Abstract
New ‘nano–meso ZSM-5’ (NM-ZSM-5) material was successfully synthesised by hydrothermal treatment using silica derived from rice husk. The synthesis of the material involves two steps of crystallisation, including the formation of ZSM-5 zeolite seed and mesoporous structure. The effect of crystallisation time on the formation of mesoporous structure was studied in the range 0–40 h. Samples were characterised by X-ray diffraction, infrared spectroscopy, transmission electron microscopy, Brunauer–Emmett–Teller and 27Al-NMR methods. The results show that the best time for crystallisation is 14 h. The synthesised material has a multiporous structure, including micropore system of ZSM-5 zeolite, mesopore system of MCM-41 and another pore system which has a diameter in the range 10–50 nm (mesoporous system) due to the burning of organic compounds that remain in the material during the calcination process. In addition, the synthesised NM-ZSM-5 achieves crystallinity of approximately 100%. The catalytic performance of NM-ZSM-5 material was evaluated by the catalytic cracking reaction to produce biofuel from vegetable oil sludge. The research proved that NM-ZSM-5 is one of the most suitable catalysts for this process. The catalytic cracking reaction over NM-ZSM-5 yields products that are similar to those of the petroleum cracking process, such as dry gases, liquefied petroleum gas, gasoline, light cycle oil and heavy cycle oil.
1. Introduction
ZSM-5 zeolite is a very active additive used in fluid catalytic cracking during the petroleum cracking process for the production of fuel. In order to improve the catalytic performance of this additive, many worldwide researches, such as synthesising MCM-41 mesoporous material based on ZSM-5 zeolite and synthesising ZSM-5/MCM41 composite by adding ZSM-5 on the MCM-41 matrix Citation1,Citation2, have been conducted. These materials have multiporous structure (both microporous and mesoporous phases) and are more active in comparison to ZSM-5 zeolite.
Tetraethylorthosilicate (TEOS), Ludox, water glass and Si-containing solutions are the resources of silica that are used in the synthesis of nano–meso ZSM-5 (NM-ZSM-5). Among them, TEOS and Ludox are very expensive. Beside water glass, the Si-containing solution from rice husk was considered. The results showed that silica extracted from rice husk has a very good quality that is required to synthesise the catalyst Citation3–6.
This study mentions the effect of the crystallisation time on the formation of NM-ZSM-5 synthesised using silica extracted from rice husk. Samples are characterised by modern techniques such as infrared spectroscopy (IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 isothermal adsorption/desorption and 27Al-NMR. The catalytic activity of material is evaluated by the catalytic cracking reaction of vegetable oil sludge to produce biofuel.
2. Experimental
2.1. Extraction of silica from rice husk
Silica was extracted from rice husk (for 30 min) using 1% sodium hydroxide solution at a temperature of 80°C. The resulting Si-containing solution was used as a silica source. The composition of the extracted solution included 9.03 g/L of SiO2 and 7.77 g/L of Na2O among others. This solution was used together with other synthetic inorganic materials to synthesise NM-ZSM-5.
2.2. Synthesis of NM-ZSM-5
A typical synthesis process of NM-ZSM-5 is described as follows:
Step 1
ZSM-5 seed was prepared using silica from rice husk, Al2(SO4)3 · 18H2O, template agent tetrapropyl ammonium bromide (TPABr) and sulphuric acid. The reaction mixture was stirred for 2 h, then transferred into an autoclave and kept at a temperature of 135°C for 10 h with stirring.
Step 2
The obtained solution of ZSM-5 zeolite seed was added to cetyltrimethylammonium bromide (CTMABr) and stirred vigorously at ambient temperature for 1 h. The final mixture was transferred into an autoclave and kept at a temperature of 135°C for different crystallisation times. The solid product was filtered and washed with deionised water to a pH of 7, dried at 100°C for 12 h and calcined at 540°C for 5 h to remove template (the final calcination temperature was reached using a ramp of 5°C/min). The obtained sample was a white-coloured powder.
2.3. Characterisation
IR spectroscopy was measured using an IMPACT-410 (Germany) at room temperature. Wave numbers are in the range 400–1300 cm−1. Sample was wafered with KBr.
Powder XRD patterns were recorded on a HUT-PCM-Bruker D8 Advance instrument diffractometer system equipped with Ni-filtered Cu-Kα radiation (operating at 40 kV, 40 mA, wavelength λ = 0.154 nm). The patterns were recorded at room temperature (298 K), 2θ values were from 0.1° to 5° and 5° to 50°, and the scanning speeds were 1 and 2 degrees/min.
TEM was performed on a Philips Tecnai-10 microscope at 100 kV.
