Abstract
In this study, we have successfully deposited N-doped SiO2/TiO2 thin films on ceramic tile substrates by sol–gel method for auto cleaning purpose. After dip coating and annealing process the film was transparent, smooth and had a strong adhesion on the ceramic tile surface. The synthesised catalysts were then characterised by using several analytical techniques such as X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscope (AFM) and UV-vis absorption spectroscopy (UV-vis). The analytical results revealed that the optical response of the synthesised N-doped SiO2/TiO2 thin films was shifted from the ultraviolet to the visible light region. The nitrogen substituted some of the lattice oxygen atoms. The surface area of co-doped catalyst increased, and its photocatalytic efficiency was enhanced. The photocatalytic tests indicated that nitrogen co-doped SiO2/TiO2 thin films demonstrated higher than of the SiO2/TiO2 activity in decolouring of methylene blue under visible light. The enhanced photocatalytic activity was attributed to an increasing of the surface area and a forming of more hydroxyl groups in the doped catalyst.
1. Introduction
Ceramic tiles are widely employed in industrial materials. Their applications are mainly as construction materials. In most cases, a periodical cleaning process is needed to maintain their transparency, also their appearance. This work is really costly and rather dangerous for workers to clean ceramic surfaces outside a skyscraper. Therefore, there is a need for self-cleaning and sterilising ceramic tiles. Titanium dioxide (TiO2) is the most widely used photocatalyst for solar energy conversion and environmental applications because of its non-toxicity, low cost and good photoactivity Citation1. The addition, SiO2 into TiO2 retards or inhibits the crystallisation of the anatase phase. A contact angle of TiO2/SiO2 thin films with 15 mol % SiO2 concentration is less than 2° and this film can maintain a super-hydrophilic property for a long time in dark conditions, thus exhibiting excellent antifogging capability Citation2. When TiO2 is irradiated by wavelength shorter than 387 nm (UV) electrons are promoted across the band gap into the conduction band, leaving holes in the valence band Citation3. Having high oxidation power, these holes react with adsorbed hydroxide ions to produce hydroxyl radicals that are the main oxidizing species responsible for the photo-oxidation of organic compounds Citation4. The major problem is that only about 4% of the solar spectrum falls in this UV range. The efficient use of sunlight becomes an appealing challenge for developing photocatalysts Citation5. One approach for achieving this objective is to sensitize TiO2 by doping with nonmetal atoms such as S, C, or N. Among these anion-doped TiO2 photocatalysts, N-doped TiO2 has been the most extensively studied, and considerable success has been achieved in enhancing the visible-light-driven photocatalytic activity by decreasing the band gap of N-doped TiO2 Citation6.
In this study, nanocrystalline N-doped SiO2/TiO2 photocatalyst were prepared by the sol–gel method. Co-doping nitrogen helps broadening photoresponse of SiO2/TiO2-based photocatalysts into the visible-light region. The self-cleaning ability of the prepared materials is assessed by water contact angle measurement and their photocatalytic activities tested under visible-light irradiation by degrading methylene blue (MB), which is known to be difficult to degrade under irradiation and is often used as a model dye contaminant to evaluate the activity of a photocatalyst Citation7. The antibacterial effects of N/SiO2–TiO2 thin films against Escherichia coli was estimated by relative number of surviving bacteria calculated from the number of viable cells on the Petri dishes.
2. Experimental procedures
2.1. Preparation of N/SiO2 –TiO2 thin films
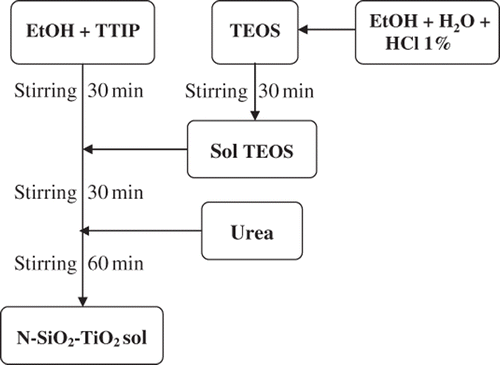
All chemicals used in the study were purchased from Aldrich, Germany, and used as received. Titanium tetraisopropoxide (TTIP) and tetraethylorthosilicate (TEOS) were used as precursors for titania and silica, respectively. First, TEOS was hydrolysed in an aqueous HCl solution, and a TTIP ethanol mixture (1 mol TTIP per 20 mol ethanol) was slowly introduced dropwise. The molar concentration of Si/Ti in the solution was chosen at an optimal value of 15 mol% Citation7. Urea was also added with various N/SiO2–TiO2 molar concentrations from 10 to 50 mol%. The detailed synthesis procedure is shown in .
