Abstract
The development of nontoxic, clean techniques for synthesising metal nanoparticles such as gold has attracted increasing attention in recent years. Many reports have been published about the synthesis of gold nanoparticles using plant extracts. However, the stability of these prepared gold nanoparticles has not been investigated. In this research, the stability of gold nanoparticles prepared by Eucalyptus camaldulensis was investigated at different temperatures (4°C, 25°C and 45°C) for 8 weeks. Transmission electron microscopy and visible absorption spectroscopy confirmed the stability of gold nanoparticles during the storage period at the mentioned condition. In addition, Fourier transform–infrared spectroscopy was used to investigate the surface chemistry of gold nanoparticles prepared by the methanol extract of E. camaldulsis. The carboxyl group was characterised on the surface of the gold nanoparticles, and this functional group may have a critical role in the stability of gold nanoparticles prepared by the mentioned plant extract at different conditions. This functional group can be used for drug delivery of amino derivative drugs using gold nanoparticles.
1. Introduction
The development of green processes for synthesising nanoparticles is evolving into an important branch of nanotechnology Citation1,Citation2. Today, nano metal particles, especially gold, have drawn the attention of scientists because of the extensive application of these particles in the development of new technologies in the areas of chemistry, electronics, medicine and biotechnology at the nanoscale Citation2–5.
Gold nanoparticles could also have many new applications in biology; for example, these nanoparticles are used for the development of biosensors and DNA labelling Citation6,Citation7. In medicine, gold nanoparticles are used for different purposes. For example, after cellular uptake, nanoparticles can act as tiny, precise and powerful heaters (thermal scalpels) to destroy cancerous cells Citation8,Citation9 and are capable of inducing apoptosis in B-chronic lymphocytic leukemia Citation10. Many reports have been published in the literature on the green synthesis of gold nanoparticles using several plant extracts such as Azadirachta indica leaves (neem) and Eucalyptus camaldulensis. Although the generated gold nanoparticles have been well characterised in previous reports Citation11,Citation12, the stability of these nanoparticles prepared using plant extracts has not been fully investigated. Moreover, few studies have been carried out to characterise the surface chemistry of gold nanoparticles synthesised by plant extracts Citation13–15.
The biogenesis of gold nanoparticles for different purposes is going to be an issue of considerable importance; thus, further studies should be carried out to characterise the stability of this nanoparticle and its surface chemistry. In this study, the methanol extract of E. camaldulensis was used to synthesise gold nanoparticles by reducing aqueous . The surface chemistry of the gold nanoparticles prepared using this extract was investigated by Fourier transform–infrared spectroscopy (FTIR). Moreover, the stability of the generated nanoparticles was studied at different temperatures (4°C, 25°C and 45°C) using transmission electron microscopy (TEM) and visible absorption spectroscopy. Throughout this study, we observed that gold nanoparticles prepared by E. camaldulensis methanol extract were stable even at the highest temperature tested (45°C), which suggested that this natural extract, for rapid synthesis of stable gold nanoparticles, could be used for additional applications. To the best of our knowledge, and based on a thorough literature survey, this is the first study on the stability of gold nanoparticles using the total extract of a plant material at different temperatures.
2. Experimental work
2.1. Synthesis and study of surface chemistry of gold nanoparticles
The E. camaldulensis leaves were air-dried at room temperature and then pulverised (50 g). The methanol extract was prepared by macerating the powder (50 g) for 48 h with three changes of the solvent (500 mL) at room temperature. The combined solvent extracts were evaporated to yield a brownish or greenish viscous residue. A stock solution (10 mg mL−1) of the plant extract was prepared in methanol for further experiments and reserved in the refrigerator. Synthesis of gold nanoparticles was carried out using the previously reported method Citation12. Aqueous chloroauric acid solution (100 mL, 10−3 M) was separately added to the reaction vessels containing 10 mL of the E. camaldulensis methanol extract (10 mg mL−1), and the resulting mixture was allowed to stand for 15 min at room temperature Citation12.
