations relating to the use of ceramicceramic hip joint prostheses. Performance of the Wear Couple BIOLOX in Hip Arthroplasty: Proceedings of the 1st Symposium on Ceramic Wear Couple, W Puhl. Ferdinand Enke Verlag, Stuttgart 1996; 84–91
- D'Antonio J, Capello W N, Manley M T. Remodeling of bone around hydroxyapatite-coated femoral stems. J Bone Joint Surg (Am) 1996; 78(8)1226–34
- D'Antonio J, Capello W, Manley M, Bierbaum B. New experience with alumina-on-alumina ceramic bearings for total hip arthroplasty. J Arthroplasty 2002; 17(4)390–7
- D'Aubigne R M, Postel M. Function al results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg (Am) 1954; 36(3)451–75
- Debrunner H U. Temperature increase of bone cement in polymerisation. Arch Orthop Unfallchir 1974; 78(4)309–18
- De Groot K, Geesink R, Klein C P, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res 1987; 21(12)1375–81
- Degussa, Kulzer. DRP patent. 1943, 973 590
- DeLee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop 1976; 121: 20–32
- D'Lima D D, Hermida J C, Chen P C, Colwell C W, Jr. Polyethylene cross-linking by two different methods reduces acetabular liner wear in a hip joint wear simulator. J Orthop Res 2003; 21(5)761–6
- Dempsey D J, Thirucote R R. Sterilization of medical devices: a review. J Biomater Appl 1989; 3(3)454–523
- Devane P A, Bourne R B, Rorabeck C H, Hardie R M, Horne J G. Measurement of polyethylene wear in metal-backed acetabular cups. I. Three-dimensional technique. Clin Orthop 1995; 319: 303–16
- Devane P A, Horne J G. Assessment of polyethylene wear in total hip replacement. Clin Orthop 1999; 369: 59–72
- Doorn P F, Mirra J M, Campbell P A, Amstutz H C. Tissue reaction to metal on metal total hip prostheses. Clin Orthop 1996; 329(Suppl)S187–205
- Doorn P, Campbell P, Benya P. The application of transmission electron microscopy to the characterization of metal wear particles from metal on metal total hp replacements. Trans Orthop Res Soc 1997; 22: 70
- Doorn P F, Campbell P A, Worrall J, Benya P D, McKellop H A, Amstutz H C. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res 1998; 42(1)103–11
- Dorr L D, Wan Z, Longjohn D B, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metal-on-metal articulation. Four to seven-year results. J Bone Joint Surg (Am) 2000; 82(6)789–98
- Dowd J E, Schwendeman L J, Macaulay W, Doyle J S, Shanbhag A S, Wilson S, Herndon J H, Rubash H E. Aseptic loosening in uncemented total hip arthroplasty in a canine model. Clin Orthop 1995; 319: 106–21
- Dowd J E, Sychterz C J, Young A M, Ench C A. Characterization of long-term femoral head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg (Am) 2000; 82(8)1102–7
- Downes S, Wood D J, Malcolm A J, Ali S Y. Growth hormone in polymethylmethacrylate cement. Clin Orthop 1990; 252: 294–8
- Draenert K. The John Charnley Award Paper. Histomorphology of the bone-to-cement interface: remodeling of the cortex and revascularization of the medullary canal in animal experiments. Hip 1981; 71–110
- Drury G B, Caress A, Corin P G. The non metallic denture bases. BrDent J 1935; 59(3)130
- Dumbleton J H, Shen C, Miller E. Study of the wear of some materials in connection with total hip replacement. 1974; 29: 163–171, Wear
- Earnes E D, Reddi A H. The effect of fluoride on bone mineral apatite. Metab Bone Dis 1979; 2: 2
- Eckardt A, Aberman H M, Cantwell H D, Heine J. Biological fixation of hydroxyapatite-coated versus grit-blasted titanium hip stems: a canine study. Arch Orthop Trauma Surg 2003; 123(1)28–35
- Edidin A A, Pruitt L, Jewett C W, Crane D J, Roberts D, Kurtz S M. Plasticity-induced damage layer is a precursor to wear in radiation-cross-linked UHMWPE acetabular components for total hip replacement. Ultra-high-molec-ular-weight polyethylene. J Arthroplasty 1999; 14(5)616–27
- Elfick A P, Hall R M, Pinder I M, Unsworth A. The influence of femoral head surface roughness on the wear of ultrahigh molecular weight polyethylene sockets in cementless total hip replacement. J Biomed Mater Res 1999; 48(5)712–8
- Eriksson A R, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent 1983; 50(1)101–7
- Essner A, Poineni V K, Wang A, Stark C, Dumbleton J H. Effect of femoral head surface roughness and crosslinking on the wear of UHMWPE acetabular inserts. 24th Trans Soc Biomater 1998; 21: 4
- Evans E M. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg (Br) 1974; 56(4)626–642
- Eyerer P, Ke Y C. Property changes of UHMW polyethylene hip cup endoprostheses during implantation. J Biomed Mater Res 1984; 18(9)1137–51
- Eyerer P, Ellwanger R, Federolf H-A, Kurth M, Madler H. Polyethylene. Consise encyclopaedia of medical and dental materials, D Williams, R Cahn. Pergamon, Oxford 1990
- Feith R. Side-effects of acrylic cement implanted into bone. A histological, (micro)angiographic, fluorescence-micro-scopic and autoradiog-aphic study in the rabbit femur. Acta Orthop Scand Suppl 1975; 161: 3–136
- Feldman L A, Hui H K. Compatibility of medical devices and materials with low-temperature hydrogen peroxide gas plasma. Med Dev Diag Ind 1997; 19: 57–62
- Ferrier G M, McEvoy A, Evans C E, Andrew J G. The effect of cyclic pressure on human monocyte-derived macrophages in vitro. J Bone Joint Surg (Br) 2000; 82(5)755–9
- Fisher D A, Tsang A C, Paydar N, Milionis S, Turner C H. Cement-mantle thickness affects cement strains in total hip replacement. J Biomech 1997; 30(11–12)1173–7
- Freeman M A, Bradley G W, Revell P A. Observations upon the interface between bone and polymethylmethacrylate cement. J Bone Joint Surg (Br) 1982; 64(4)489–93
- Freeman M A, Plante-Bordeneuve P. Early migration and late aseptic failure of proximal femoral prostheses. J Bone Joint Surg (Br) 1994; 76(3)432–8
- Fritsch E W, Gleitz M. Ceramic femoral head fractures in total hip arthroplasty. Clin Orthop 1996; 328: 129–36
- Fujita H, Ido K, Matsuda Y, Iida H, Oka M, Kitamura Y, Nakamura T. Evaluation of bioactive bone cement in canine total hip arthroplasty. J Biomed Mater Res 2000; 49(2)273–88
- Furnes O, Lie S A, Havelin L I, Vollset S E, Engesaeter L B. Exeter and charnley arthroplasties with Boneloc or high viscosity cement. Comparison of 1,127 arthroplasties followed for 5 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 1997; 68(6)515–20
- Geesink R G, de Groot K, Klein C P. Chemical implant fixation using hydroxyl-apatite coatings. The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin Orthop 1987; 225: 147–70
- Geesink R G, Hoefnagels N H. Six-year results of hydroxya-patite-coated total hip replacement. J Bone Joint Surg (Br) 1995; 77(4)534–47
- Gibbons D F. Materials for orthopaedic joint prostheses. Biocompatibility of orthopaedic implants, D F Williams. CRC Press, Boca Raton, FL 1982; I: 112–3
- Gillespie W J, Henry D A, O'Connell D L, Kendrick S, Juszczak E, McInneny K, Derby L. Development of hematopoietic cancers after implantation of total joint replacement. Clin Orthop 1996; 329(Suppl)S290–6
- Gillis A M, Furman B D, Li S. Influence of ultra high molecular weight polyethylene resin type and manufacturing method on real time oxidation. Trans Orthop Res Soc 1998; 23: 360
- Goh J C, Low S L, Bose K. Effect of femoral rotation on bone mineral density measurements with dual energy X-ray absorptiometry. Calcif Tissue Int 1995; 57(5)340–3
- Gomez-Barrena E, Li S, Furman B S, Masri B A, Wright T M, Salvati E A. Role of polyethylene oxidation and consolidation defects in cup performance. Clin Orthop 1998; 352: 105–17
- Goodman S B, Fornasier V L, Kei J. The effects of bulk versus particulate polymethylmethacrylate on bone. Clin Orthop 1988; 232: 255–62
- Green T R, Fisher J, Stone M, Wroblewski B M, Ingham E. Polyethylene particles of a 'critical size' are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998; 19(24)2297–302