N/SiO2–TiO2 thin films were deposited by the dip coating process on ceramic tile substrates having dimensions of 26 × 76 mm2 at room temperature. The substrates were immersed into the as-prepared N/SiO2–TiO2 solution for 1 min, and were withdrawn from the solution at a velocity 4 mm/s. After each layer was deposited, substrates were annealed at 300°C on a hotplate for 5 min. The procedure from coating to drying was repeated three times to achieve a film thickness of about 200 nm. Afterwards, substrates were calcined at 500°C for 2 h. In our work, N/SiO2–TiO2 powders were also prepared using the same calcination procedure.
2.2. Characterisation of film structure and morphology
X-ray diffraction (XRD) were obtained patterns of the calcined gels were obtained with a Cu-Kα radiation (Siemens Kristalloflex diffractometer) to determine the crystal phase composition of the prepared photocatalysts. The average crystal size was estimated by applying the Scherrer equation to the apparent full width at half maximum intensity (FWHM) of the (101) peak of anatase TiO2 Citation8, as follows:
2.3. Self-cleaning activity measurements
The self-cleaning activities of synthesised films were evaluated by analysing a decrease in concentration of MB during exposure to visible-light irradiation. Ceramic tile samples (26 × 26 mm2) were placed in a container filled with 10 ml of 10 ppm MB. This container was then exposed to the visible light provided by a compact Philips lamp (18 W). Then the MB solution was taken out after being exposed for 2 h, and the concentration of MB was determined by using UV-Visible spectrometry. The changes in concentration of MB were estimated by absorbance peak for MB at 664 nm. The obtained results showed that the concentration of MB decreased when the exposing time was increased. The efficiency of decomposition of MB can be calculated by applying the following formula Citation9:
2.4. Antibacterial activity measurements
E. coli bacteria were grown overnight (20 to 24 h) at 35°C. The cultured bacteria were added in 10 ml saline solution to reach approximately the concentration of 109 CFU/ml. Samples were placed in sterilised Petri dishes. Then 100 µl saline solution was added dropwise onto the surface of films, and a normal ceramic substrate used as a blank. A 18 W compact lamp is then used to expose the samples. The number of surviving bacteria on the Petri dishes were counted after incubation for 24 h at 35°C.
3. Results and discussion
3.1. X-ray diffraction
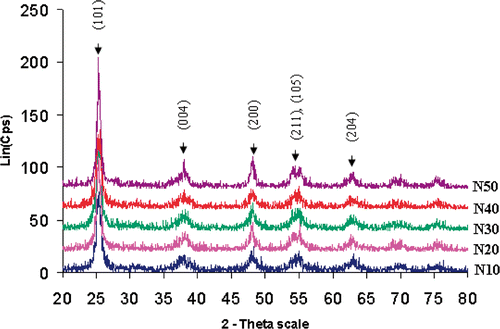
The XRD diagrams of powder samples with different N contents calcined at 500°C are shown in . As seen, all samples contain only the anatase phase with high intensity, and other crystal phases (rutile or brookite) are not detected. Bragg's reflections at angles of 25.4°, 38°, 48°, 54° and 55° correspond to (101), (004), (200), (105) and (211) tetragonal crystal planes of TiO2 anatase phase, respectively. For obtaining transparent self-cleaning N/SiO2–TiO2 films on ceramic substrates, calcination procedure is carried out at 500°C for 2 h. The existence of anatase phase in our synthesised films is a very important result because it is well known that anatase exhibits the highest photocatalytic activity. From the full-width at half-maximum (FWHM) of the strongest peak (101) anatase phase, crystallite sizes were calculated by using Scherrer's equation ().