SEM was carried out on a Jeol JSM-7500F instrument. Sample was dried, mounted on a thin plate and coated by a flimsy platinum layer before recording.
The Brunauer–Emmett–Teller (BET) surface area and pore volume were measured by nitrogen adsorption/desorption isotherms method using ASAP2010 equipment (Micrometrics, USA). The sample was treated in vide pressure at 120°C for 4 h and at 350°C for 9 h.
The presence of aluminium within the framework has been evaluated through 27Al solid state magic angle spinning-NMR method. Spectrum was obtained with a Bruker MSL400 spectrometer for 27Al (at 104.3 MHz).
2.4. Catalytic cracking reaction
The vegetable oil sludge was obtained from Van Dao, a Vietnamese Company. The composition is as follows: triglycerides (61%), free fatty acid (37%) and impurities (2%). The hydrocarbon chains of triglycerides and free fatty acid are mainly C16 (30%) and C18 (36%), the others consisted of C12–C17 hydrocarbon chains.
Micro-activity test (MAT 5000) system of Zeton, Canada, was used for catalytic cracking. The procedures of catalytic cracking applied ASTM D5154 testing method and the main parameters of catalytic cracking and pyrolysis processes are given in .
Table 1. Main parameters of pyrolysis and catalytic cracking conditions.
The coke formation was evaluated in situ by combustion with air and quantified by CO2 infrared analyser (1440 Gas Analyser, Servomex).
The gas product was collected in a glass bottle containing brine water and analysed by refinery gas analyser (TRACE GC, Thermo Electron).
The distribution of boiling point (BP) of the liquid product was determined by SIMDIST GC (Thermo Electron) system, flame ionisation detector integrated TriPlus auto-sampler. The gas was calibrated according to RGA standard of MESA International Technologies, Inc., while the liquid for distillation simulation was according to ASTM D2887 Calibration mix (RESTEK).
The composition of the liquid fraction of vegetable oil sludge cracking reaction was analysed by GC–MS method (GC-HP HEWLETT 5890 PACKARD SERIES II; MS-HEWLETT 5989B PACKARD).
3. Results and discussion
3.1. Synthesis of NM-ZSM-5
Temperature and time of the crystallisation are the key factors which have a considerable influence on the formation of the porous inorganic materials. We investigated the influence of the crystallisation time of step 2 on the synthesis of NM-ZSM-5. The synthesis conditions are given in .
Table 2. The crystallisation conditions of NM-ZSM-5 samples.
3.2. Characterisation of NM-ZSM-5
3.2.1. IR spectra
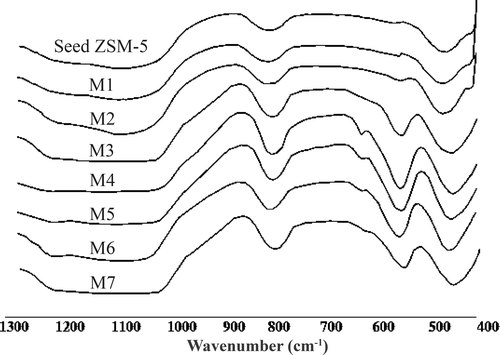
shows the IR spectra of the ZSM-5 seed and the NM-ZSM-5 samples with silica source extracted from rice husk. Spectra range from 400 to 1300 cm−1.
The IR spectra of all samples show peaks that are similar to those of the standard ZSM-5 sample Citation4. The peak at 550 cm−1 is attributed to the fluctuation of 5-1 rings in ZSM-5 structure, which is typical for the crystalline status of a material and is usually used as a standard band to determine the content of the crystalline phase. According to Kulkarni et al. Citation7, the crystallinity of synthesised material is 100% if the intensive ratio of the peaks at 550 to 450 cm−1 is 0.8. shows that the ratio of the samples increase from M1 to M4 and then decrease from M4 to M7. In addition, the ratio of the peaks at 550 to 450 cm−1 of M4 is approximately 0.8. Consequently, these results show that the crystallinity of the M4 sample is the highest in comparison to other samples and reaches approximately 100%.
3.2.2. X-ray diffraction
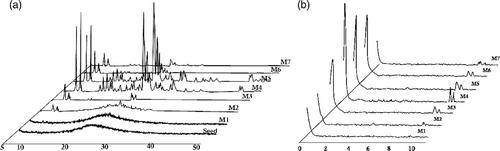
The results obtained from IR method can be proved again more clearly by XRD method. The XRD patterns of the NM-ZSM-5 samples are shown in (wide-angle X-ray) and (small-angle X-ray). The ZSM-5 structure of the samples was confirmed by the presence of the peaks at 9.05° and 24.05° which characterise the MFI structure Citation7.
