Abstract
The interaction of citrate- and polyethylene imine (PEI)-functionalised gold nanoparticles (GNP) with cancer cell lines with respect to the cellular response was studied. It was found that GNP/citrate nanoparticles were able to induce apoptosis in human carcinoma lung cell lines A549, but GNP/PEI did not show any reduction in the viability of the cells in human breast cancer cell line MCF-7 and A549 cell lines. FACS data confirmed that the number of apoptotic cells increased with increase in the concentration of GNP/citrate nanoparticles. Decline in cellular expansion and changes in the nuclear morphology were noted after the treatment of GNP/citrate nanoparticles on A549 cell lines, which itself is a direct response for stress induction. The induction of cellular apoptosis was further confirmed by DNA fragmentation assay. These data confirm the potential of GNP/citrate nanoparticle to evoke cell-specific death response in the A549 cell lines.
1. Introduction
Explorations in the field of nanoscience and technology is accelerating nowadays at a tremendous pace for the better understanding of its potentials for enhancing economic growth and for improving life quality through therapeutics and medical diagnostics. When considering the consumer applications of nano-sized materials, sufficient attention must be paid in evaluating the potential long-term adverse effects as well as their possible hazards on biological systems. Thus, Nanoscale engineering is one of the most dynamic domains at the interface between electronics, physics, biology and medicine. At cellular level, much of the important understandings have come from the studies on cellular response of carbon nanotubes Citation1,Citation2 cadmium selenide and gold nanoparticles (GNP) Citation3,Citation4. Bulk gold is known to be an inert element and is specifically approved by the FDA for internal pharmacological studies Citation5,Citation6. However, the above-cited reports also indicate a new type of hazard to our environment, where toxicity can be produced simply by size, shape and chemical functionalisation.
While focusing on tumour therapeutics that fundamentally uphold the principles of increased specificity and differential accumulation of drug/drug-loaded particles, it has been reported that the tumour vasculature is relatively more permeable than that of healthy tissue Citation7. Hence, much emphasise has been given on particle size of such tumour therapeutic agents Citation8. This fact is widely exploited nowadays for the targeting and accumulation of nanoparticles at a higher concentration within the tumour site. The particle tracking studies done in mammalian single-cell cancer systems, particularly during the radio therapeutic treatment of sinus endothelium – liver cancers revealed that it has openings of 150 nm in diameter, whereas the spleen filters out particles greater than 250 nm in size Citation9. It is also evident that normal tissues contain capillaries with tight junctions that are less permeable to nano-sized particles. Thus, particles can be designed accordingly in controlled nano-size scale so that the particles get retained in and around the targeted sites but can pass freely through the other organs and systems. Passive targeting of non-toxic nanoparticles can therefore enhance the drug concentrations in solid tumours several-fold relative to those obtained with free drugs. At the outset, we have synthesised and used GNP functionalised with sodium citrate and PEI to study the cellular response and functional variation between two human cancer cell lines. The selection of GNP for this study was mainly owing to the chemical inertness of the gold itself that directly facilitates the passive targeting of GNP to the mammalian single cell cancer systems.
2. Materials and methods
2.1. Materials
Gold hydrochloride (HAuCl4) and polyethylene imine (PEI) used for nanoparticles synthesis were purchased from Sigma-Aldrich and Trisodium citrate was obtained from Merck. The Annexin V assay kit was purchased from Invitrogen. The MTT assay kit for cell viability estimation was purchased from Sigma-Aldrich. Trizol reagent (Invitrogen) was used for total DNA isolation from cancer cell lines. The ladder sequence that was used as a reference was purchased from Takara Bio Inc. Human lung carcinoma type II epithelial cells, A549 cell line and human breast cancer cells MCF-7 were obtained from National Centre for Cell Science, Pune, India.
