Abstract
We present the synthesis and detailed characterisation of the Rb x Mn[Fe(CN)6] compound. The composition of the material significantly depends on the applied preparative conditions. Analysis of spectroscopic results (EDX, Raman) and X-ray powder diffraction data yielded a further assessment of the difference in structural features. The characteristic individual magnetic behaviour, as well as the metal-to-metal charge transfer capabilities of the various samples, could be related to significant changes within the structures that appear to be associated with the synthetic method used.
Keywords:
1. Introduction
Recently, the development of photomagnetic molecular materials, in which magnetic properties can be controlled by light, has drawn increasing interest due to their potential relevance for high-density data storage and other technological applications. Several types of molecular materials with photomagnetic properties have been investigated in the past few years. Among them, RbMn[Fe(CN)6] represents an appealing object for further study aimed at the exploration of its potential for applications in molecular electronics. A vital advantageous criterion supporting this is the possibility of reaching a maximal photoefficiency, which is a corollary of its fairly stoichiometric Mn : Fe ratio Citation1–3. However, the researches on Prussian blue analogues have been focused mainly on properties of their bulk forms. In the recent years, nano-sized Prussian blue analogues have emerged as a promising subject for applications to the nanomagnetic devices Citation4,Citation5. These materials often exhibit novel size-dependent properties, which show different properties from their bulk form Citation6. In particular, the magnetic properties of nanoparticles can be different from those of bulk materials and may exhibit anisotropy originating from the low-dimensional character of the systems. The high surface to volume ratios of nanoparticles are often advantageous in magneto-optical studies since more photons can interact with the system without being attenuated. There have been several techniques for preparing such materials. Here, we report a new approach for the growth of nanoparticles of cyano-bridged molecule-based magnets using an organic solvent, formamide and investigate structural, optical and magnetic properties of the prepared products.
2. Experimental
The synthesis of Rb x Mn[Fe(CN)6] sample has been carried out by a route similar to that described in Citation6 with some modifications. A starting mixture of RbCl, MnCl2 and K3[Fe(CN)6] was used as the precursor. The particle size of Rb x Mn[Fe(CN)6] was found to be dependent on the ratio of formamide content to water content. Thus, the particle size was controlled by varying the formamide–water ratio. In this study, two different types of samples are compared: the first type is made using water solvent only, hereafter called the ‘water’ sample, and the second type is made using formamide solvent only, hereafter called the ‘formamide’ sample.
Structural characterisation was performed by means of X-ray diffraction (XRD) using a D5005 diffractometer with Cu-Kα radiation. The field emission scanning electron microscopy (FE-SEM) observation was carried out by using a S4800 (Hitachi) microscope. The magnetic properties of the samples were investigated by a magnetic property measurement system PPMS 6000 (Quantum Design). In the magnetisation measurements, the temperature was first varied from 2 to 40 K (or 2 to 140 K) in the absence of a magnetic field (zero field cooling, ZFC); the field was then kept constant at 0.01 T and the temperature was varied from 40 to 2 K (or 140 to 2 K; field cooling, FC).
The Raman measurements were performed using the 514.5 nm line of Ar ion laser in a backscattering geometry using Jobin Yvon T 64000 triple spectrometer equipped with a cryogenic charge-coupled device (CCD) array detector.
3. Results and discussion
shows the typical FE-SEM images of the samples. From the images, we can see that the Rb x Mn[Fe(CN)6] particles are of a cubic shape and the particle sizes are not uniform for the ‘water’ sample (a), and 100–150 nm for the ‘formamide’ sample (b). Clearly, the size of the Rb x Mn[Fe(CN)6] particles synthesised using formamide solvent only is much smaller than that of the particles synthesised using water only as a solvent. This indicates that the formamide solvent has prevented the growth of the Rb x Mn[Fe(CN)6] particles.
