Abstract
A facile and efficient template method was developed for the fabrication of Ag and Ag2S nanoparticle chains. The morphologies and structures of products were characterised by X-ray powder diffraction and transmission electron microscopy. The result shows that the length of Ag and Ag2S nanoparticle chains are up to several micrometres. Systematic studies exhibit that the concentration of template (polyacrylamide) reagent, reaction solution and ageing time are important factors on the control synthesis of nanoparticle chains. The possible mechanism for the formation of Ag and Ag2S nanoparticle chains was also discussed. The physical and chemical properties of the Ag and Ag2S nanoparticle chains were investigated and their phytotoxicities were also researched for the first time. The phytotoxic result shows that the Ag and Ag2S nanoparticle chains have obvious inhibition of seed germination.
1. Introduction
It is well known that silver has extraordinary, inhibitory and bactericidal properties since ancient times. The broad-spectrum antibacterial properties of silver have been found in industrial fields as well as in the human bodies Citation1–3. At the same time, silver is relatively nontoxic to human cells. Because of these characteristics, silver having various morphologies is ideally suited for extensive applications in industrial and medical products, such as urinary catheters, suture rings, food packaging and the antimicrobial component Citation4–7. Ag2S semiconductor materials attracted attentions because of its promising optoelectronic and thermoelectric properties which are used in photovoltaic cells, photoconductors, infrared (IR) detectors, magnetic field sensors, hybrid films, chemical sensor, solid state electrochemical sensors, optical filters and superionic conductors Citation8–15. Recently, some preparing methods of Ag and Ag2S nanoparticle chains have been reported, such as freeze-drying Citation16, microwave-hydrothermal Citation17, solvent extraction–reduction Citation18, low-temperature synthesis Citation19, aqueous solutions, etc. Citation20,Citation21. In this article, we have developed a simple approach for the fabrication of one-dimensional Ag and Ag2S nanoparticle chains, at the same time, the phytotoxicities of the Ag and Ag2S nanoparticle chains were investigated for the first time. The phytotoxic result shows that the Ag and Ag2S nanoparticle chains have obvious inhibition of seed germination.
2. Experimental section
2.1. Preparation of Ag and Ag2S nanoparticle chains
All reagents are of analytical purity grade and are used without further purificaiton. Twenty-five miligrams of polyacrylamide was added into 25 mL 0.01 mol L−1 Na2S · 9H2O solution. Then, 25 mL 0.02 mol L−1 AgNO3 solution was added into the previously mentioned solution within 60 min under stirring. The obtained mixture was kept at room temperature for 2 h until the Ag2S nanoparticle chains were formed. The resulting products were separated in a centrifuge with 1500 rpm and followed by washing with deionised water and alcohol for three times. The final products were collected and dried in a desiccator.
A total of 0.015 g of polyacrylamide was added into 15 mL 0.01 mol L−1 AgNO3 aqueous solution. The reaction system was adjusted to a pH of 10 by adding ethanediamine, then the system was reduced by 15 mL hydrazine hydrate (N2H4 · H2O, 30 wt.%) solution during 30 min under continuous stirring. The mixture was kept at room temperature for 2 h and the silver nanoparticle chains were formed. The products were separated in a centrifuge with 1500 rpm and followed by washing with deionised water and alcohol for three times. The final products were collected and dried in atmosphere.
The morphologies of the products were observed through transmission electron microscopy (TEM, Hitachi-800). The crystal phase and structure of the products were determined by X-ray powder diffraction (XRD) with Shimadzu XD-3A diffractometer with graphite monochromatised Cu-Ka radiation (50 kV, 100 mA). The optical properties of the Ag and Ag2S nanoparticle chains in alcohol solution were studied through UV-Vis spectroscopy (Agilent 8453).
2.2. Phytotoxicities of Ag and Ag2S nanoparticle chains
The mung beans of about 4 mm in diameter without any biological contamination were used as phytotoxicities subject of Ag and Ag2S nanoparticle chains. The mung beans were randomly divided into three groups of 20 mung beans each. There were 10 mL of 0.01 mol L−1 Ag nanoparticle chains in group A, 10 mL of 0.01 mol L−1 Ag2S in group B and 10 mL of tap water in group C. These three groups were kept at room temperature. The aforesaid experiments were repeated five times in order to obtain the average results.