2.2. Preparation of citrate and PEI functionalised GNP
The functionalisation of GNP with citrate was carried out as per the reported method Citation10. Both PEI- and citrate-mediated reduction were carried out at 100°C. The reduction with citrate was done by heating HAuCl4 dispersion (1 mM) at 100°C using a thermostat for uniform temperature. The formation of nanoparticles was initiated by adding 40 mM of tri-sodium citrate. Addition of 0.01 wt% of PEI dispersion instead of citrate would result in the formation of GNP functionalised with PEI, as PEI will then act as a reducing as well as a stabilising agent. The change in colour from light yellow to wine red is marked as the reaction endpoint and a stable dispersion of gold was obtained. After the colour change, the dispersion was allowed to cool and subjected to high-speed centrifugation at 15,000 rpm for 12 min. The GNP were then resuspended in water at pH 7.0.
2.3. Characterisation
UV-visible absorbance spectrum of the synthesised nanoparticles was recorded with a UV-VIS 1700 spectrophotometer (Shimadzu).The mean size and size distribution for the prepared nanoparticles were determined by dynamic light scattering (DLS-ZP/Particle Sizer Nicomp™ 380 ZLS) measurements. Size of the particles was further confirmed using SEM (JEOLJSM-6490LA) and AFM (JEOL JSPM-5200). Surface charge and thereby the stability of the nanoconjugate systems were obtained through zeta potential measurements (DLS-ZP/Particle Sizer Nicomp™ 380 ZLS). FT-IR spectra were recorded on Perkin Elmer Spectrum RX1 to obtain evidence for the surface conjugation of GNP by citrate ions and PEI. The samples for FT-IR analysis were pelletised using KBr. MTT assay for cytotoxicity studies were done using a micro plate reader (BioTek PowerWave XS). Nuclear morphology changes were analysed using inverted fluorescent microscope (Olympus-BX-51).
2.4. In vitro cell culture studies
Human lung carcinoma type II epithelial cells, A549 cell line and human breast cancer cells MCF-7, were routinely maintained in T-25 tissue culture flasks in minimal essential medium (MEM) along with 10% heat-inactivated foetal bovine serum and 100 units/mL, penicillin/streptomycin and incubated at 37°C in a humidified 5% CO2 incubator. GNP/citrate and GNP/PEI were used to identify the cellular response. GNP dispersion was prepared in 2 mM stock concentration by dissolving in PBS (pH 7.2).
2.5. Assessment of cellular responses
2.5.1. MTT assay
The cells were treated with various concentrations of GNP for a period of 48 h. After treatment, the culture medium was replaced with serum-free medium containing 0.5 mg/mL. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and incubated for 3 h. The blue MTT formazan crystals formed were dissolved in a buffer containing isopropyl alcohol with the specified percentage of HCl, and the absorbance was measured using a UV-VIS spectrophotometer. The untreated cells were used as a positive control (i.e., 100% viable), and all values from the experiment were correlated with this set of data.
2.5.2. Flow cytometry-Annexin V assay
The cell lines were treated with GNP/citrate and GNP/PEI and incubated for a period of 48 h to account for any apoptotic response. The cells were then detached with 0.05% trypsin–EDTA solution and pelleted. Apoptosis assay was conducted using FITC-conjugated Annexin V assay kit. After staining, the cells were washed and resuspended in PBS and analysed using FACS Aria II system (BD biosciences). The percentage of viable/apoptotic cells was then estimated using the FACS Diva software.
2.5.3. Analysis of cellular morphology change
The cells were grown over circular cover slips on a 24-well culture plate with a seeding density of 3 × 103 cells per well. The cells were incubated with GNP/citrate and GNP/PEI particles for 48 h. Following incubation, the cells were fixed with 2.5% glutaraldehyde. The cover slips were then washed with sterile PBS to remove the glutaraldehyde and dehydrated with a graded series of ethanol. Further dehydration was performed using acetone and then with isoamyl acetate. The cover slips were coated with an ultra-thin platinum layer and observed under SEM.