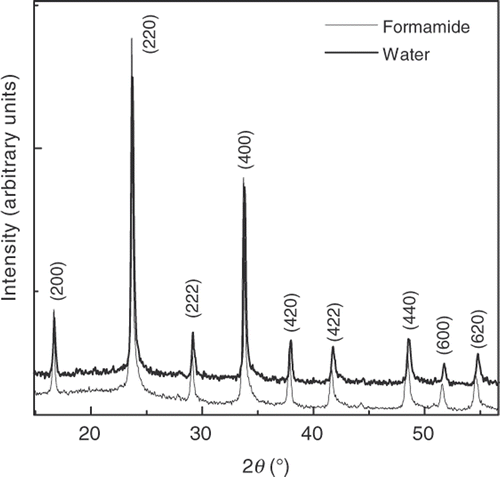
The powder XRD patterns of the samples are shown in . All the diffraction peaks are readily indexed to a pure face-centred cubic (fcc) phase (space group Fm3m (no. 225)) Citation7 with a lattice constant of a = 10.37 Å, and no other impurity phases were found. The broadening of the XRD peaks of the ‘formamide’ samples is attributed to the lattice disorder and a dramatic reduction in the particle size. It is a known fact that RbMn[Fe(CN)6] exhibits a phase transition from a high-temperature cubic structure (HT phase) to a low-temperature tetragonal one
(LT phase) around 250 K Citation8. This structural transition is related to an entropy-driven charge transfer from the Mn ion to the Fe ion Citation6, which is described as Fe3+(
, S = 1/2)–CN–Mn2+(
S = 5/2) → Fe2+(
, S = 0)–CN–Mn3+(
S = 2). The tetragonal nature of the LT phase is due to the Jahn–Teller distortion of the Mn3+ ion because the depopulation of the
orbital produces a shortening of the Mn–N distances in the four N atoms headed by that eg
orbital.
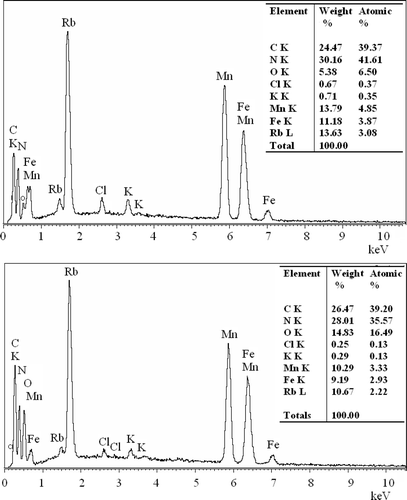
The compositions of sample are also confirmed by an energy-dispersive X-ray analysis (EDX), which reveals the presence of carbon, nitrogen, oxygen, iron, manganese, sodium and potassium ( and inset Table). Two testing results are accordant and the amounts of C and N are considerably large by counting. This result proves that the substances with two different morphologies mentioned above are Rb x Mn[Fe(CN)6] Prussian blue. However, there remains the small amount of K and Cl in the sample. Moreover, there are small amounts of chlorine and potassium impurities in the samples, because the potassium elements, which were introduced from the starting materials, such as MnCl2 and K3[Fe(CN)6], were not completely washed out by water or ethanol. However, the EDX result proves that both the samples are actually Rb x Mn[Fe(CN)6] Prussian blue, although their morphologies are quite different from each other.
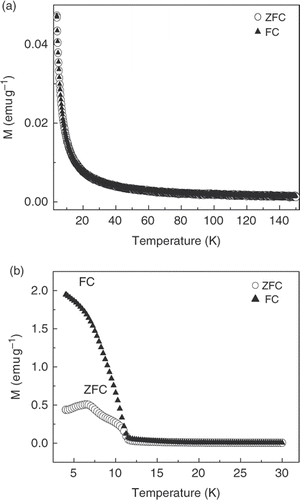
shows the ZFC and FC magnetisation curves in the range of 2–40 K or 2–140 K obtained for the Rb x Mn[Fe(CN)6] samples. corresponds to the water sample, and to the formamide sample, respectively. The FC and ZFC of water sample coincided (), which means that the magnetic property is similar to bulk sample. The ZFC curve of the formamide sample shows a narrow peak at 7 K, which indicates the blocking temperature (T B) of the nanoparticles. The FC curve increases with decreasing temperature and never reaches saturation, indicating that, even at the lowest investigated temperature, a fraction of the particles is still in the superparamagnetic state. By magnetic measurement of Ni3[Cr(CN)6]2 nanocubes, Mallah and Co-workers Citation9 have confirmed that these nanoparticles behaved like the bulk material at high temperature. However, as shown in , there is a divergence between the FC and ZFC magnetisation curves at 11 K, i.e. well below the Curie temperature, indicating that nanoparticles are single-domain superparamagnets with a blocking temperature of 7 K, which corresponds to the maximum in the ZFC curve.