3. Results and discussions
3.1. Morphologies and structures
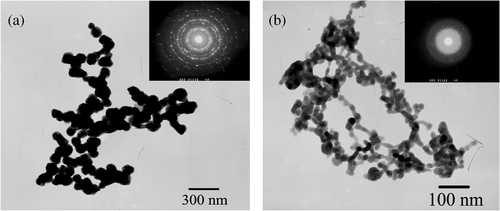
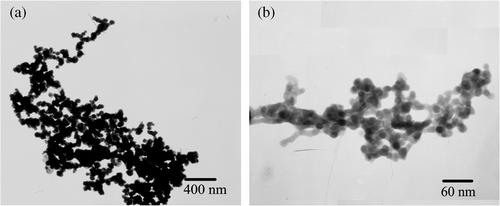
The TEM images of the Ag products are shown in . It can be clearly observed that the Ag products form parallel aligned nanoparticle chains and the sizes of nanoparticles are uniform with a diameter about 42 nm. These nanoparticles are in close contact with each other to form nanoparticle chains with a length of several micrometres (up to 3 µm). The results show that the Ag nanoparticle chains could be prepared through the facile template method. The selected region diffraction (ED) demonstrates that the nanoparticle chains are polycrystalline.
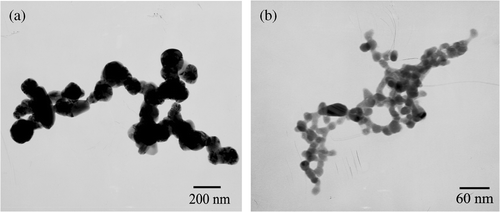
The TEM images of the Ag2S products are shown in . It clearly displays that the Ag2S products form parallel aligned nanoparticle chains and the nanoparticles have uniform sizes with a diameter of about 14 nm. These nanoparticles are closely linked to each other to form nanoparticle chains, which is several micrometres (up to 1.2 µm) in length. The ED indicates that the nanoparticle chains are also polycrystalline.
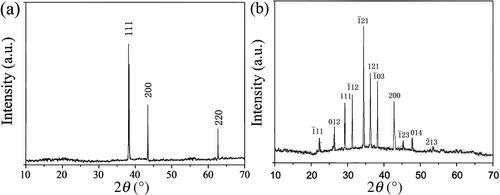
The XRD pattern for the Ag nanoparticle chains is shown in . All of the peaks match well with Bragg reflections of the standard face-centred cubic structure (JCPDS no. 65-2871). The XRD pattern of the Ag2S nanoparticle chains is shown in . All of the peaks agree well with Bragg reflections of the standard face-centred cubic structure (JCPDS no. 14-0072). The four strong peaks at 31.5°, 34.4°, 37.7° and 43.4° can be assigned to their characteristic ,
,
and
indices. Relatively broadened diffraction peaks indicate that the Ag2S crystals of nanoparticle chains are small in size.
3.2. Optical properties
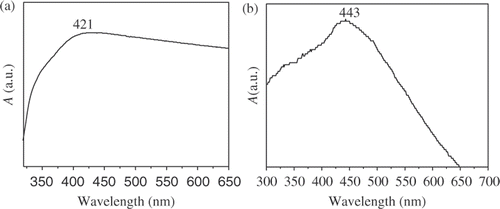
The sample for UV-Vis absorption spectra was dispersed in anhydrous ethanol at room temperature. shows the UV-Vis absorption spectrum of the Ag nanoparticle chains. The UV-Vis absorption spectrum shows that the maximum absorption peak of nanoparticle chains is located at 421 nm. The absorption peaks have obvious blueshift compared with that of Ag bulk material Citation22,Citation23. The UV-Vis absorption spectrum of Ag2S nanoparticle chains () shows obvious absorption at 443 nm Citation24–26.