On the other hand, the XRD patterns also show that the M4 samples have the highest content of crystalline phase. This consideration is in good agreement with the above-mentioned IR result.
The XRD patterns () show the presence of the peaks at 2θ region of 0–2°, which are characteristic of mesophase Citation8. Also, the M4 sample has the highest intensity of peaks. The obtained results prove that the optimum crystallisation time for NM-ZSM-5 synthesis is 24 h (step 1: 10 h; step 2: 14 h).
3.2.3. BET characterisation
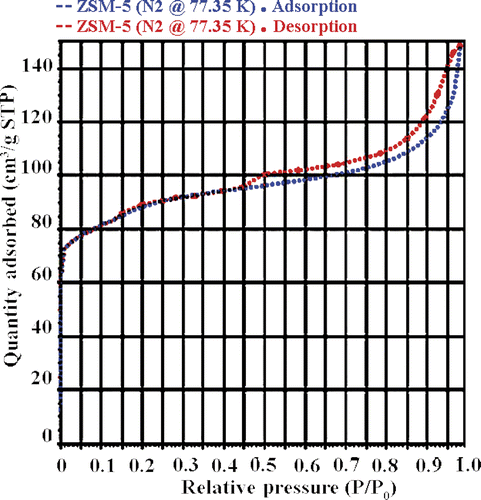
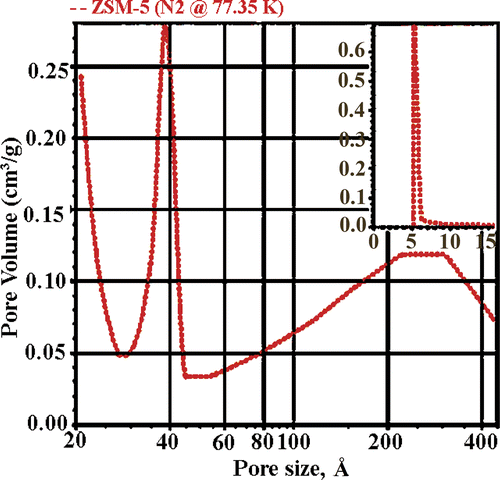
The mesostructure of the NM-ZSM-5 sample was also observed by N2 adsorption/desorption isotherms. shows the appearance of a wide hysteresis loop. This typical shape is characteristic of mesoporous structure Citation8. shows that the sample has two pore systems: micropore with a size of 5.5 Å and mesopore of around 40–200 Å; and the specific surface area is ∼522 m2/g.
3.2.4. SEM and TEM images
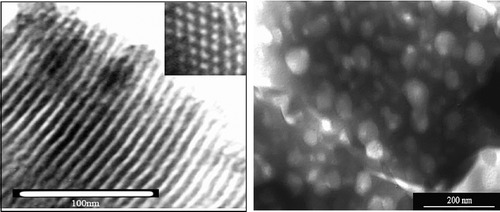
The SEM and TEM images show the morphology of NM-ZSM-5 sample. The SEM images () clearly indicate that MCM-41 formed with a uniform size of 350 nm in the shape of hexagons. The ZSM-5 zeolite seeds assembled to create MCM-41-ordered mesoporous structure under direction of the organic template.
The existence of long-range dimensional mesophase of the material is shown in the TEM image (). The mesopores in the structure of MCM-41 are uniform with a size of 4 nm.
In addition, the observed TEM image shows a very interesting feature of the synthesised sample. Besides the MCM-41 mesoporous system, there is another porous system which has a diameter in the range 10–50 nm. The exits of this pore system can be explained by the carbon that is present in the treated rice husk, which is still being removed in the calcination process. The removal of carbon formed special mesoporous system of synthesised material and this result tally with the result obtained from BET method in the pore distribution chart.
3.2.5. Nuclear magnetic resonance-27Al-NMR
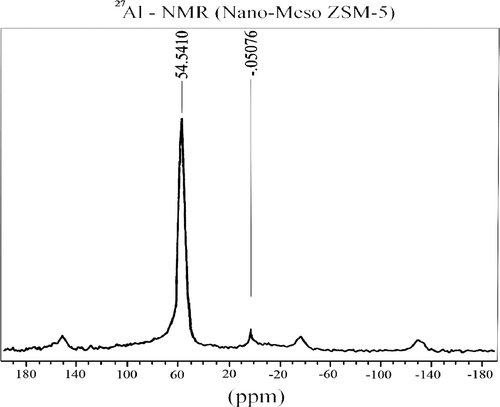
The exits of Al3+ ion in zeolite framework relates with the acidity of the material (due to Al3+ ion tetrahedrally coordinated in the framework of zeolite). From the obtained result, the acidity of the material can be indirectly measured via the determination of the presence of Al3+ in zeolite framework. So, to determine the distribution of Al3+ ion of synthesised material, the 27Al-NMR method was applied. shows the 27Al-NMR of NM-ZSM-5.