Table 1. Crystallite sizes of N/SiO2–TiO2 powders with different N contents.
As shown in , an effect of the N-doped concentration on the crystallite size of N/SiO2–TiO2 powders is demonstrated. It is revealed that the crystallite size increases from 9.25 to 15.59 nm when N-doped concentration increases from 10 to 40 mol%. The difference in the crystal size may be agglomerates of primary particle that affect the crystal development, which are in agreement with similar report Citation10. The size decreases to 13.07 nm at for 50 mol%. It can be said that the larger the amount of N-doping is, the better the crystallisation gets, and bigger the grain size of N/SiO2–TiO2 powders gets. But when nitrogen concentration increases above 40%, the crystallite size decreases. This proves a retard effect of N on the crystallinity of N/SiO2–TiO2 powders when concentration of dopant is too much.
3.2. Surface morphology observation
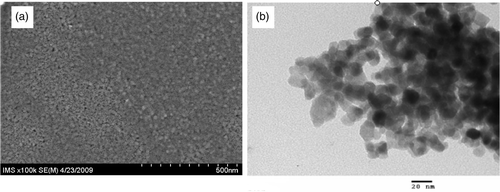
As far as the surface geometry is concerned, the hydrophilic properties are well known to be enhanced for films with a fine roughness. Therefore, the controlling surface microstructure of films is a solution to improve the hydrophilic property of the synthesised films Citation11. shows SEM and TEM images of N40. We observed that the film surfaces are smooth, homogeneous without cracks. Moreover, the surface consists of small granular crystallites with a size of 15 nm that is similar with that obtained by the XRD study. It can be said that synthesised films without agglomeration of big clusters results in an increase of specific surface area, and subsequently enhances desired photocatalytic properties of films.
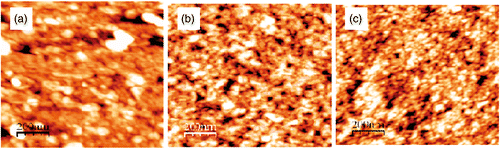
The AFM images in indicate that film roughness changed according to the same rule in XRD or UV-Vis studies: the larger the amount of N-doping, the better the crystallisation, the bigger the grain size of N/SiO2–TiO2 powders, the smaller the grain boundary, and the smaller the surface roughness Citation12. But there is an upper limit value of nitrogen concentration at which the surface roughness (RMS) does not decrease, that is threshold N40. These RMS values are around 1 nm.
3.3. Optical properties of as-prepared N/SiO2–TiO2 thin films
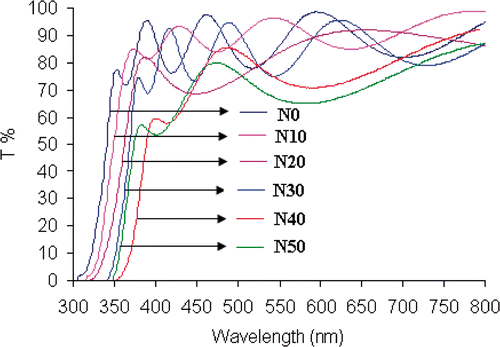
The optical absorbance spectra of N/SiO2–TiO2 thin films with different doping concentrations measured in the region of 300–800 nm are shown in . The transmittance within visible and near infrared region is higher than 70%, which reveals superior optical properties of N/SiO2–TiO2 material produced in this work. The spectra show shoulders at near 350 nm and cut-off wavelength approaching 300 nm. The transmittance quickly decreases for wavelength below 350 nm due to the light absorption caused by the electrons excitation from the valence band to the conduction band of TiO2. The absorption edge shifts towards longer wavelengths (i.e. red shifted) with the increase of nitrogen concentration from 0 to 40 mol%. A shift towards shorter wavelengths (i.e. blue shifted) is observed when the amount of nitrogen is more than 40 mol%.