Chloroauric acid was purchased from Merck, Germany. Two aliquots of the prepared gold colloids (10 mL) were centrifuged for 45 min at 14000 rpm and, subsequently, washed with distilled water. The process of centrifugation and re-dispersion in distilled water was repeated three times to ensure better separation of free entities from metal nanoparticles. The washing procedure was repeated with acetone and chloroform, and the purified pellets were then dried at room temperature and subjected to FTIR (Nicolet 550). In a separate experiment, the purified pellet was re-dispersed in a methanol–acetic acid solution (9:1) and incubated for 24 h. Subsequently, the colloid was centrifuged for 45 min at 14000 rpm and washed three times with distilled water. After being centrifuged in the described condition, the residue was dried and studied with FTIR spectroscopy.
2.2. Stability test and characterisation of gold nanoparticles
The aliquots of the prepared colloids (100 mL) were incubated in sealed containers at different temperatures (4°C, 25°C and 45°C). The stability of the gold colloids prepared using the methanol extract of E. camaldulensis was monitored by sampling the aqueous component (2 mL) and measuring the UV-Vis spectrum of the solutions at different intervals (0, 1, 2, 3, 4, 5, 6, 7 and 8 weeks). All samples were diluted three times with distilled water. Digitised UV-Vis absorbency spectra were collected using a GBC Cintra 6 spectrophotometer, with 1-cm quartz cells and a scan rate of 1000 nm min−1. All spectral measurements were performed using blank solution as a reference. The digitised spectra with 1 nm increment were collected. Furthermore, the shapes and sizes of the gold nanoparticles at the start of experiment and after the storage period (8 weeks) at different temperatures were studied with TEM (model EM 208 Philips).
2.3. Statistical analysis
One-way repeated-measure analysis of variance (ANOVA) and one-way ANOVA were used to compare the average of maximum wavelength (λ max) in the spectra of the samples during the mentioned period of time and at different temperatures, respectively.
3. Results and discussion
3.1. Stability of gold nanoparticles at different temperatures
The chemical reduction of the aqueous solution of chloroauric acid (HAuCl4) is one of the most widely used methods for synthesising gold colloids. Different reducing agents and plant extracts have been reported to prepare gold colloids. However, the gold nanoparticles should have sufficient stability during the synthesis and storage periods for further application. Different chemicals such as nonionic or ionic surfactants have been reported as stabilisers of different metal colloids Citation16–18. These compounds cap the surface of the nanoparticles and prevent the nanoparticles from aggregating Citation19. No reports have been published on the stability of gold nanoparticles prepared by plant extracts. The stability of gold nanoparticles was studied at different temperatures for 2 months (8 weeks). All storage mixtures were characterised by UV-Vis spectroscopy. The technique outlined above proved to be very useful for the analysis of the nanoparticles Citation20–22. As illustrated in , a strong, broad absorption band with a maximum located at 533 nm was observed for the gold colloids prepared using the methanol extract of E. camaldulensis. This peak is assigned to a surface plasmon, a phenomenon that is well documented for various metal nanoparticles with sizes ranging from 2 to 100 nm Citation20–22. Aliquots of this colloid sample were reserved in sealed containers at different temperatures (4°C, 25°C and 45°C). The UV-Vis spectra of these samples were recorded during the incubation time at different intervals, and the maximum wavelengths (λ max) obtained are reported in . Moreover, shows the spectra of all the reserved samples after the final incubation period (8 weeks). Statistical analysis using repeated measure ANOVA showed that there were no differences between the average of the maximum wavelength (λ max) of the samples during 8 weeks (p > 0.05). In addition, further analysis of the data with one-way ANOVA showed that different temperatures (4°C, 25°C and 45°C) had no significant effect on the average of the maximum wavelength in the samples (p > 0.05). No aggregation in gold nanoparticles was observed for all colloids stored in sealed containers for 8 weeks at the mentioned temperatures.
Figure 1. UV-visible spectra of gold colloids: spectra recorded after the methanol extract of E. camaldulensis (10 mL) was added to 90 mL of the chloroauric acid solution (1 mM). The curves are recorded after periods of 15 min (a) and 8 weeks (b).