The products were formed through a self-assembly process, which were under the control of the template Citation27–30. The two reacting ions reacted to form the Ag or Ag2S nucleus, and then the nucleus grew under the control of the template to form of the Ag or Ag2S nanoparticle chains.
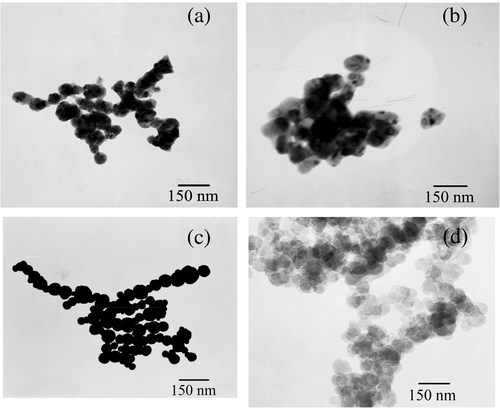
When the ammonia replaced the ethylenediamine to adjust the pH to 10 in the presence of polyacrylamide, the structure of Ag crystals were sphere clusters with about 100 nm in diameter (). The results indicate that the ethylenediamine can assist polyacrylamide template to control the formation and growth of the silver nucleus. The suitable dripping race is 0.4–0.6 mL min−1. If the race is too quick (such as 4 mL min−1, ), the further growth is limited and nanometre scale products were obtained; however, the formed Ag crystal nucleus cannot be dispersed well, so the nanoparticle will be aggregated. In addition, the concentration of reaction solution is an important factor of controlling the synthesis of nanoparticle chains. When the concentration of Ag+ is lower (such as 0.001 mol L−1), nanoparticle chains with uniform morphologies can be obtained (), however, the yield is too low. If the concentration of Ag+ ions is higher, the products will aggregate easily (0.5 mol L−1 or more, ). We have done a series of experiments to look for the suitable conditions for the formation of silver nanoparticle chains. The results show that the suitable concentration of the Ag+ ions is 0.005–0.02 mol L−1, the suitable concentration of the polyacrylamide is 1.0–2.0 g L−1, and the aging time is 1–3 h. If the ageing time is too long, the aggregation of the products will appear ().
3.3. Phytotoxicity
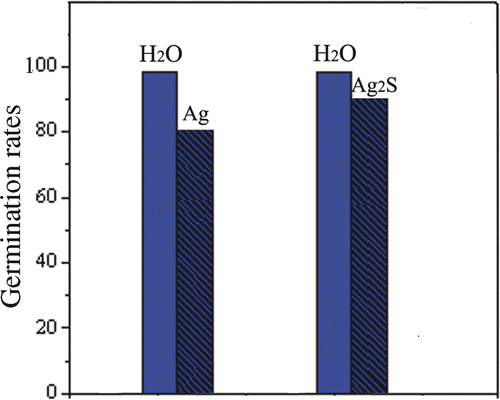
The Ag nanomaterials have been found to have intrinsic toxicities in a certain extent to generation of plants in the recent years. Therefore, the effects of Ag nanomaterials on plants have received considerable attention Citation31,Citation32. However, there are few reports in general about the toxicity of Ag nanomaterials on seed germination Citation33,Citation34. Especially, the phytotoxicities of the Ag and Ag2S nanoparticle chains have not been reported. In our research, the phytotoxicities of the Ag and Ag2S nanoparticle chains on seed germination were investigated for the first time. The images of Ag and Ag2S nanoparticle chains in phytotoxicity experiments are shown in . The formula for germination rates (GR) of the mung beans is GR = n g/n × 100 (n g, the number of seed germination and n the total number of experimental seeds). The results () demonstrate that the Ag and Ag2S nanoparticle chains all have phytotoxicities and the Ag nanoparticle chains are more phytotoxic than the Ag2S nanoparticle chains. This mechanism will be researched in the future.
4. Conclusions
In summary, the Ag and Ag2S nanoparticle chains were synthesised through polyacrylamide as a template. The products have good optical properties which allow them to have many potential applications in many fields such as nanobiosensor, catalyst materials, etc. The template method is facile and efficient, and could be used to synthesise other one-dimensional assembly nanomaterials. It is worth mentioning that the Ag and Ag2S nanoparticle chains have phytotoxicities. In this regard, we will carry out the further value study.