- Greer K W, Schmidt M B, Hamilton J V. The hip simulator wear of gamma-vacuum, gamma-air, and ethylene oxide sterilized UHMWPE following a severe oxidative challenge. 44th Trans Orthop Res Soc 1998; 23: 52
- Griffith M J, Seidenstein M K, Williams D, Charnley J. Socket wear in Charnley low friction anhroplasty of the hip. Clin Orthop 1978; 137: 37–47
- Grobbelaar C J, Weber F A, Spirakis A, DuPlesis T A, Cappaert G, Cakic J N. Clinical experience with gamma irradiation cross-linked polyethylene. A 14- to 20 year followup report. S Afr Bone Joint Surg 1999; 9: 140–7
- Grobbelaar C J, du Plessis T A, Marais F. The radiation improvement of polyethylene prostheses. A preliminary study. J Bone Joint Surg (Br) 1978; 60(3)370–4
- Gruen T A, McNeice G M, Amstutz H C. “Modes of failure” of cemented stem-type femoral components. A radiographic analysis of loosening. Clin Orthop 1979; 141: 17
- Hall R M, Unsworth A, Siney P, Wroblewski B M. Wear in retrieved Charnley acetabular sockets. Proc Inst Mech eng[H] 1996; 210(3)197–207
- Hall R M, Siney P, Unsworth A, Wroblewski B M. The effect of surface topography of retrieved femoral heads on the wear of UHMWPE sockets. Med Eng Phys 1997; 19: 711–79
- Hallert B. Scientific research and measuring technic. Sver Tandlakarforb Tidn 1970; 62(13)663–8
- Hamada Y, Horiuchi T, Sano Y, Usui I. The wear of a polyethylene socket articulating with a zirconia ceramic femoral head in canine total hip arthroplasty. J Biomed Mater Res 1999; 48(3)301–8
- Hamilton J V, Wang H C, Sung C. The effect of fusion defects on the mechanical properties of UHMWPE. 5th Trans World Biomater Conf. 1996; 2: 511
- Hamilton J V, Schmidt M B, Shah C. The eflects of sterilization dose on UHMWPE wear rates. 43rd Trans Orthop Res Soc 1997; 782
- Hannouche D, Nich C, Bizot P, Meunier A, Nizard R, Sedel L. Fractures of ceramic bearings: history and present status. Clin Orthop 2003; 417: 19–26
- Haraguchi K, Sugano N, Nishii T, Miki H, Oka K, Yoshikawa H. Phase transformation of a zirconia ceramic head after total hip arthroplasty. J Bone Joint Surg (Br) 2001; 83(7)996–1000
- Hardinge K, Porter M L, Jones P R, Hukins D W, Taylor C J. Measurement of hip prostheses using image analysis. The maxima hip technique. J Bone Joint Surg (Br) 1991; 73(5)724–8
- Harrigan T P, Harris W H. A three-dimensional non-linear finite element study of the effect of cement-prosthesis debonding in cemented femoral total hip components. J Biomech 1991; 24(11)1047–58
- Harris W H. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg (Am) 1969; 51(4)737–55
- Harris W H. The first 32 years of total hip arthroplasty. One surgeon's perspective. Clin Orthop 1992; 274: 6–11
- Harris W H. Current status of highly Crosslinked polyethylene. 32nd open meeting of the hip society. 2004; 15
- Hasegawa M, Ohashi T, Tani T. Poor outcome of 44 cemented total hip arthroplasties with alumina ceramic heads: clinical evaluation and retrieval analysis after 10-16 years. Acta Orthop Scand 2001; 72(5)449–56
- Hasegawa M, Sudo A, Hirata H, Uchida A. Ceramic acetabular liner fracture in total hip arthroplasty with a ceramic sandwich cup. J Arthroplasty 2003; 18(5)658–61
- Hatton A, Nevelos J E, Nevelos A A, Banks R E, Fisher J, Ingham E. Alumina-alumina artificial hip joints Part I: a histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials 2002; 23(16)3429–40
- Hatton A, Nevelos J E, Matthews J B, Fisher J, Ingham E. Effects of clinically relevant alumina ceramic wear particles on TNF-alpha production by human peripheral blood mononuclear phagocytes. Biomaterials 2003; 24(7)1193–1204
- Havelin L I, Espehaug B, Vollset S E, Engesaeter L B. The effect of the type of cement on early revision of Charnley total hip prostheses. A review of eight thousand five hundred and seventy-nine primary arthroplasties from the Norwegian Arthroplasty Register. J Bone Joint Surg (Am) 1995; 77(10)1543–50
- Havelin L I, Espehaug B, Engesaeter L B. The performance of two hydroxyapatite-coated acetabular cups compared with Charnley cups. From the Norwegian Arthroplasty Register. J Bone Joint Surg (Br) 2002; 84(6)839–45
- Haynes D R, Boyle S J, Rogers S D, Howie D W, Vernon-Rob-erts B. Variation in cytokines induced by particles from different prosthetic materials. Clin Orthop 1998; 352: 223–30
- Hendrich C, Bahlmann J, Eulert J. Migration of the uncemented Harris-Galante acetabular cup: results of the einbildroentgenanalyse (EBRA) method. J Arthroplasty 1997; 12(8)889–95
- Hennig W, Blencke B A, Bromer H, Deutscher K K, Gross A, Ege W. Investigations with bioactivated polymethylmethacrylates. J Biomed Mater Res 1979; 13(1)89–99
- Henssge E J, Bos I, Willman G. AL203 against AL203 combination in hip endoprostheses. Histologic investigations with semiquantitative grading of revision and autopsy cases and abrasion measures. J Mater Sci Mater Med 1994; 5: 657–61
- Herberts P, Malchau H. How outcome studies have changed total hip arthroplasty practices in Sweden. Clin Orthop 1997; 344: 44–60
- Hernandez J R, Keating E M, Faris P M, Meding J B, Ritter M A. Polyethylene wear in uncemented acetabular components. J Bone Joint Surg (Br) 1994; 76(2)263–6
- Hernigou P, Bahrami T. Zirconia and alumina ceramics in comparison with stainless-steel heads. Polyethylene wear after a minimum ten-year follow-up. J Bone Joint Surg (Br) 2003; 85(4)504–9
- Hirashima Y, Ishiguro N, Kondo S, Iwata H. Osteoclast induction from bone marrow cells is due to pro-inflamma-tory mediators from macrophages exposed to polyethylene particles: a possible mechanism of osteolysis in failed THA. J Biomed Mater Res 2001; 56(2)177–83
- Hopper R H, Jr, Young A M, Orishimo K F, McAuley J P. Correlation between early and late wear rates in total hip arthroplasty with application to the performance of marathon cross-linked polyethylene liners. J Arthroplasty 2003a; 18(7 Suppl 1)60–7
- Hopper RH, Jr, Young A M, Orishimo K F, Engh C A, Jr. Effect of terminal sterilization with gas plasma or gamma radiation on wear of polyethylene liners. J Bone Joint Surg (Am) 2003b; 85(3)464–8
- Howie D W, Vernon-Roberts B, Oakeshott R, Manthey B. A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. J Bone Joint Surg (Am) 1988; 70(2)257–63
- Howie D W, Haynes D R, Rogers S D, McGee M A, Pearcy M J. The response to particulate debris. Orthop Clin North Am 1993; 24(4)571–81
- Huiskes R, Weinans H, Van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effect of flexible materials. Clin Orthop 1992; 274: 124–34
- Hultmark P, Hostner J, Herberts P, Karrholm J. Radiographic evaluation of Charnley cups used in first-time revision: repeated observations for 7-15 years. J Arthroplasty 2003; 18(8)1005–15
- Hunt S M, McKenna S P, McEwen J, Williams J, Papp E. The Nottingham Health Profile: subjective health status and medical consultations. Soc Sci Med [A] 1981; 15(3 Pt 1)221–9
- Ilchmann T, Mjoberg B, Wingstrand H. Measurement accuracy in acetabular cup wear. Three retrospective methods compared with Roentgen stereophotogrammetry. J Arthroplasty 1995; 10(5)636–42
- Illgren R L, Laurent M P, Watanuki K, Hagenauer M E, Bhambri S K, Pike J W, Blanchard C R, Forsythe T M. Highly crosslinked vs conventional polyethylene particles. An in vitro comparison of biologic activities. 49th Trans Orthop Res Soc 2003; 1438
- Ingram J H, Fisher J, Stone M, Ingham E. Effect of crosslinking on biological activity of UHMWPE wear debris. 49th Trans Orthop Res Soc 2003; 1439
- Ito A, Tateishi T, Niwa S, Tange S. In-vitro evaluation of the cytocompatibility of wear particles generated by UHMWPE/zirconia friction. Clin Mater 1993; 12(4)203–9
- Ivarsson I, Wahlstrom O, Djerf K, Jacobsson S A. Revision of infected hip replacement. Two-stage procedure with a temporary gentamicin spacer. Acta Orthop Scand 1994; 65(1)7–8