The 27Al-NMR of NM-ZSM-5 gives rise to one resonance only at 55 ppm with high intensity due to the presence of aluminium in a tetrahedrally coordinated environment, which is typical for aluminium zeolitic framework Citation7,Citation8. However, it also shows one more resonance at 0 ppm with very low intensity and it is negligible in comparison with the intensity of resonance at 55 ppm. According to Jiao et al. Citation9 and Hunger and Brunner Citation10, this resonance is attributed to Al3+ which is not present in zeolite framework. This indicates that the quantity of aluminium used is almost present in zeolite framework.
3.3. Catalytic performance
In order to evaluate the catalytic activity of the synthesised material, the vegetable oil sludge cracking reaction was performed. The reaction was carried out on a MAT5000 system of Hanoi University of Technology (Vietnam) with input parameters listed in .
The catalytic activity is measured via product yield and conversion of the cracking process. Main products of the cracking reaction are as follows:
| • | Dry gasses: mainly consisting of H2, CO, CO2, CH4, C2H6 and C2H4. | ||||
| • | Liquefied petroleum gas (LPG): consisting of C3–C4 (C3, C3=, iC4, nC4, C4=, propandien). | ||||
| • | Gasoline: BP is less than 221°C and also consisting of C5 + gases in gas product. | ||||
| • | Light cycle oil (LCO): BP ranges between 221°C and 343°C. | ||||
| • | Heavy cycle oil (HCO): BP is more than 343°C and other fragments which are not converted from initial vegetable oil sludge. | ||||
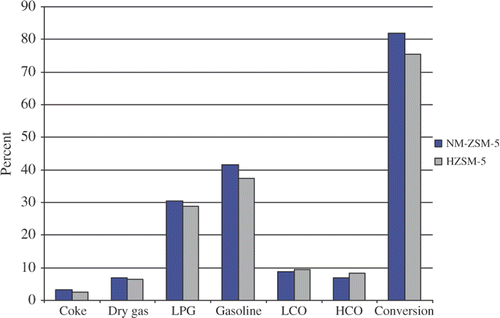
shows the products obtained from the catalytic cracking reaction over NM-ZSM-5 and HZSM-5 catalyst materials in the form of a bar chart. The result indicates that both NM-ZSM-5 and HZSM-5 are very active for the vegetable oil sludge cracking reaction to produce biofuel. On the other hand, it shows that the total of LPG, gasoline (desirous products) and conversion of the catalytic cracking reaction over NM-ZSM-5 is higher than that of HZSM-5. On the contrary, HCO and LCO (undesirable products) of NM-ZSM-5 are lower than that of HZSM-5.
Figure 8. Products and conversion of vegetable oil sludge cracking reaction over HZSM-5 and NM-ZSM-5.
The products obtained from the cracking reaction are classified into gas, liquid and solid (coke) types. Gas product is analysed by gas chromatography system (refinery gas analyser; TRACE GC, Thermo Electron). describes the gas product of catalytic cracking over HZSM-5 and NM-ZSM-5 and of pyrolysis. The total quantities of gas product of the cracking reaction over HZSM-5 and NM-ZSM-5 catalysts are higher than that of the pyrolysis. It indicates that the catalytic cracking underwent more deeply than the pyrolysis did. Moreover, the presence of i-butane in gas product of catalytic cracking over NM-ZSM-5 shows that an isomerisation has occurred.
Table 3. The composition of gas product.
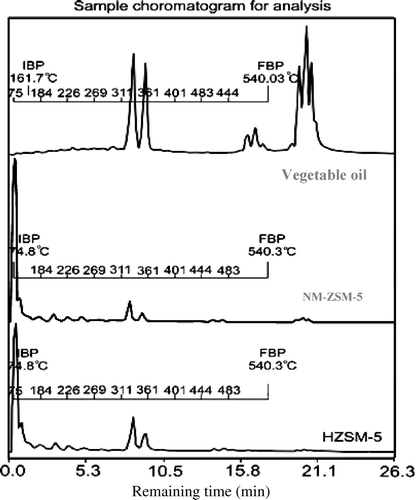
The liquid product of the cracking reaction is analysed by SIMDIST GC Thermo Electron system. The composition of liquid fraction is measured via the distribution of BP (). It shows that the vegetable oil sludge has three areas of BP. All of them are over 343°C, corresponding to the segments of HCO and LCO. The spectra of the catalytic cracking reaction over both HZSM-5 and NM-ZSM-5 also show that the peaks occur near 75°C and 343°C; the intensity of the former being much higher than that of the latter. It shows that the cracking reaction over these catalysts have a total of LPG and gasoline that is much higher than those of HCO and LCO. However, the intensity of peaks at near 343°C in the spectra of NM-ZSM-5 is lower than that of HZSM-5 and the ratio of peaks at 75°C to 343°C is much higher than that of HZSM-5. This indicates that the obtained gasoline and LPG fractions in the product of cracking reaction over NM-ZSM-5 is higher than that of HZSM-5.