Rb x Mn[Fe(CN)6] was also studied dynamically using Raman spectroscopy, as shown in . There are two strong vibrational bands at 2080 and 2125 cm−1, respectively, which are most likely caused by the stretching vibration of C≡N group of Prussian blue Citation10. The cyanide stretching modes are known to be sensitive to the oxidation state of the coordinating metals. Therefore, changes in the Raman spectra are due to oxidation state changes in Mn and Fe. Cyanide coordinated to Mn(II) has two low-wavenumber peaks at 2125 cm−1, whereas cyanide coordinated to Mn(III) has a single resolvable high-wavenumber peak at 2080 cm−1. This analysis indicates that there exist various valence states of both Mn and Fe resulting in the effect on the exchange interaction, and consequently, affecting the transition temperature. The Raman spectra also show the shift of the peaks. It is clear from the figure that the Raman band broadens and shifts to higher wavenumber with decreasing particle size. Xu et al. Citation11 have tried to explain the variation in the Raman bands with a phonon confinement model for the anatase. In accordance with the relationship between particle size and phonon position as shown in Citation12:
4. Conclusion
In this study, we showed that the Prussian blue nanoparticle samples exhibit significantly different Raman scattering and magnetic properties than those of the bulk Prussian blue samples. We have developed a new approach for the growth of Rb x Mn[Fe(CN)6] nanoparticle samples using the formamide solvent, and the physical properties have been measured and compared with those of the large particle Prussian blue sample. A divergence between the FC and ZFC magnetisation curves is observed for the nanoparticle Prussian blue sample at 11 K, indicating that nanoparticles in the sample are single-domain superparamagnets with a blocking temperature of 7 K.
Acknowledgements
This study was supported by Hanoi National University of Education and Vietnam's National Foundation for Science and Technology Development (NAFOSTED).
References
- Loutete-Dangui , ED , Codjovi , E , Tokoro , H , Dahoo , PR , Ohkoshi , S and Boukheddaden , K . 2008 . Spectroscopic ellipsometry investigations of the thermally induced first-order transition of RbMn[Fe(CN)6] . Phys. Rev. B , 78 : 014303 – 014312 .
- Luzon , J , Castro , M , Vertelman , EJ , Gengler , RY , van Koningsbruggen , PJ , Molodtsova , O , Knupfer , M , Rudolf , P , van Loosdrecht , PH and Broer , R . 2008 . Prediction of the equilibrium structures and photomagnetic properties of the Prussian blue analogue RbMn[Fe(CN)6] by density functional theory . J. Phys. Chem. A , 112 : 5742 – 5748 .
- Suemoto , T , Ohki , K , Fukaya , R , Nakajima , M , Tokoro , H and Ohkoshi , S . 2009 . Dynamics of the charge transferred states relevant to magnetic phase transition in rubidium manganese hexacyanoferrate . J. Luminescence , 129 : 1775 – 1778 .
- Zhou , PH , Xue , DS , Luo , HQ and Chen , XG . 2002 . Fabrication, structure, and magnetic properties of highly ordered Prussian blue nanowire arrays . Nano Lett. , 2 : 845 – 847 .
- Berseth , PA , Sokol , JJ , Shores , MP , Heinrich , JL and Long , JR . 2000 . High-nuclearity metal-cyanide clusters: Assembly of a Cr8Ni6(CN)24 cage with a face-centered cubic geometry . J. Am. Chem. Soc. , 122 : 9655 – 9662 .
- Vien , V , Minh , NV , Lee , HI , Kim , JM , Kim , Y and Kim , SJ . 2008 . A new route for obtaining Prussian blue nanoparticles . Mater. Chem. Phys. , 107 : 6 – 8 .
- Azaroff , LV . 1968 . Elements of X-ray Crystallography , New York : McGraw-Hill .
- Ohkoshi , S-I , Tokoro , H , Utsunomiya , M , Mizuno , M , Abe , M and Hashimoto , KJ . 2002 . Observation of spin transition in an octahedrally coordinated Manganese(II) compound . Phys. Chem. B , 106 : 2423 – 2425 .
- Mallah , T , Thiebaut , S , Verdaguer , M and Veillet , P . 1993 . High-Tc molecular-based magnets: Ferrimagnetic mixed-valence Chromium(III)-Chromium(II) cyanides with Tc at 240 and 190 Kelvin . Science , 262 : 1554 – 1557 .
- Xia , L and McCreery , RL . 1999 . Structure and function of ferricyanide in the formation of chromate conversion coatings on aluminum aircraft alloy . J. Electrochem. Soc. , 146 : 3696 – 3701 .
- Xu , CY , Zhang , PX and Yan , L . 2001 . Blue shift of Raman peak from coated TiO2 nanoparticles . J. Raman Spectrosc. , 32 : 862 – 865 .
- Choi , HC , Jung , YM and Kim , SB . 2005 . Size effects in the Raman spectra of TiO2 nanoparticles . Vib. Spectrosc. , 37 : 33 – 38 .
![Figure 1. SEM images of Rb x Mn[Fe(CN)6] (a) water and (b) formamide samples.](/cms/asset/359c3f03-64ff-4c0c-82f2-4e494910d5d3/tjen_a_533294_o_f0001g.gif)
![Figure 5. Raman spectra of Rb x Mn[Fe(CN)6] samples.](/cms/asset/7d334f24-ab67-4a62-86ee-41f7f47effba/tjen_a_533294_o_f0005g.gif)