- Iwase T, Warashina H, Yamauchi K, Sugiura S, Hasegawa Y. Early head penetration into cemented Hylamer Ogee socket. J Arthroplasty 2003; 18(7)920–4
- Jacobs J J, Rosenbaum D H, Hay R M, Gitelis S, Black J. Early sarcomatous degeneration near a cementless hip replacement. A case report and review. J Bone Joint Surg (Br) 1992; 74(5)740–4
- Jacobs J J, Shanbhag A, Glant T T, Black J, Galante J O. Wear Debris in Total Joint Replacements. J Am Acad Orthop Surg 1994; 2(4)212–220
- Jacobs J J, Skipor A K, Doorn P F, Campbell P, Schmalzried T P, Black J, Amstutz H C. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop 1996; 329(Suppl)S256–63
- Jacobsson S A, Djerf K, Wahlstrom O. A comparative study between McKee-Farrar and Charnley arthroplasty with long-term follow-up periods. J Arthroplasty 1990; 5(1)9–14
- Jaffe W L, Scott D F. Total hip arthroplasty with hydroxyapa-tite-coated prostheses. J Bone Joint Surg (Am) 1996; 78(12)1918–34
- Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop 1981; 157: 259–78
- Jasty M, Bragdon C, Jiranek W, Chandler H, Maloney W, Harris W H. Etiology of osteolysis around porous-coated cementless total hip arthroplasties. Clin Orthop 1994; 308: 111–26
- Jasty M, Goetz D D, Bragdon C R, Lee K R, Hanson A E, Elder J R, Harris W H. Wear of polyethylene acetabular components in total hip arthroplasty. An analysis of 128 components retrieved at autopsy or revision. J Bone Joint Surg (Am) 1997; 79(3)349–358
- Judet J, Judet R. The use of an artificial femoral head for arthroplasty of the hip joint. J Bone Surg. (Br) 1956; 32: 166
- Kadoya Y, Revell P A, Kobayashi A, al-Saffar N, Scott G, Freeman M A. Wear particulate species and bone loss in failed total joint arthroplasties. Wear particulate species and bone loss in failed total joint arthroplasties. Clin Orthop 1997; 340: 118–29
- Kamikawa K, Harada Y, Nagata K, Tsuchida T, Moriya H. Differential effects of oxidized and non-oxidized polyethylene particles on human monocyte/macrophages in vitro. 44th Trans Orthop Res Soc 1998; 786
- Keener J D, Callaghan J J, Goetz D D, Pederson D R, Sullivan P M, Johnston R C. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: a concise follow-up of a previous report. J Bone Joint Surg (Am) 2003a; 85(6)1066–72
- Keener J D, Callaghan J J, Goetz D D, Pederson D, Sullivan P, Johnston R C. Long-term function after Charnley total hip arthroplasty. Clin Orthop 2003b; 417: 148–56
- Kim K J, Kobayashi Y, Itoh T. Osteolysis model with continuous infusion of polyethylene particles. Clin Orthop 1998; 352: 46–52
- Kim Y H, Kim J S, Cho S H. A comparison of polyethylene wear in hips with cobalt-chrome or zirconia heads. A prospective, randomised study. J Bone Joint Surg (Br) 2001; 83(5)742–50
- Kiratli B J, Heiner J P, McBeath A A, Wilson M A. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res 1992; 10(6)836–44
- Kobayashi A, Donnelly W J, Scott G, Freeman M A. Early radiological observations may predict the long-term survival of femoral hip prostheses. J Bone Joint Surg (Br) 1997; 79(4)583–9
- Korovessis P, Petsinis G, Repanti M. Zweymueller with metal-on-metal articulation: clinical, radiological and histological analysis of short-term results. Arch Orthop Trauma Surg 2003; 123(1)5–11
- Kothari M, Bartel D L, Booker J F. Surface geometry of retrieved McKee-Farrar total hip replacements. Clin Orthop 1996; 329(Suppl)S141–7
- Krause W R, Miller J, Ng P. The viscosity of acrylic bone cements. J Biomed Mater Res 1982; 16(3)219–43
- Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech 1995; 28(10)1225–36
- Krismer M, Stockl B, Fischer M, Bauer R, Mayrhofer P, Ogon M. Early migration predicts late aseptic failure of hip sockets. J Bone Joint Surg (Br) 1996; 78(3)422–6
- Krismer M, Biedermann R, Stockl B, Fischer M, Bauer R, Haid C. The prediction of failure of the stem in THR by measurement of early migration using EBRA-FCA. Einzel-Bild-Roentgen-Analyse-femoral component analysis. J Bone Joint Surg (Br) 1999; 81(2)273–80
- Kroon P O, Freeman M A. Hydroxyapatite coating of hip prostheses. Effect on migration into the femur. J Bone Joint Surg (Br) 1992; 74(4)518–22
- Kubo T, Sawada K, Hirakawa K, Shimizu C, Takamatsu T, Hirasawa Y. Histiocyte reaction in rabbit femurs to UHMWPE, metal, and ceramic particles in different sizes. J Biomed Mater Res 1999; 45(4)363–9
- Kuhn A T. Corrosion of Co-Cr alloys in aqueous environments. Biomaterials 1981; 2(2)68–77
- Kurtz S M, Muratoglu O K, Evans M, Edidin A A. Advances in the processing, sterilization, and crosslinking of ultrahigh molecular weight polyethylene for total joint arthroplasty. Biomaterials 1999; 20(18)1659–88
- Kurtz S M, Pruitt L A, Jewett C W, Foulds J R, Edidin A A. Radiation and chemical crosslinking promote strain hardening behavior and molecular alignment in ultra high molecular weight polyethylene during multi-axial loading conditions. Biomaterials 1999b; 20: 1449–1462
- Kusaba A, Kuroki Y. Femoral component wear in retrieved hip prostheses. J Bone Joint Surg (Br) 1997; 79(2)331–6
- Kyi M S, Holton J, Ridgway G L. Assessment of the efficacy of a low temperature hydrogen peroxide gas plasma sterilization system. J Hosp Infect 1995; 31: 275–84
- Kärrholm J. Roentgen stereophotogrammetry. Review of orthopedic applications. Acta Orthop Scand 1989; 60(4)491–503
- Kärrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated, and porous-coated stems with roentgen stereophotogrammetric analysis. J Bone Joint Surg (Am) 1994; 76(11)1692–705
- Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses: Review of methodology and clinical results. Clin Orthop 1997; 344: 94–110
- Kärrholm J, Nivbrant B, Thanner J, Anderberg C, Börlin N, Herberts P, Malchau H. Radiostereometric evaluation of hip implant design and surface finish. Scientific exhibition at 67th Annual Meeting of the American Academy of Orthopaedic Surgeons. Orlando March, 2000
- Lacefield W R. Hydroxyapatite coatings. Ann N Y Acad Sci 1988; 523: 72–80
- Lalor P A, Namba R, Mitchell S L, Bearcroff J, Beals N, Sledge C B, Spector M. Migration of polyethylene particles around stable implants in an animal model. J Long Term Eff Med Implants 1999; 9(4)261–72
- Lancaster J G, Dowson D, Isaac G H, Fisher J. The wear of ultra-high molecular weight polyethylene sliding on metallic and ceramic counterfaces representative of current femoral surfaces in joint replacement. Proc Inst Mech Eng [H] 1997; 211(1)17–24
- Landy M M, Walker P S. Wear of ultra-high-molecular-weight polyethylene components of 90 retrieved knee prostheses. J Arthroplasty 1988; 3(Suppl)S73–85
- Langkamer V G, Case C P, Collins C, Watt I, Dixon J, Kemp A J, Atkins R M. Tumors around implants. J Arthroplasty 1997; 12(7)812–8
- Lappalainen R, Selenius M, Anttila A, Konttinen Y T, Santavirta S S. Reduction of wear in total hip replacement prostheses by amorphous diamond coatings. J Biomed Mater Res 2003; 15 66B(1)410–3
- Lec A JC, Ling R S, Vangal S S. Some clinically relevant variables affecting the mechanical behaviour of bone cement. Arch Orthop Traumat Surg 1978; 92: 1–18
- Lee I Y, Skinner H B, Keyak J H. Effects of variation of prosthesis size on cement stress at the tip of a femoral implant. J Biomed Mater Res 1994; 28(9)1055–60
- Lee S H, Brennan F R, Jacobs J J, Urban R M, Ragasa D R, Glant T T. Human monocyte/macrophage response to cobalt-chromium corrosion products and titanium particles in patients with total joint replacements. J Orthop Res 1997; 15(1)40–9
- Lemons J E. Hydroxyapatite coatings. Clin Orthop 1988; 235: 220–3
- Lerouge S, Huk O, Yahia L, Witvoet J, Sedel L. Ceramicceramic and metal-polyethylene total hip replacements: comparison of pseudomembranes after loosening. J Bone Joint Surg (Br) 1997; 79(1)135–9
- Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res 1997; 38(2)155–82
- Li S, Burstein A H. Ultra-high molecular weight polyethylene. The material and its use in total joint implants. J Bone Joint Surg (Am) 1994; 76(7)1080–90
- Lidén H, Bengtsson P, Hassander H, Kärrholm J, Wesslen B. Evaluation of bone cements in laboratory THA model. 44th Trans Orthop Res Soc 1998; 840
- Linder L. Bone cement monomer. University of Göteborg, GöteborgSweden 1976, Thesis
- Linder L, Hansson H A. Ultrastructural aspects of the interface between bone and cement in man. Report of three cases. J Bone Joint Surg (Br) 1983; 65(5)646–9
- Linder L, Carlsson A S. The bone-cement interface in hip arthroplasty. A histologic and enzyme study of stable components. Acta Orthop Scand 1986; 57(6)495–500
- Litner F, Bohm G, Huber M, Scholz R. Histology of tissue adjacent to an HAC-coated femoral prosthesis. A case report. J Bone Joint Surg (Br) 1994; 76: 824–830
- Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg (Am) 1990; 72(4)518–28
- Livingston B J, Chmell M J, Spector M, Poss R. Complications of total hip arthroplasty associated with the use of an acetabular component with a Hylamer liner. J Bone Joint Surg (Am) 1997; 79(10)1529–38
- Lombardi A V, Jr, Mallory T H, Vaughn B K, Drouillard P. Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads. J Bone Joint Surg (Am) 1989; 71(9)1337–42
- Lombardi A V, Mallory T H, Alexiades M M, Cuckler J M, Faris P M, Jaffe K A, Keating E M, Nelson C L, Jr, Ranawat C S, Williams J, Wixson R, Berend K R, Dodds K L, Adams J B. Mid-term results of a polyethylene-free metal on metal articulation. J Arthroplasty 2004; 19(2)260
- MacDonald S J, McCalden R W, Chess D G, Bourne R B, Rorabeck C H, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop 2003; 406: 282–96
- Maezawa K, Nozawa M, Hirose T, Matsuda K, Yasuma M, Shitoto K, Kurosawa H. Cobalt and chromium concentrations in patients with metal-on-metal and other cementless total hip arthroplasty. Arch Orthop Trauma Surg 2002; 122(5)283–7
- Magnan B, Gabbi C, Regis D. Sodium fluoride sustainedrelease bone cement: an experimental study in vitro and in vivo. Acta Orthop Belg 1994; 60(1)72–9
- Malchau H, Herberts P, Ahnfelt L. Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978-1990. Acta Orthop Scand 1993; 64(5)497–506
- Malchau H, Karrholm J, Wang Y X, Herberts P. Accuracy of migration analysis in hip arthroplasty. Digitized and conventional radiography, compared to radiostereometry in 51 patients. Acta Orthop Scand 1995; 66(5)418–24
- Malchau H, Herberts P, Söderman P, Oden A. Prognosis of total hip replacement. Update and validation of results from the Swedish national hip arthroplasty register 1979–1998. Department of Orthopaedics, Göteborg University, Sweden 2000
- Mann K A, Bartel D L, Wright T M, Burstein A H. Coulomb frictional interfaces in modeling cemented total hip replacements: a more realistic model. J Biomech 1995; 28(9)1067–78
- Manning D, Chiang P, Martell J, Harris W H. In vivo comparison of traditional vs. highly crosslinked polyethylene wear. J Arthroplasty 2004; 19(2)262
- Martell J M, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg (Am) 1997; 79(11)1635–41
- Martell J M, Verner J J, Incavo S J. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty 2003; 18(7 Suppl 1)55–9
- Martin D P, Engelberg R, Agel J, Swiontkowski M F. Comparison of the Musculoskeletal Function Assessment questionnaire with the Short Form-36, the Western Ontario and McMaster Universities Osteoarthritis Index, and the Sickness Impact Profile health-status measures. J Bone Joint Surg (Am) 1997; 79(9)1323–35
- Mathiesen E B, Lindgren U, Reinholt F P, Sudmann E. Wear of the acetabular socket. Comparison of polyacetal and polyethylene. Acta Orthop Scand 1986; 57(3)193–6
- Matsuda Y, Ido K, Nakamura T, Fujita H, Yamamuro T, Oka M, Shibuya T. Prosthetic replacement of the hip in dogs using bioactive bone cement. Clin Orthop 1997; 336: 263–77
- Matthews J B, Green T R, Stone M H, Wroblewski B M, Fisher J, Ingham E. Comparison of the response of primary human peripheral blood mononuclear phagocytes from different donors to challenge with model polyethylene particles of known size and dose. Biomaterials 2000a; 21(20)2033–44
- Matthews J B, Besong A A, Green T R, Stone M H, Wroblewski B M, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res 2000b; 52(2)296–307
- McKee G K. Artificial hip joint. J Bone Joint Surg (Br). Proceedings of the east Anglian orthopaedic club. 1951; 33: 465
- McKellop H, Clarke I, Markolf K, Amstutz H. Friction and wear properties of polymer, metal, and ceramic prosthetic joint materials evaluated on a multichannel screening device. J Biomed Mater Res 1981; 15(5)619–53
- McKellop H A, Rostlund T V. The wear behavior of ionimplanted Ti-6A1-4V against UHMW polyethylene. J Biomed Mater Res 1990; 24(11)1413–25
- McKellop H, Lu B, Benya P. Friction, lubrication and wear of cobalt-chromium, alumina and zirconia hip prostheses compared on a joint simulator. 38th Trans Orthop Res Soc 1992; 17: 402
- McKellop H A, Campbell P, Park S H, Schmalzried T P, Grigoris P, Amstutz H C, Sarmiento A. The origin of submicron polyethylene wear debris in total hip arthroplasty. Clin Orthop 1995; 311: 3–20
- McKellop H, Park S H, Chiesa R, Doorn P, Lu B, Normand P, Grigoris P, Amstutz H. In vivo wear of three types of metal on metal hip prostheses during two decades of use. Clin Orthop 1996; 329(Suppl)S128–40
- McKellop H, Shen F-W, Salovey R. Extremely low wear of gamma-crosslinked remelted UHMW polyethylene acetabular cups. 44th Trans Orthop Res Soc 1998; 98–117
- McKellop H, Shen F W, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res 1999; 17(2)157–67
- McKellop H, Shen F W, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. A hip-simulator study. J Bone Joint Surg (Am) 2000; 82(12)1708–25
- McKellop H A. Bearing surfaces in total hip replacements. Instr Course Lect. state of the art and future developments. 2001; 50: 165–79
- McMinn D, Treacy R, Lin K, Pynsent P. Metal on metal surface replacement of the hip. Experience of the McMinn prothesis. Clin Orthop 1996; 329(Suppl)S89–98
- Medley J B, Chan F W, Krygier J J, Bobyn J D. Comparison of alloys and designs in a hip simulator study of metal on metal implants. Clin Orthop 1996; 329(Suppl)S148–59
- Michaud R J, Rashad S Y. Spontaneous fracture of the ceramic ball in a ceramic-polyethylene total hip arthroplasty. J Arthroplasty 1995; 10(6)863–7
- Minari C, Baleani M, Cristofolini L, Baruffaldi F. The effect on the fatigue strength of bone cement of adding sodium fluoride. Proc Inst Mech Eng [H] 2001; 215: 251–3
- Mishra A K, Davidson J A, Poggie R A, Kovacs P, FritzGerald T J. Mechanical and tribological properties and biocompatibiliiy of diffusionh hardened T-13Nb-13Zr: A new titanium alloy for surgical implants. Medical Applications of Titanium and Its alloy: The Material and Biological Issue, S A Brown, J E Lemons. ASTM, West Conshohocken, PA 1996; 96–112
- Mittelmeier H, Heisel J. Sixteen-years' experience with ceramic hip prostheses. Clin Orthop 1992; 282: 64–72
- Mjöberg B. Loosening of the cemented hip prosthesis. The importance of heat injury. Acta Orthop Scand Suppl 1986; 221: 1–40
- Mjöberg B, Franzen H, Selvik G. Early detection of pros-thetic-hip loosening. Comparison of low- and high-vis-cosity bone cement. Acta Orthop Scand 1990; 61: 273–4
- Mogensen B, Ekelund L, Hansson L I, Lidgren L, Selvik G. Surface replacement of the hip in chronic arthritis. A clinical, radiographic and roentgen stereophotogrammetric evaluation. Acta Orthop Scand 1982; 53(6)929–36
- Moilanen T, Stocks G W, Freeman M A, Scott G, Goodier W D, Evans S J. Hydroxyapatite coating of an acetabular prosthesis. Effect on stability. J Bone Joint Surg (Br) 1996; 78(2)200–5