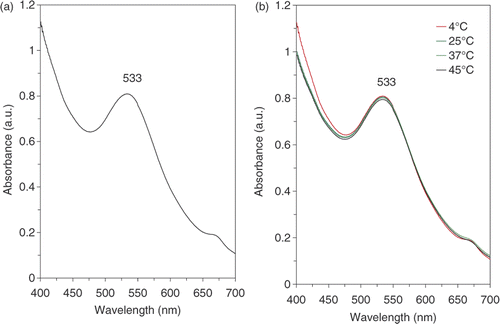
Table 1. The maximum wavelength (nm) of the colloid samples obtained during the stability test of the gold nanoparticles prepared by the methanol extract of E. camaldulensis.
3.2. Particle size and its chemical composition
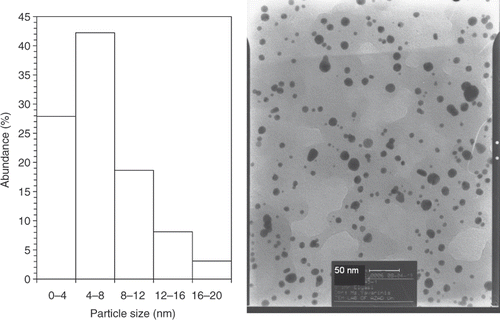
shows representative TEM images recorded from the drop-coated film of the gold nanoparticles synthesised by treating the chloroauric acid solution with plant extracts of E. camaldulensis. The particle size histogram of the as-prepared gold particles shows that the particles range in size from 1.25 to 17.5 nm, and possess an average size of 5.5 nm (left illustration in ). The colloids that incubated in harsh temperatures (4°C and 45°C) were further subjected to TEM analysis ( and ) at the end of the storage period (8 weeks), and we did not observe any particle agglomeration in the TEM micrographs (right images in and ) for the samples reserved at different temperatures (4°C, 25°C and 45°C). In addition, no significant changes in the particle size and shape of the nanoparticles were observed for the samples before and after incubation at the mentioned temperatures for 8 weeks (the left particle size histograms in and ). The gold nanoparticles were predominantly spherical and polydispersed with diameters in the range of 1.25 to 17.5 nm before and after the incubation period and different temperatures. The average size of the gold nanoparticles samples stored at 4°C and 45°C was about 6 nm.
Figure 2. TEMs recorded from a small region of a drop-coated film of chloroauric acid solution treated with the methanol extracts of E. camaldulensis (right picture) for 15 min (scale bars correspond to 50 nm). The related particle size distribution histograms (left picture) obtained after 350 individual particles were counted.
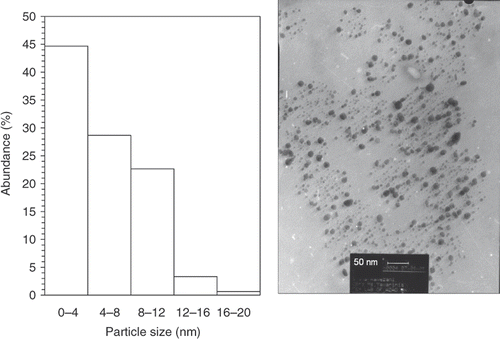
Figure 3. TEMs recorded from a small region of a drop-coated film of gold colloid prepared using methanol extracts of E. camaldulensis and reserved at 4°C (right picture) for 8 weeks (scale bars correspond to 50 nm). The related particle size distribution histograms (left picture) obtained after 350 individual particles were counted.
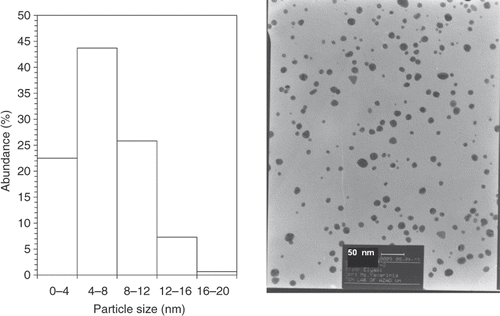
Figure 4. TEMs recorded from a small region of a drop-coated film of gold colloid prepared using the methanol extract of E. camaldulensis and reserved at 45°C (right picture) for 8 weeks (scale bars correspond to 50 nm). The related particle size distribution histograms (left picture) obtained after 350 individual particles were counted.