3.4. Hydrophilic properties of N40 thin films
illustrates the dependence of photo-induced change in water contact angle of N40 films, which were treated after 2 h of visible-light irradiation and then kept overnight in a dark environment. The hydrophilic ability of samples may be explained by the water contact angle on the surface. The super-hydrophilic property of film surfaces allows water to spread completely across the surface rather than to remain as droplets. The observed result means that N40 coated ceramic tile is a good material for self-cleaning purposes. Moreover, shows a very low water contact angle (<2°) on N40 coated sample, while water contact angles are at large values for normal ceramic tile substrate (30.1°). In addition, after storing samples overnight in ambient condition, the water contact angle on N40 coated ceramic was increased a little to 6° (). This result means that coated samples are able to maintain super-hydrophilic capability for a long time in dark environment.
3.5. Self-cleaning property of N/SiO2–TiO2 thin films
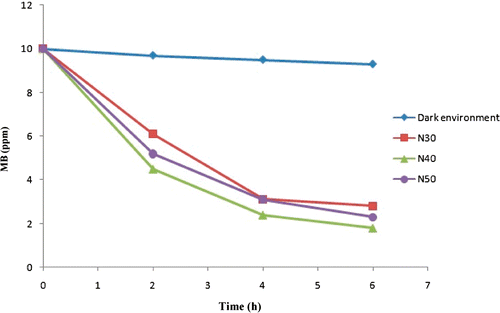
Photocatalytic performance capabilities of N/SiO2–TiO2 thin films were evaluated by degrading MB under visible light exposure. shows the result of measuring the decomposition rate of MB for the prepared films under visible light exposure. The obtained results show that the degradation increased with increasing visible light exposure time. The self-cleaning properties of the films were increased to about 71.76%, 82.29%, and 68.8% with an addition of 30 mol%, 40 mol% and 50 mol% of nitrogen dopant (N30, N40, N50), respectively. Therefore, it can be said that a significant improvement of self-cleaning performance can be achieved by choosing appropriate composition for the synthesised films, and N-doping further improved the self-cleaning properties of synthesised films.
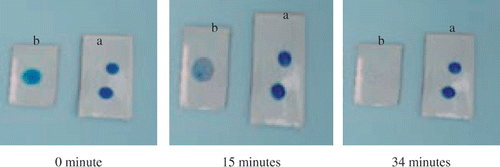
Finally, we have qualitatively analysed photocatalysis property of N40 film on ceramic tile substrate by decoloration of MB, and shows the photocatalytic decoloration of MB on N40 coated ceramic tile substrate and normal substrate. It can be seen that the concentration of MB on the N40 coated substrate was decreased 50% after a period of 15 min, and almost no MB was detected after a period of 34 min.
3.6. Antibacterial activity of N40 coated on the ceramic tile substrate
In order to study bacterial inactivation characteristics of synthesised films, the method of counting the number of surviving bacteria is used. Results are shown in .
Figure 9. (Colour online) Test results on E. coli after 24 h on (a) normal ceramic tile substrate and (b) N40 coated ceramic tile substrate.
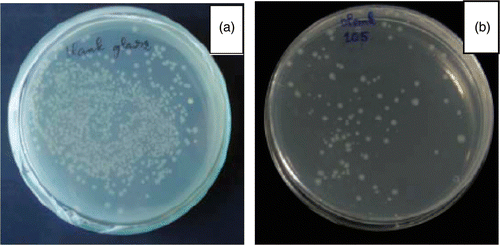
As for the N40 coated substrate, the growth inhibition effect on E. coli is about 70% after 24 h. It is the photocatalytic property of synthesised films that makes it to have a sterilising capability. Under the illumination of visible light, the N/SiO2–TiO2 thin films can spontaneously decompose positive-negative, electron (e−) and hole (h+), which can form the electron-cavity, the cavities oxidize the OH− and H2O, which are absorbed on the film surfaces to OH, which can decompose the cell membrane of E. coli.