- Morberg P H. On bone tissue reactions to acrylic cement (Thesis). University Gothenburg, GothenburgSweden 1991
- Mouzin O, Soballe K, Bechtold J E. Loading improves anchorage of hydroxyapatite implants more than titanium implants. J Biomed Mater Res 2001; 58(1)61–8
- Muratoglu O K, Bragdon C R, O'Connor D O, Jasty M, Harris W H, Gul R, McGarry F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials 1999; 20(16)1463–70
- Muratoglu O K, Bragdon C R, O'Connor D O, Skelan H, Delaney J, Jasty M, Harris W H. The comparison of the wear behavior of four different types of cross-linked acetabular components. 46th Trans Orrhop Res Soc 2000; 25: 566
- Muratoglu O K, Bragdon C R, O'Connor D O, Jasty M, Harris W H. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. Recipient of the 1999 HAP Paul Award. J Arthroplasty 2001a; 16(2)149–60
- Muratoglu O K, Bragdon C R, O'Connor D, Perinchief R S, Estok D M, 2nd, Jasty M, Harris W H. Larger diameter femoral heads used in conjunction with a highly crosslinked ultra-high molecular weight polyethylene: a new concept. J Arthroplasty 2001b; 16: 24–30
- Muratoglu O K, O'Connor D O, Bragdon C R, Delaney J, Jasty M, Harris W H, Merrill E, Venugopalan P. Gradient crosslinking of UHMWPE using irradiation in molten state for total joint arthroplasty. Biomaterials 2002; 23(3)717–24
- Murray D W, Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg (Br) 1990; 72(6)988–92
- Nakashima Y, Sun D H, Trindade M C, Chun L E, Song Y, Goodman S B, Schurman D J, Maloney W J, Smith R L. Induction of macrophage C-C chemokine expression by titanium alloy and bone cement particles. J Bone Joint Surg (Br) 1999; 81(1)155–62
- Nashed R S, Becker D A, Gustilo R B. Are cementless acetabular components the cause of excess wear and osteolysis in total hip arthroplasty?. Clin Orthop 1995; 317: 19–28
- Nevelos J E, Ingham E, Doyle C, Fisher J, Nevelos A B. Analysis of retrieved alumina ceramic components from Mittelmeier total hip prostheses. Biomaterials 1999; 20(19)1833–40
- Nevelos J E, Prudhommeaux F, Hamadouche M, Doyle C, Ingham E, Meunier A, Nevelos A B, Sedel L, Fisher J. Comparative analysis of two different types of aluminaalumina hip prosthesis retrieved for aseptic loosening. J Bone Joint Surg (Br) 2001; 83(4)598–603
- Nivbrant B, Karrholm J, Rohrl S, Hassander H, Wesslen B. Bone cement with reduced proportion of monomer in total hip arthroplasty: preclinical evaluation and randomized study of 47 cases with 5 years' follow-up. Acta Orthop Scand 2001; 72(6)572–84
- Nizard R S, Sedel L, Christel P, Meunier A, Soudry M, Witvoet J. Ten-year survivorship of cemented ceramicceramic total hip prosthesis. Clin Orthop 1992; 282: 53–63
- Norton M R, Yarlagadda R, Anderson G H. Catastrophic failure of the Elite Plus total hip replacement, with a Hylamer acetabulum and Zirconia ceramic femoral head. J Bone Joint Surg (Br) 2002; 84(5)631–5
- Nunn D, Freeman M A, Hill P F, Evans S J. The measurement of migration of the acetabular component of hip prostheses. J Bone Joint Surg (Br) 1989; 71(4)629–31
- Nyren O, McLaughlin J K, Gridley G, Ekbom A, Johnell O, Fraumeni J F, Jr, Adami H O. Cancer risk after hip replacement with metal implants: a population-based cohort study in Sweden. J Natl Cancer Inst 1995; 87(1)28–33, 4
- Nyström L, Soderkvist I, Wedin P A. A note on some identification problems arising in roentgen stereo photogrammetric analysis. J Biomech 1994; 27(10)1291–4
- Oonishi H, Takayama Y, Tsuji E. Improvement of polyethylene by irradiation in artificial joints. Radiat Phys Chem 1992; 396: 495–504
- Oonishi H, Ishimaru H, Kato A. Effect of cross-linkage by gamma irradiation in heavy doses to low wear polyethylene in total hip prostheses. J Mater Sci Mater Med 1996; 7: 753–763
- Oonishi H, Kuno M, Tsuji E, Fujisima A. The optimum dose of gamma irradiation. Heavy doses to low wear polyethylene in total hip prostheses. J Mater Sci Mater Med 1997; 8: 11–18
- Oonishi H, Nishida M, Kawanabe K. In vitro wear of AL203/ AL203 implant combination with over 10 million cycles duration. 45th Trans Orthop Res Soc 1999; 24: 50
- Oonishi H, Kadoya Y. Wear of high-dose gamma-irradiated polyethylene in total hip replacements. J Orthop Sci 2000; 5(3)223–8
- Oral E, Hawkins N E, O'Keefe M C, Wannomae K K, Harris W H, Muratoglu O K. α-Tocopherol rich cross-linked UHMWPE has high fatigue resistance and low wear. 50th Trans Orthop Res Soc 2004a; 213
- Oral E, Greenbaum E S, Harris W H, Muratoglu O K. Blending α-Tocopherol with UHMWPE powder for oxidation resistance. 50th Trans Orthop Res Soc 2004b; 1485
- Orishimo K F, Robert H, Hopper R H, Jr, Engh C A. Long-Term In Vivo Wear Performance OF Porous-Coated Acetabular Components Sterilized with Gamma Irradiation in air or Ethylene oxide. J Arthroplasty 2003; 18: 546–552
- Parfitt A M, Kleerekoper M, Villanueva A R. Increased bone age: Mechanism and consequences. Osteoporosis, International Symposium on Osteoporosis, C Christiansen, J S Johanssen, J Riis. Denmark Oktober, 1987; 301
- Parisi A N, Young W E. Sterilization with ethylene oxide and other gases. Sterilization and Preservation 4, S S. Lea & Febiger, Philadelphia 1991; 580–595
- Pedersen J G, Lund B, Reimann I. Depressive effects of acrylic cement components on bone metabolism. Isotope release and phosphatase production studied in vitro. Acta Orthop Scand 1983; 54(6)796–801
- Phillips N J, Stockley I, Wilkinson J M. Direct plain radiographic methods versus EBRA-Digital for measuring implant migration after total hip arthroplasty. J Arthroplasty 2002; 17(7)917–25
- Piconi C, Burger W, Richter H G, Cittadini A, Maccauro G, Covacci V, Bruzzese N, Ricci G A, Marmo E. Y-TZP ceramics for artificial joint replacements. Biomaterials 1998; 19(16)1489–94
- Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials 1999; 20(1)1–25
- Pietrabissa R. Biomateriali per protesi e organi artificiali. Collana di Ingegneria Biomedica. Patron Editore, Bologna 1996; 1
- Pizzoferrato A, Cenni E, Ciapetti G. In Vitro Cytocompatibility and Tissue Reaction to Ceramics. Bioceramics and the Human Body, A Raviglioli, A Krajewski. Elsevier, Amsterdam 1992; 288–291
- Pocock S J. Clinical trials: A practical approach. Wiley, Chichester 1983
- Polyethylene resins. Modern plastics encyclopaedia for 1962. McGraw-Hill Publications, New York 1961
- Premnath V, Harris W H, Jasty M, Merrill E W. Gamma sterilization of UHMWPE articular implants: an analysis of the oxidation problem. Ultra High Molecular Weight Poly Ethylene. Biomaterials 1996; 17(18)1741–53
- Prudhommeaux F, Nevelos J, Doyle C. Analysis of Wear Behavior of Alumina/Alumina Hip Prothesis After 10 Years of Implantation. Proceedings of the 11th International Symposium on Ceramics in Medicine, R Z Legeros, J P Legeros. World Scientific Publishing Co, New York 1998
- Pruitt L, Koo J, Rimnac C M, Suresh S, Wright T M. Cyclic compressive loading results in fatigue cracks in ultra high molecular weight polyethylene. J Orthop Res 1995; 13(1)143–6
- Puolakka T J, Keranen J T, Juhola K A, Pajamaki K J, Halonen P J, Nevalainen J K, Saikko V, Lehto M U, Jarvinen M. Increased volumetric wear of polyethylene liners with more than 3 years of shelf-life time. Int Orthop 2003; 27(3)153–9
- Quinn J M, Neale S, Fujikawa Y, McGee J O, Athanasou N A. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int 1998; 62(6)527–31
- Rahbek O, Overgaard S, Jensen T B, Bendix K, Soballe K. Sealing effect of hydroxyapatite coating: a 12-month study in canines. Acta Orthop Scand 2000; 71(6)563–73
- Rahbek O, Overgaard S, Lind M, Bendix K, Bunger C, Soballe K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles. An experimental study in dogs. J Bone Joint Surg (Br) 2001; 83(3)441–7