4. Conclusion
NM-ZSM-5 catalyst was successfully synthesised from rice husk silica. The results prove that the optimum crystallisation time for the synthesis of NM-ZSM-5 is 24 h. Besides the ZSM-5 microporous and the MCM-41 mesoporous systems with a size of 5 Å and 4 nm, respectively, there is another porous system which has a diameter in the range 10–50 nm (mesoporous system) due to the burning of organic compounds that remain in the material during the calcination process.
NM-ZSM-5 proved that it is a good catalyst in the cracking reaction of vegetable oil sludge with higher conversion and selectivity for LPG and gasoline products in comparison to HZSM-5 zeolite. The obtained results have found a new way in the salvage of agricultural waste to produce promising catalysts which can be applied not only to catalysis and adsorption fields, but also to produce biofuel from vegetable oil sludge replacing the exhausted natural petroleum oil resource.
Acknowledgements
The authors are thankful to the Vietnamese Ministry of Science and Technology, the Vietnamese Ministry of Industry and Trade and the Belgian Federal Science Policy Office for providing financial support.
References
- S. Selene-Ramírez, J.M. Domínguez, L.A. García-Serrano, and M.A. Mantilla, Synthesis of hybrid materials composed by crystalline zeolites and noncrystalline MCM-41 phases at low temperature, 18th North American Meeting, Cancun, Mexico, (2003), poster-p. 179
- Chen , HY , Xi , HX , Cai , XY and Yu , Q . 2009 . Experimental and molecular simulation studies of a ZSM-5-MCM-41 micro-mesoporous molecular sieve . Microporous Mesoporous Mater. , 118 : 396 – 402 .
- L.T.H. Nam, B.L. Su, N.D. Tuyen, N.N. Triu, N.T.T. Loan, D.X. Do, V.D.S. Tho, and N.A. Vu, Study on synthesis and characterization of ‘Nano-meso ZSM-5’ using silica source from Vietnamese rice husk, Proceedings of First International Workshop on Nanotechnology and Application, Vung Tau, Vietnam, 15–17 November 2007, pp. 293–299
- Katsuki , H , Furuta , S , Watari , T and Komarneni , S . 2005 . ZSM-5 zeolite/porous carbon composite: Conventional and microwave hydrothermal synthesis from carbonized rice husk . Microporous Mesoporous Mater. , 86 ( 1–3 ) : 145 – 151 .
- Prasetyoko , D , Ramli , Z , Endud , S , Hamdan , H and Sulikowski , B . 2006 . Conversion of rice husk ash to zeolite beta . Waste Manage. , 26 : 1173 – 1179 .
- L.T.H. Nam, N.D. Tuyen, N.T.T. Loan, T.Q. Vinh, D.X. Dong, L.T.K. Lan, N.T. Anh, L.Q. Du, V.T.M. Hong, and B.-L. Su, Influence of silica resource from rice husk on structure of the HZSM-5 zeolite, Proceedings of APCTP-ASEAN Workshop on Advanced Materials Science and Nanotechnology, Nha Trang, Vietnam, 15–20 September 2008, pp. 637–641
- Kulkarni , SB , Shiralkar , VP , Kosthane , AN , Borade , RB and Ratnasamy , P . 1982 . Studies in the synthesis of ZSM-5 zeolites . Zeolites , 2 : 313 – 318 .
- P.A. Webb and C. Orr, Analytical Methods in Fine Particle Technology, Micromeritics Instrument Corporation, Norcross, GA, 1997, pp. 55–56
- Jiao , J , Ray , SS , Wang , W , Weitkamp , J and Hunger , M . 2005 . Effect of dehydration on the local structure of framework silicon atoms in zeolites Y investigated by solid-state NMR spectroscopy . Z. Anorg. Allg. Chem. , 631 : 484 – 490 .
- Hunger , M and Brunner , E . 2004 . NMR Spectroscopy . Mol. Sieves , 4 : 201 – 293 .