3.3. Surface chemistry of prepared gold nanoparticles
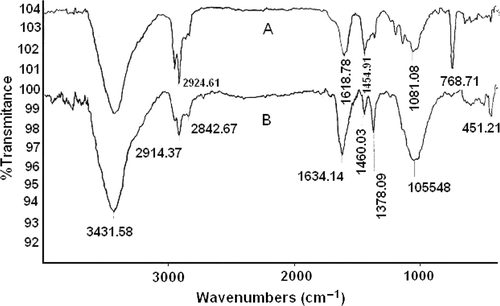
FTIR measurements were carried out to recognise the possible functional group(s) responsible for efficient stabilisation of gold nanoparticles prepared by the E. camaldulensis methanol extract. The as-prepared gold nanoparticles were centrifuged at 14000 rpm for 45 min; the pellet obtained was washed with aqueous and non-aqueous solvents to isolate the metal nanoparticles from free natural constituents or other compounds present in the gold colloids before the FTIR experiment. The gold nanoparticle sample shows peaks at 3426 (broad), 2950, 2924, 2858, 1618 (CO), 1460, 1374, 1230, 1040, 983 and 620 cm−1 (, spectrum A). The observed peaks are more characteristic of carboxylic acid (COO−) residues on the surface of gold nanoparticles prepared using E. camaldulensis methanol extract. In the second experiment, the gold nanoparticles were treated with diluted acetic acid, and the carboxylic residue (COOH) was shifted to 1634 cm−1(, spectrum B). The characterisation of nonionised and ionised carboxylic acid confirmed that the gold nanoparticles were functionalised by some acidic organic natural compounds. The presence of carboxylic acid adsorbed on the surface of the nanoparticles can be responsible for the good stability of gold nanoparticles prepared by the methanol extract of E. camaldulensis at different temperatures.
4. Conclusions
This study shows that the gold nanoparticles prepared using the methanol extract of E. camaldulensis have good stability even at the highest temperature tested (45°C), which suggests that this extract can be used for rapid synthesis of stable gold nanoparticles for additional applications. The carboxyl group was characterised on the surface of gold nanoparticles using FTIR analysis, and this functional group may have a critical role in the stability of gold nanoparticles prepared by a methanol extract. In addition, this surface and functional group (carboxyl residue) may be conjugated with the amino residues of different drugs for drug delivery in the future.
Acknowledgements
This study was financially supported by the Medicinal Plants Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences. The support of the Iranian Nanotechnology Initiative is also gratefully acknowledged. Furthermore, the authors appreciate Mr. Emadedin Haratifar's and Mr Mojtaba Shakibaie's for their excellent technical assistance.
References
- Raveendran , P , Fu , J and Wallen , SL . 2006 . A simple and ‘green’ method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles . Green Chem. , 8 : 34 – 38 .
- V. Armendariz, J.L. Gardea-Torresdey, M. Jose-Yacaman, J. Gonzalez, I. Herrera, and J.G. Parsons, Proceedings of the 2002 Conference on Application of Waste Remediation Technologies to Agricultural Contamination of Water Resources, Hazardous Substance Research Center, Kansas City, MO, USA, 2002
- Magudapathy , P , Gangopadhyay , P , Panigrahi , BK , Nair , KGM and Dhara , S . 2001 . Electrical transport studies of Ag nanocrystallites embedded in glass matrix . Physica B , 299 : 142 – 146 .
- Joerger , R , Klaus , T and Granqvist , CG . 2000 . Biologically produced silver-carbon composite materials for optically functional thin-film coatings . Adv. Mater. , 12 : 407 – 409 .
- Tanaka , K . 1999 . Nanotechnology towards the 21st century . Thin Solid Films, , 341 : 120 – 125 .