- Rasquinha V J, Mathews V, Rodriguez J A, Ranawat C S. Polyethylene Wear: Ram Extruded Versus isostatic molded machined liners: a matched pair analysis. 69th Annual Meeting of the American Academy of Orthopaedic Surgeons. Dalas February, 2002; 664
- Reikeras O, Gunderson R B. Failure of HA coating on a gritblasted acetabular cup: 155 patients followed for 7-10 years. Acta Orthop Scand 2002; 73(1)104–8
- Rieker C, Kottig P, Schon R, Windler M, Wyss U. Clinical tribological performance of 144 metal-on-metal hip articulations. METASUL: A Metal-On-Metal Bearing, C Rieker, M Windler, U Wyss. Hans Huber, BernSwitzerland 1999; 83–91
- Ries M D, Weaver K, Beals N. Safety and efficacy of ethylene oxide sterilized polyethylene in total knee arthroplasty. Clin Orthop 1996; 331: 159–63
- Ries M D, Weaver K, Rose R M, Gunther J, Sauer W, Beals N. Fatigue strength of polyethylene after sterilization by gamma irradiation or ethylene oxide. Clin Orthop 1996; 333: 87–95
- Ries M D, Salehi A, Widding K, Hunter G. Polyethylene wear performance of oxidized zirconium and cobalt-chro-mium knee components under abrasive conditions. J Bone Joint Surg (Am) 2002; 2(Suppl)129–35
- Rimnac C M, Klein R W, Betts F, Wright T M. Post-irradia-tion aging of ultra-high molecular weight polyethylene. J Bone Joint Surg (Am) 1994; 76(7)1052–6
- Ritter M A, Albohm M J, Keating E M, Faris P M, Meding J B. Comparative outcomes of total joint arthroplasty. J Arthroplasty 1995; 10(6)737–41
- Rock M. Oncogenesis. Handbook of Biomaterial Properties, J Black, G Hastings. Chapmann & Hall, London. 1998: 529–539
- Roe R J, Grood E S, Shastri R, Gosselin C A, Noyes F R. Effect of radiation sterilization and aging on ultrahigh molecular weight polyethylene. J Biomed Mater Res 1981; 15(2)209–30
- Rokkum M, Reigstad A. Polyethylene wear with an entirely HA-coated total hip replacement. Acta Orthop Scand 1998; 69: 253–258
- Rokkum M, Reigstad A, Johansson C B. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2-8 years after implantation. Acta Orthop Scand 2002; 73(3)298–306
- Rooker G D, Wilkinson J D. Metal sensitivity in patients undergoing hip replacement. A prospective study. J Bone joint Surg (Br) 1980; 62: 502–505
- Rose R M, Nusbaum H J, Schneider H, Ries M, Paul I, Crugnola A, Simon S R, Radin E L. On the true wear rate of ultra high -molecular-weight polyelhylene in the total hip prosthesis. J Bone Joint Surg (Am) 1980; 62: 537–549
- Rose R M, Cimino W R, Ellis E, Crugnola A N. Exploratory investigations on the structure dependence of the wear resistance of polyethylene. Wear 1982; 77: 89–104
- Rothman R H, Sharkey P, Hozack W, Martell J. Wear performance of conventional (ARCOM) and highly crosslinked acetabular liners in bilateral total hip arthroplasty. J Arthroplasty 2004; 19(2)261
- Russe W. Roentgen photogrammetry of the artificial hip joint acetabulum. Aktuelle Probl Chir Orthop 1988; 32: 1–80
- Sabokbar A, Pandey R, Quinn J M, Athanasou N A. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch Orthop Trauma Surg 1998; 117(3)136–40
- Saha S, Pal S. Mechanical properties of bone cement: a review. J Biomed Mater Res 1984; 18(4)435–62
- Saikko V, Nevalainen J, Revitzer H, Ylinen P. Metal release from total hip articulations in vitro: substantial from CoCr/CoCr, negligible from CoCr/PE and alumina/PE. Acta Orthop Scand 1998; 69(5)449–54
- Santavirta S S, Lappalainen R, Pekko P, Anttila A, Konttinen Y T. The counterface, surface smoothness, tolerances, and coatings in total joint prostheses. Clin Orthop 1999; 369: 92–102
- Santavirta S. Compatibility of the totally replaced hip. Reduction of wear by amorphous diamond coating. Acta Orthop Scand Suppl 2003; 74(S310)1–19
- Sauer W L, Anthony M E. Predicting the clinical wear performance of orthopaedic bearing surfaces. Alternative Bearing Surfaces in Totat Joint Replacement, J J Jacobs, T L Craig. ASTM, West Conshohocken, PA 1998; 1–29
- Schaffer A W, Pilger A, Engelhardt C, Zweymueller K, Ruediger H W. Increased blood cobalt and chromium after total hip replacement. J Toxicol Clin Toxicol 1999; 37(7)839–44
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg (Am) 1992a; 74(6)849–63
- Schmalzried T P, Kwong L M, Jasty M, Sedlacek R C, Haire T C, O'Connor D O, Bragdon C R, Kabo J M, Malcolm A J, Harris W H. The mechanism of loosening of cemented acetabular components in total hip arthroplasty. Analysis of specimens retrieved at autopsy. Clin Orthop 1992b; 274: 60–78
- Schmalzried T P, Szuszczewicz E S, Akizuki K H, Petersen T D, Amstutz H C. Factors correlating with long term survival of McKee-Farrar total hip prostheses. Clin Orthop 1996a; 329(Suppl)S48–59
- Schmalzried T P, Fowble V A, Ure K J, Amstutz H C. Metal on metal surface replacement of the hip. Technique, fixation, and early results. Clin Orthop 1996b; 329(Suppl)S106–14
- Schmalzried T P, Scott D L, Zahiri C A, Dorey F J, Sanford W M, Kem L, Humhrey W. Variables affecting wear in vivo: analysis of 1080 hips with computer-assisted technique. 24th Trans Soc Biomater 1998a; 21: 216
- Schmalzried T P, Dorey F J, McKellop H. The multitactorial nature of polyethylene wear in vivo. J Bone Joint Surg (Am) 1998b; 80(8)1234–1242
- Schmalzried T P, Callaghan J J. Current Concepts Review-Wear in total hip and knee replacements. J Bone Joint Surg (Am) 1999; 81(1)115–136
- Schmidt M B, Hamilton J V. The effects of calcium stearate on the properties of UHMWPE. 42nd Trans Orthop Res Soc 1996a; 21: 22
- Schmidt M, Weber H, Schon R. Cobalt chromium molybdenum metal combination for modular hip prostheses. Clin Orthop 1996; 329(Suppl)S35–47
- Schnebel E. Reichsverb. Deutscher Dentisten. German Patent 1940; 760351
- Schneider P M. Low temperature sterilization alternatives in the 1990's. Tappi J 1994; 77: 115–119
- Scott M, Widding K, Ries M, Shanbhag A. Wear particles analyses of conventional and crosslinked UHMWPE tested in an anatomic hip simulator. 47th Trans Orthop Res Soc 2001; 1
- Sedel L, Nizard R S, Kerboull L, Witvoet J. Alumina-alu-mina hip replacement in patients younger than 50 years old. Clin Orthop 1994; 298: 175–83
- Sedel L. Evolution of alumina-on-alumina implants: a review. Clin Orthop 2000; 379: 48–54
- Selvik G. Roentgen Stereophotogrammetry: A Method for the Study of the Kinematics of the Skeletal System. University of Lund, LundSweden 1974; 232: 1–51, Thesis, Reprinted in: Acta Orthop Scand 60: Suppl 1989
- Semlitsch M, Willert H G. Clinical wear behaviour of ultrahigh molecular weight polyethylene cups paired with metal and ceramic ball heads in comparison to metal-on-metal pairings of hip joint replacements. Proc Inst Mech Eng [H] 1997; 211(1)73–88
- Shanbhag A S, Jacobs J J, Black J, Galante J O, Glant T T. Macrophage/particle interactions: effect of size, composition and surface area. J Biomed Mater Res 1994; 28(1)81–90
- Shanbhag A S, Jacobs J J, Black J, Galante J O, Glant T T. Human monocyte response to particulate biomaterials generated in vivo and in vitro. J Orthop Res 1995; 13(5)792–801
- Shanbhag A S, Jacobs J J, Black J, Galante J O, Glant T T. Effects of particles on fibroblast proliferation and bone resorption in vitro. Clin Orthop 1997; 342: 205–17
- Shea K G, Lundeen G A, Bloebaum R D, Bachus K N, Zou L. Lymphoreticular dissemination of metal particles after primary joint replacements. Clin Orthop 1997; 338(219)26
- Shen C, Dumbleton J H. The friction and wear behaviour of irradiated very high molecular weight polyethylene. Wear 1974; 30: 349–364
- Simon J A, Dayan A J, Ergas E, Stuchin S A, Di Cesare P E. Catastrophic failure of the acetabular component in a ceramic-polyethylene bearing total hip arthroplasty. J Arthroplasty 1998; 13(1)108–13
- Skinner J A, Kroon P O, Todo S, Scott G. A femoral component with proximal HA coating. An analysis of survival and fixation at up to ten years. J Bone Joint Surg (Br) 2003; 85(3)366–70