4. Conclusion
In this work, we have successfully deposited N/SiO2–TiO2 thin films on ceramic tile substrates. Solution of N/SiO2–TiO2 was prepared by sol–gel method. Nitrogen co-doping helps broadening the photoresponse of SiO2/TiO2 photocatalysts (with 15 mol% SiO2) into visible-light region. The N/SiO2–TiO2 thin film with 40 mol% nitrogen concentration (N40) gives optimal results about crystalline structure, optical and photocatalysis properties. The self-cleaning properties of prepared thin films were evaluated by degrading methylene blue under visible-light. It was shown that photocatalytic performance can be improved by adding active N elements. In this study, the N40 films exhibited the highest photocatalytic performance, demonstrated by methylene blue decomposition nearly completed after only 6 h of visible light exposure. N40 films also show high antibacterial activity by eliminating E. coli. Ceramic tiles coated N/SiO2–TiO2 thin films are believed to be useful for self-cleaning and sterilising ceramic tiles.
Acknowledgments
The authors highly appreciate the financial support of the Department of Science and Technology, Ho Chi Minh City, Vietnam.
References
- Guan , K . 2005 . Relationship between photocatalytic activity, hydrophilicity and self- cleaning effect of TiO2/SiO2 films . Surf. Coatings Technol. , 191 : 155 – 160 .
- Chien , DM , Viet , NN , Van , NTK and Phong , NTP . 2009 . Characteristics modification of TiO2 thin films by doping with silica and alumina for self-cleaning application . J. Exp. Nanosci. , 4 : 221 – 232 .
- Chen , X and Mao , SS . 2007 . Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications . Chem. Rev. , 107 : 2891 – 2959 .
- Trapalis , CC , Keivanidis , P , Kordas , G , Zaharescu , M , Crisan , M , Szatvanyi , A and Gartner , M . 2003 . TiO2 (Fe3+) nanostructured thin films with antibacterial properties . Solids. , 433 : 186 – 190 .
- Asahi , R , Morikawa , T and Ohwaki. , T . 2001 . Visible-light photocatalysis in nitrogen-doped titanium oxides . Science , 293 : 269 – 271 .
- Ghicov , A , Macak , JM , Tsuchiya , H , Kunze , J , Haeublein , V , Frey , L and Schmuki , P . 2006 . Ion implantation and annealing for an efficient N-doping of TiO2 NAnotubes . Nano Lett. , 6 : 1080 – 1082 .
- Severino , D , Junqueira , HC , Gugliotti , M , Gabrielli , DS and Bapista , MS . 2003 . Influence of negatively charged interfaces on the ground and excited state properties of methylene blue . Photochem. Photobiol. , 77 : 459 – 468 .
- Borgarello , E , Kiwi , J , Pelizzetti , E , Visca , M and Graetzel , M . Sustained water cleavage by visible light, J. Am. Chem. Soc. 103 (1981), pp. 6324–6329
- Jing , W , Zong-zhe , J , Jin-sheng , L , Zhi-jiang , J and Xue-wu , Y . 2001 . Study on the antibacterial test method for ceramics [J] . Jiangsu Ceram. , 34 : 11 – 17 .
- Dong , F , Zhao , W , Wu , Z and Guo , S . 2009 . Band structure and visible light photocatalytic activity of multi-type nitrogen doped TiO2 nanoparticles prepared by thermal decomposition . J. Hazardous Mater. , 162 : 763 – 770 .
- Bersani , D , Lottici , PP and Ding , X-Z . 1998 . Phonon confinement effects in the Raman scattering by TiO2 nanocrystals . Appl. Phys. Lett. , 72 : 1 – 5 .
- Le , DD , Dang , TMD , Chau , VT and Dang , MC . 2010 . The fabrication of visible light responsive Ag–SiO2 co-doped TiO2 thin films by the sol–gel method . Adv. Nat. Sci.: Nanosci. Nanotechnol. , 1 : 1 – 5 .
- Chien , DM , Viet , NN , Van , NTK and Phong , NTP . 2009 . SiO2–TiO2 thin film and its photocatalyst properties . Adv. Nat. Sci. , 10 : 31 – 38 .