- Kohler , JM , Csaki , A , Reichert , J , Moller , R , Straube , W and Fritzsche , W . 2001 . Selective labeling of oligonucleotide monolayers by metallic nanobeads for fast optical readout of DNA-chips . Sens. Actuators B , 76 : 166 – 172 .
- Lazarides , AA , Kelly , KL , Jensen , TR and Schatz , GC . 2000 . Optical properties of metal nanoparticles and nanoparticle aggregates important in biosensors . J. Mol. Struct. , 529 : 59 – 63 .
- El-Sayed , IH , Huang , X and El-Sayed , MA . 2006 . Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles . Cancer Lett. , 239 : 129 – 135 .
- Salata , OV . 2004 . Applications of nanoparticles in biology and medicine . J. Nanobiotech. , 2 : 3 – 9 .
- Mukherjee , P , Bhattacharya , R , Bone , N , Lee , YK , Patra , CR , Wang , S , Lu , L , Secreto , C , Banerjee , PC , Yaszemski , MJ , Kay , NE and Mukhopadhyay , D . 2007 . Potential therapeutic application of gold nanoparticles in B-chronic lymphocytic leukemia (BCLL): Enhancing apoptosis . J. Nanobiotech. , 5 : 4 – 17 .
- Shankar , SS , Rai , A , Ahmad , A and Sastry , M . 2004 . Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachta indica) leaf broth . J. Colloid Interface Sci. , 275 : 496 – 502 .
- Ramezani , N , Ehsanfar , Z , Shamsa , F , Amin , GR , Shahverdi , HR , Monsef Esfahani , HR , Shamsaie , A , Dowlatabadi , R and Shahverdi , AR . 2008 . Screening of medicinal plant methanol extracts for the synthesis of gold nanoparticles by their reducing potential . Z. Naturforsch. , 63 : 903 – 908 .
- Badri Narayanam , K and Sakthivel , N . 2008 . Coriander leaf mediated biosynthesis of gold nanoparticles . Mater. Lett. , 62 : 4588 – 4590 .
- Huang , J , Li , Q , Sun , D , Lu , Y , Su , Y , Yang , X , Wang , H , Wang , Y , Shao , W , He , N , Hong , J and Chen , C . 2007 . Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf . Nanotech. , 18 : 105104 – 105115 .
- Shankar , SS , Rai , A , Ankamwar , B , Singh , A , Ahmad , A and Sastry , M . 2004 . Biological synthesis of triangular gold nanoprisms . Nat. Mater. , 3 : 482 – 488 .
- Lu , C , Zu , Y and Yam , VW . 2007 . Nonionic surfactant-capped gold nanoparticles as postcolumn reagents for high-performance liquid chromatography assay of low-molecular-mass biothiols . J. Chromatogr. A , 1163 : 328 – 332 .
- Mastalir , A , Rac , B , Kiraly , Z , Tasi , G and Molnar , A . 2008 . Preparation of monodispersed Pt nanoparticles in MCM-41, catalytic applications . Catal. Cammun. , 9 : 762 – 768 .
- Dorjnamjin , D , Ariunaa , M and Shim , YK . 2008 . Synthesis of silver nanoparticles using hydroxyl functionalized ionic liquids and their antimicrobial activity . Int. J. Mol. Sci. , 9 : 807 – 820 .
- Xu , J , Han , X , Liu , H and Hu , Y . 2005 . Synthesis of monodispersed gold nanoparticles stabilized by Gemini surfactant in reverse micelles . J. Dispersion Sci. Technol. , 26 : 473 – 476 .
- Singaravelua , G , Arockiamary , JS , Ganesh Kumarb , V and Govindaraju , K . 2007 . A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville . Colloids Surf. B , 57 : 97 – 101 .
- Henglein , A . 1993 . Physicochemical properties of small metal particles in solution: ‘Microelectrode’ reactions, chemisorption, composite metal particles, and the atom-to-metal transition . J. Phys. Chem. B , 97 : 5457 – 5471 .
- Sastry , M , Mayya , KS and Bandyopadhyay , K . 1997 . pH Dependent changes in the optical properties of carboxylic acid derivatized silver colloidal particles . Colloids Surf. A , 127 : 221 – 228 .