- Snorrason F, Karrholm J, Holmgren C. Fixation of cemented acetabular prostheses. The influence of preoperative diagnosis. J Arthroplasty 1993; 8: 83–90
- Soballe K. ydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand Suppl 1993a; 255: 1–58
- Soballe K, Hansen E S, Brockstedt-Rasmussen H, Bunger C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg (Br) 1993b; 75(2)270–8
- Sochart D H. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop 1999; 363: 135–50
- St John K R, Zardiackas L D, Poggie R A. Wear evaluation of cobalt-chromium alloy for use in a metal-on-metal hip prosthesis. J Biomed Mater Res 2004; 68B(1)1–14
- Streicher R M. Influence of ionizing irradiation in air and nitrogen for sterilizarion of surgical grade polyethylene for implants. Radiat Phys Clem 1988; 31: 693–698
- Strömberg C. Thesis: Cemented revision total hip replacement. Dept. of Orthopaedic Surgery. Inst. of Surgical Sciences Göteborg. University of Göteborg. 1995
- Sturup J, Nimb L, Kramhoft M, Jensen J S. Effects of polymerization heat and monomers from acrylic cement on canine bone. Acta Orthop Scand 1994; 65(1)20–3
- Sudmann E, Havelin L I, Lunde O D, Rait M. The Charnley versus the Christiansen total hip arthroplasty. A comparative clinical study. Acta Orthop Scand 1983; 54(4)545–52
- Sun D C, Schmidig G, Stark C, Dumbleton J H. Free radicals in irradiated UHMWPE are “Visible”. Proceedings of the Society for Biomaterials 24th Annual Meering. San Diego, CA 1998
- Sun Y, Sturmer T, Gunther K P, Brenner H. Reliability and validity of clinical outcome measurements of osteoarthritis of the hip and knee—a review of the literature. Clin Rheumatol 1997; 16(2)185–98
- Sundfeldt M, Widmark M, Wennerberg A, Kärrholm J, Johansson C B, Carlsson L V. Does sodium fluoride in bone cement affect implant fixation - Part I: Bone tissue response, implant fixation and histology in nine rabbits. Journal of Materials Science: Materials in Medicine 2002a; 13: 1037–1043
- Sundfeldt M, Persson J, Swanpalmer J, Wennerberg A, Kärrholm J, Johansson C A, Carlsson L V. Does sodium fluoride in bone cement affect implant fixation - Part II: Evaluation of the effect of sodium fluoride additions to acrylic bone cement and the fixation of titanium implants in ovariectomized rabbits. Journal of Materials Science: Materials in Medicine 2002b; 13: 1045–1050
- Sutherland C J, Wilde A H, Borden L S, Marks K E. A ten-year follow-up of one hundred consecutive Muller curvedstem total hip-replacement arthroplasties. J Bone Joint Surg (Am) 1982; 64(7)970–82
- Sutula L C, Collier J P, Saum K A, Currier B H, Currier J H, Sanford W M, Mayor M B, Wooding R E, Sperling D K, Williams I R, Kasprzak D J, Surprenant B A. The Otto Aufranc Award. Impact of gamma sterilization on clinical performance of polyethylene in the hip. Clin Orthop 1995; 319: 28–40
- Swanpalmer J, Kullenberg R, Hansson T. The feasibility of triple-energy absorptiometry for the determination of bone mineral, Ca and P in vivo. Physiol Meas 1998a; 19(1)1–15
- Swanpalmer J, Kullenberg R, Hansson T. Measurement of bone mineral using multiple energy x-ray absorptiometry. Phys Med Biol 1998b; 43(2)379–87
- Sychterz C J, Shah N, Engh C A. Examination of wear in Duraloc acetabular components: two- to five-year evaluation of Hylamer and Enduron liners. J Arthroplasty 1998; 13(5)508–14
- Sychterz C J, Yang A M, McAuley J P, Engh C A. Two-dimen-sional versus three-dimensional radiographic measurements of polyethylene wear. Clin Orthop 1999; 365: 117–23
- Söderkvist I, Wedin P A. Determining the movements of the skeleton using well-configured markers. J Biomech 1993; 26: 1473–1477
- Thanner J, Freij-Larsson C, Karrholm J, Malchau H, Wesslen B. Evaluation of Boneloc. Chemical and mechanical properties, and a randomized clinical study of 30 total hip arthroplasties. Acta Orthop Scand 1995; 66(3)207–14
- Thanner J, Karrholm J, Herberts P, Malchau H. Porous cups with and without hydroxylapatite-tricalcium phosphate coating: 23 matched pairs evaluated with radiostereometry. J Arthroplasty 1999; 14(3)266–71
- Thanner J, Karrholm J, Herberts P, Malchau H. Hydroxyapatite and tricalciumphosphate-coated cups with and without screw fixation: a randomized study of 64 hips. J Arthroplasty 2000; 15: 405–12
- Thoren B, Sahlstedt B. Influence of pelvic position on radiographic measurements of the prosthetic acetabular component. An experimental study on a pelvic model. Acta Radiol 1990; 31(2)133–6
- Toksvig-Larsen S, Franzen H, Ryd L. Cement interface temperature in hip arthroplasty. Acta Orthop Scand 1991; 62(2)102–5
- Toni A, Terzi S, Sudanese A, Tabarroni M, Zappoli F A, Stea S, Giunti A. The use of ceramic in prosthetic hip surgery. The state of the art. Chir Organi Mov 1995; 80(2)125–37
- Tonino A J, Therin M, Doyle C. Hydroxyapatite-coated femoral stems. Histology and histomorphometry around five components retrieved at post mortem. J Bone Joint Surg (Br) 1999; 81(1)148–54
- Topoleski L D, Ducheyne P, Cuckler J M. Microstructural pathway of fracture in oly(methyl methacrylate) bone cement. Biomaterials 1993; 14(15)1165–72
- Ueno M, Amino H, Okimatu H, Oonishi H. Wear, friction, and mechanical investigation and development ofalu-mina-to-alumina combination total hip joint. Joint Arthroplasty, S Imura, M Wada, H Omori. Springer, TokyoJapan 1999; 119–131
- Urban R M, Jacobs J J, Tomlinson M J, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am 2000; 82(4)457–76
- Urban J A, Garvin K L, Boese C K, Bryson L, Pedersen D R, Callaghan J J, Miller R K. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results. J Bone Joint Surg (Am) 2001; 83(11)1688–94
- Valstar E R, Spoor C W, Nelissen R G, Rozing P M. Roentgen stereophotogrammetric analysis of metal-backed hemispherical cups without attached markers. J Orthop Res 1997; 15(6)869–73
- Valstar E R, de Jong F W, Vrooman H A, Rozing P M, Reiber J H. Model-based Roentgen stereophotogrammetry of orthopaedic implants. J Biomech 2001; 34(6)715–22
- Van Der Vis H M, Marti R K, Tigchelaar W, Schuller H M, Van Noorden C J. Benign cellular responses in rats to different wear particles in intra-articular and intramedullary environments. J Bone Joint Surg (Br) 1997; 79(5)837–43
- Verdonschot N, Huiskes R. Acrylic cement creeps but does not allow much subsidence of femoral stems. J Bone Joint Surg (Br) 197l; 79(4)665–9
- Versieck J, Cornelis R. Trace Elements in Human Plasma or Serum. CRC Press, Boca Raton 1989
- Vose G P. Factors affecting the precision of radiographic densitometry of the lumbar spine and femoral neck. Progress m development of methods in bone densitometry, G D Whedon, W F Neuman, D W Jenkins. NASA, Washington, DC 1966; 47–63
- Vrooman H A, Valstar E R, Brand G J, Admiraal D R, Rozing P M, Reiber J H. Fast and accurate automated measurements in digitized stereophotogrammetric radiographs. J Biomech 1998; 31(5)491–8
- Walker P S, Mai S F, Cobb A G, Bentley G, Hua J. Prediction of clinical outcome of THR from migration measurements on standard radiographs. A study of cemented Charnley and Stanmore femoral stems. J Bone Joint Surg (Br) 1995; 77(5)705–14
- Walter A. On the material and the tribology of alumina-alumina couplings for hip joint prostheses. Clin Orthop 1992; 282: 31–46
- Wang A, Polineni V K, Stark C, Dumbleton J H. Effect of femoral head surface roughness on the wear of ultrahigh molecular weight polyethylene acetabular cups. J Arthroplasty 1998; 13(6)615–20
- Warashina H, Sakano S, Kitamura S, Yamauchi K I, Yamaguchi J, Ishiguro N, Hasegawa Y. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials 2003; 24(21)3655–61
- Waugh W. John Chamley, the Man and the Hip. Springer, New York 1990
- Weber B G. Experience with the Metasul total hip bearing system. Clin Orthop 1996; 329(Suppl)S69–77