Acknowledgements
This study was supported by the State Key Laboratory of Pollution Control and Resource Reuse Foundation, China (no. PCRRF09005).
References
- Feng , QL , Wu , J , Chen , GQ , Cui , FZ , Kim , TN and Kim , JO . 2000 . A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus . J. Biomed. Mater. , 52 : 662 – 668 .
- Lee , D , Cohen , RE and Rubner , MF . 2005 . Antibacterial properties of Ag nanoparticle-loaded multilayers and formation of magnetically directed antibacterial microparticles . Langmuir , 21 : 9651 – 9659 .
- Costerton , JW , Stewart , PS and Greenberg , EP . 1999 . Bacterial biofilms: A common cause of persistent infections . Science , 284 : 1318 – 1322 .
- Panacek , A , Kvitek , L , Prucek , R , Kolar , M , Vecerova , R , Pizurova , N , Sharma , VK , Nevecna , T and Zboril , R . 2006 . Silver colloid nanoparticles: Synthesis, charaterization, and their antibacterial activity . J. Phys. Chem. B , 110 : 16248 – 16253 .
- Shi , Z , Neoh , KG and Kang , ET . 2004 . Surface-grafted viologen for precipitation of silver nanoparticles and their combined bactericidal activities . Langmuir , 20 : 6847 – 6852 .
- Balogh , L , Swanson , DR , Tomalia , DA , Hagnauer , GL and McManus , AT . 2001 . Dendrimer − silver complexes and nanocomposites as antimicrobial agents . Nano Lett. , 1 : 18 – 21 .
- Lok , CN , Ho , CM , Chen , R , He , QY , Yu , WY , Sun , H , Tam , PKH , Chiu , JF and Che , CM . 2006 . Proteomic analysis of the mode of antibacterial action of silver nanoparticles . J. Proteome. Res. , 5 : 916 – 924 .
- Kobayashi , M . 1990 . Review on structural and dynamical properties of silver chalcogenides . Solid State Ionics. , 39 : 121 – 149 .
- Ferhat , M and Nagao , J . 2000 . Thermoelectric and transport properties of β-Ag2Se compounds . J. Appl. Phys. , 88 : 813 – 816 .
- Schoen , DT , Xie , C and Cui , Y . 2007 . Electrical switching and phase transformation in silver selenide nanowires . J. Am. Chem. Soc. , 129 : 4116 – 4117 .
- Liang , YC and Tada , K . 1988 . Dependence of silver distributions in electron-beam-exposed regions on dosage as well as on the thicknesses of dry-sensitized layers and chalcogenide glass films . J. Appl. Phys. , 64 : 4494 – 4499 .
- Hodes , G , Manassen , J and Cahen , D . 1976 . Photoelectrochemical energy conversion and storage using polycrystalline chalcogenide electrodes . Nature , 261 : 403 – 404 .
- Santhosh-Kumar , MC and Pradeep , B . 2002 . Structural electrical and optical properties of silver selenide thin films . Semicond. Sci Technol. , 17 : 261 – 265 .
- Zhang , SW , Liu , J and Xu , CH . 2007 . Preparation of silver nanopowder by freeze-drying procedure . Dry Technol. , 27 : 529 – 533 .
- Purcar , V , Donescu , D , Petcu , C , Luque , R and Macquarrie , DJ . 2009 . Efficient preparation of silver nanoparticles supported on hybrid films and their activity in the oxidation of styrene under microwave irradiation . Appl. Catal. A , 363 : 122 – 128 .
- Eftekhari , A . 2001 . Silver hexacyanoferrate(II) film direct modified electrode as amperometric sensor for the determination of silver . Anal. Lett. , 34 : 1087 – 1095 .
- Kong , JM , Wong , CV , Gao , ZQ and Chen , XT . 2008 . Preparation of silver nanoparticles by microwave-hydrothermal technique . Synth. React. Inorg. Met.-Org., and Nano-Met. Chem. , 38 : 186 – 188 .