- Weightman B, Freeman M A, Revell P A, Braden M, Albrektsson B E, Carlson L V. The mechanical properties of cement and loosening of the femoral component of hip replacements. J Bone Joint Surg (Br) 1987; 69(4)558–64
- Weightman B. The stress in total hip prosthesis femoral stems: a comparative experimental study in Advances in Artificial Hip and Knee Joint Technology. D Springer-Verlag, a cura di M. Schaldach, Hohmann, Berlin, Heidelberg, New York 1990; 138–147
- Wetherell R G, Amis A A, Heatley F W. Measurement of acetabular erosion. The effect of pelvic rotation on common landmarks. J Bone Joint Surg (Br) 1989; 71(3)447–51
- Wiklund I, Romanus B. Nottingham health Profile. Livskvalitetsbedömning hjälp vid operationsprioritering. Läkartidningen 1988; 85(38)3060–1
- Wiles P. The surgery of the osteoarthritic hip. (Br) J Surg 1957; 45: 488–97
- Wilkinson J M, Peel N F, Elson R A, Stockley I, Eastell R. Measuring bone mineral density of the pelvis and proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 2001; 83(2)283–8
- Wilkinson J M, Hamer A J, Elson R A, Stockley I, Eastell R. Precision of EBRA-Digital software for monitoring implant migration after total hip arthroplasty. J Arthroplasty 2002; 17(7)910–6
- Wilkinson J M, Gordon A, Stockley I. Experiences with the Plasmacup—early stability, wear, remodelling, and outcome. Int Orthop 2003; 27(Suppl 1)S16–9
- Willert H G, Buchhorn G H, Gobel D, Koster G, Schaffner S, Schenk R, Semlitsch M. Wear behavior and histopathology of classic cemented metal on metal hip endoprostheses. Clin Orthop 1996; 329(Suppl)S160–86
- Willert H G, Ludwig J, Semlitsch M. Reaction of bone to methacrylate after hip rthroplasty: a long-term gross, light microscopic, and scanning electron microscopic study. J Bone Joint Surg (Am) 1974; 56(7)1368–82
- Willmann G. Ceramics for total hip replacement: What a surgeon should know. Orthopedics 1998; 21: 173–177
- Willmann G. New generaration ceramics. Bioceramics in Hip Joint Replacement. Proceedings of the 5th International Ceram Tec Symposium, G Willmann, K Zweymuller. Georg Thieme, StuttgartGemany 2000; 127–135
- Winter M, Griss P, Scheller G, Moser T. Ten- to 14-year results of a ceramic hip prosthesis. Clin Orthop 1992; 282: 73–80
- Wirganowicz P Z, Thomas B J. Massive osteolysis after ceramic on ceramic total hip arthroplasty. A case report. Clin Orthop 1997; 338: 100–4
- Woods J S. Hematopoietic system. Metal Toxicology, R A Goyer, C D Klaassen, M P Waalkes. Academic Press, San Diego 1995
- Wooley P H, Petersen S, Song Z, Nasser S. Cellular immune responses to orthopaedic implant materials following cemented total joint replacement. J Orthop Res 1997; 15: 874–880
- Wright T M, Bartel D L. The problem of surface damage in polyethylene total knee components. Clin Orthop 1986; 205: 67–74
- Wright T M, Astion D J, Bansal M, Rimnac C M, Green T, Insall J N, Robinson R P. Failure of carbon fiber-reinforced polyethylene total knee-replacement components. J Bone Joint Surg (Am) 1988a; 70(6)926–32, A report of two cases
- Wright T M, Rimnac C M, Faris P M, Bansal M. Analysis of surface damage in retrieved carbon fiber-reinforced and plain polyethylene tibial components from posterior stabilized total knee replacements. J Bone Joint Surg (Am) 1988b; 70(9)1312–9
- Wroblewski B M, Taylor G W, Siney P. Charnley low-friction arthroplasty: 19- to 25-year results. Orthopedics 1992; 15(4)421–4
- Wroblewski B M, Siney P D, Dowson D, Collins S N. Prospective clinical and joint simulator studies of a new total hip arthroplasty using alumina ceramic heads and crosslinked polyetethylene cups. J Bone Joint Surg (Br) 1996; 78: 280–285
- Wroblewski B M. Wear of the high-density polyethylene socket in total hip arthroplasty and its role in endosteal cavitation. Proc Inst Mech Eng [H] 1997; 211(1)109–18
- Wroblewski B M, Siney P D, Fleming P A. Low-friction arthroplasty of the hip using alumina ceramic and crosslinked polyethylene. A ten-year follow-up report. J Bone Joint Surg (Br) 1999; 81(1)54–5
- Wroblewski B M, Siney P D, Fleming P A. Wear of enhanced ultra-high molecular-weight polyethylene (Hylamer) in combination with a 22.225 mm diameter zirconia femoral head. J Bone Joint Surg (Br) 2003; 85(3)376–9
- Wykman A G. Acetabular cement temperature in arthroplasty. Effect of water cooling in 19 cases. Acta Orthop Scand 1992; 63(5)543–4
- Yamaguchi M, Hashimoto Y, Akisue T, Bauer T W. Polyethylene wear vector in vivo: A three-dimensional analysis using retrieved acetabular components and radiographs. J Orthop Res 1999; 17: 695–702
- Yeom B, Yu Y J, McKellop H A, Salovey R. Profile of oxidation in irradiated polyethylene. J. Polymer Sci 1998; 36: 329–339, Part A
- Yetkinler D N, Litsky A S. Viscoelastic behaviour of acrylic bone cements. Biomaterials 1998; 19(17)1551–9
- Yoon T R, Rowe S M, Jung S T, Seon K J, Maloney W J. Osteolysis in association with a total hip arthroplasty with ceramic bearing surfaces. J Bone Joint Surg (Am) 1998; 80(10)1459–68
- Zysk S P, Gebhard H H, Pellengahr C, Refior H J, Plitz W, Messmer K, Veihelmann A. inflammatory responses to wear particles in vivo: a novel model in the murine knee joint. Orthopade 2003; 32(4)305–11
- Önsten I, Bengner U, Besjakov J. Socket migration after Charnley arthroplasty in rheumatoid arthritis and osteoarthritis. A roentgen stereophotogrammetric study. J Bone Joint Surg (Br) 1993; 75(5)677–80
- Önsten I, Akesson K, Besjakov J, Obrant K J. Migration of the Charnley stem in rheumatoid arthritis and osteoarthritis. A roentgen stereophotogrammetric study. J Bone Joint Surg (Br) 1995; 77(1)18–22
- Önsten I, Carlsson A S, Sanzen L, Besjakov J. Migration and wear of a hydroxyapatite coated hip prosthesis. A controlled roentgen stereophotogrammetric study. J Bone Joint Surg (Br) 1996; 78(1)85–91
- Önsten I, Carlsson A S, Besjakov J. Wear in uncemented porous and cemented polyethylene sockets: a randomised, radiostereometric study. J Bone Joint Surg (Br) 1998; 80(2)345–50
- Önsten I, Berzins A, Shott S, Sumner D R. Accuracy and precision of radiostereometric analysis in the measurement of THR femoral component translations: human and canine in vitro models. J Orthop Res 2001; 19(6)1162–7
APPENDIX
Questionnarie used in study I, II and III
Hur många timmar per vecka promenerar du eller spelar golf etc?
□inga □mindre än 5 □5-10 □10 – 20 □mer än 20
Hur många timmar per vecka gör du lättare kroppsarbete som att jobba i trädgård eller liknande?
□inga □mindre än 1 □1-3 □3 – 7 □mer än 7
Hur många timmar per vecka cyklar eller simmar du?
□inga □mindre än 1 □1-3 □3 – 7 □mer än 7
Hur många timmar joggar du eller dansar/gympar/per vecka?
□inga □mindre än 1 □1-3 □3 – 7 □mer än 7
Hur många timmar per vecka tränar du tennis/badminton eller liknande?
□aldrig □mindre än 1 □1 – 3 □mer än 3
Utövar du tungt arbete som skogsarbete, tunga lyft eller liknande?
□sällan eller aldrig □1–2 ggr/månad □1–2 ggr/vecka □mer än 2 ggr/vecka
Utövar du ibland extra ansträngande aktiviteter någon vecka som fjällvandring, slalom eller liknande?
□aldrig □1 vecka/år □2-3 veckor/år □mer än 4 veckor/år
Använder du käpp? □aldrig □1 käpp □2 käppar/rollator
Går du mycket i trappor? □aldrig □normalt □ofta
Hur uppskattar du själv din aktivitetsnivå?
□mycket låg □låg □normal □hög □mycket hög
Table 1. Repeatability of the questionnaire in 20 patients
Table 2. Study IV: The maximum width (mm) of radiolucent lines in various Gruen regions postoperatively and at 2 years. Values are number, mean ± SE
Table 3. Study IV: Distribution of radiolucent lines (0 / 1–49 / 50–99 / 100%) in the various Gruen regions postoperative and at two years
Table 4. Study V: Maximum width of radiolucent lines in each region (mean ± SE)
Table 5. Study V: Distribution of radiolucent lines (0 / 1–49 / 50–99 / 100%) related to region (1-6).