- Shi , HQ , Fu , X , Zhou , XD and Hu , ZS . 2005 . Preparation of organic fluid containing Ag nanoparticles with extractant cyanex 301 . J. Dispers. Sci. Technol. , 26 : 315 – 319 .
- Sergeev , BM , Sergeev , GB , Kasaikin , VA , Litmanovich , EA and Prusov , AN . 2001 . Low-temperature synthesis and properties of silver nanoparticles stabilised by acrylic polymers . Mol. Cryst. Liq. Cryst. , 356 : 121 – 129 .
- Li , HD , Ying , C , Yang , L and Li , LL . 2008 . Synthesis of faceted and cubic Ag2S nanocrystals in aqueous solutions . Colloid Interface Sci. , 317 : 485 – 492 .
- Wang , D , Xie , T , Peng , Q and Li , YD . 2008 . Ag, Ag2S, and Ag2Se nanocrystals: Synthesis, assembly, and construction of mesoporous structures . J. Am. Chem. Soc. , 130 : 4016 – 4022 .
- Zhong , XH , Xie , RG , Zhang , Y , Basche , T and Knöll , W . 2005 . High-quality violet- to red-emitting ZnSe/CdSe core/shell nanocrystals . Chem. Mater. , 17 : 4038 – 4041 .
- Liu , JK , Yang , XH and Tian , XG . 2008 . Preparation of silver/hydroxyapatite nanocomposite spheres . Powder Technol. , 184 : 21 – 24 .
- Yan , SC , Wang , HT , Zhang , YP , Li , SC and Xiao , ZD . 2008 . A simple route to large-scale synthesis of silver sulfide nanowires . J. Non-Crys. Solids , 354 : 5559 – 5562 .
- Savarimuthu , PA . 2009 . Synthesis of Ag2S and Ag2Se nanoparticles in self assembled block copolymer micelles and nano-arrays fabrication . Mater. Lett. , 63 : 773 – 776 .
- Rajeshi , K . 2005 . Electrochemical synthesis of conical Ag2S nanostructures and their optical properties . J. Mater. Sci. , 40 : 3523 – 3525 .
- Sung , KM , Mosley , DW , Peelle , BR , Zhang , S and Jacobson , JM . 2004 . Synthesis of monofunctionalized gold nanoparticles by fmoc solid-phase reactions . J. Am. Chem. Soc. , 126 : 5064 – 5065 .
- Liu , JK , Lu , Y , Hu , XJ and Mu , J . 2007 . Facile synthesis of copper nanoparticle chains . NANO , 2 : 31 – 34 .
- Pal , U and Santiago , P . 2005 . Controlling the morphology of ZnO nanostructures in a low-temperature hydrothermal process . J. Phys., Chem. B , 109 : 15317 – 15321 .
- Liu , MX , Gan , LH , Zeng , YL , Xu , ZJ , Hao , ZX and Chen , LW . 2008 . Self-assembly of CdTe nanocrystals into two-dimensional nanoarchitectures at the air−liquid interface induced by Gemini surfactant of 1,3-bis(hexadecyldimethylammonium) propane dibromide . J. Phys. Chem. C , 112 : 6689 – 6694 .
- Navarro , E , Piccapietra , F , Wagner , B , Marconi , F , Kaegi , R , Odzak , N , Sigg , L and Behra , R . 2008 . Toxicity of silver nanoparticles to Chlamydomonas reinhardtii . Environ. Sci. Technol. , 42 : 8959 – 8964 .
- Brar , SK , Verma , M , Tyagi , RD and Surampalli , RY . 2010 . Engineered nanoparticles in wastewater and wastewater sludge: Evidence and impacts . Waste Management , 30 : 504 – 520 .
- Stampoulis , D , Sinha , SK and White , JC . 2009 . Assay-dependent phytotoxicity of nanoparticles to plants . Environ. Sci. Technol. , 43 : 9473 – 9479 .
- Barrena , R , Casals , E , Colón , J , Font , X , Sánchez , A and Puntes , V . 2009 . Evaluation of the ecotoxicity of model nanoparticles . Chemosphere , 75 : 850 – 857 .