2.5.4. Analysis of nuclear morphology change
To study the nuclear-specific modifications, the cells were grown over circular cover slips on a 24-well culture plate with a seeding density of 3 × 103 cells per well. The cells were added with GNP/citrate and GNP/PEI particles and incubated for 48 h. After incubation, the cells were fixed with 2% para formaldehyde. The cover slips were then washed with sterile PBS to remove the para formaldehyde and stained with DAPI. The cover slips containing cells were mounted over clean grease-free glass slide and imaged in different magnifications using oil-immersion objectives under a fluorescent microscope equipped with CCD camera.
2.5.5. DNA fragmentation assay
The A549 cells were incubated for 48 h with GNP/citrate and GNP/PEI. Following incubation, the cells were detached using 0.05% trypsin–EDTA solution and pelleted by centrifugation. The pellet was resuspended in sterile PBS. The total genomic DNA was isolated using Trizol reagent and stored at −20°C in TE buffer. The isolated DNA was checked by electrophoresis using 1% agrose gel. Standard wide-range DNA ladder was used as the reference marker. The gel was then imaged under gel documentation unit (Bio-Rad Chemi Doc XRS System).
2.6. Statistics
Statistical analysis of the data was performed via one-way analysis of variance (ANOVA) using origin software; a value of p<0.05 was considered significant (n = 3).
3. Results and discussion
3.1. Characterisations
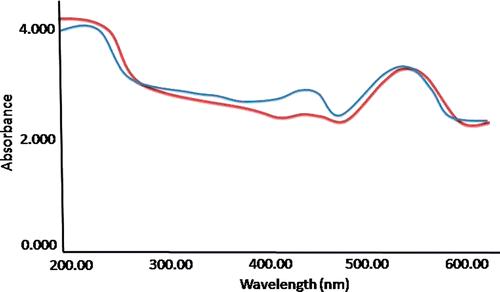
The characteristic UV spectrometric peaks for plasmon resonance (λ max) of GNP/citrate and GNP/PEI were found to be as 515 and 517 nm, respectively (). The existence of strong plasmon response (SPR) was also evident because of the substantial absorbance of gold nanoparticles recorded at around 520 nm. This phenomenon is reported earlier Citation11 and considered as responsible for imparting the ruby red colour to the conventional gold colloids. From this experiment, it was evident that surface conjugation of GNP with citrate is not creating any differences in the fundamental affluence of GNP, but only modifying its surface texture.
Figure 1. (Colour online) Absorption spectra of GNP functionalised with citrate in blue and GNP functionalised with PEI in red.
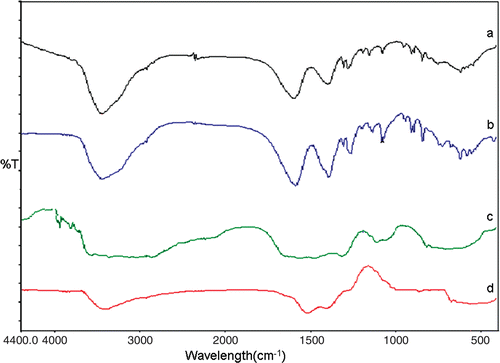
The evidence of the surface functionalisation of GNP by citrate ions was obtained by FT-IR measurements. The characteristic peaks at 1395 and 1586 cm−1 () correspond to the symmetric and anti-symmetric stretching of COO−. These data confirm the interaction between citrate ions and GNP. The formation of spherical GNP was found to be dependent highly on the stabilising capacity of citrate ions present at the surface. Surface functionalisation of GNP using citrate ions can be performed in a controlled manner. On the other hand, the GNP was functionalised with PEI showed a characteristic carbonyl absorption of imide and ester at around 3000 and 3400 cm−1 (), respectively, which was reported earlier by Kim et al. Citation12. Poly(ethyleneimine) is a branched chain polymer having a ratio of 1:2:1 of primary:secondary:tertiary amines with a branching site for every 3–3.5 nitrogen atoms and a general backbone of (CH2CH2NH) x . Indeed, every third atom of PEI is a protonable amino nitrogen atom, which makes the polymeric network an effective ‘proton sponge’. The optimal cation/anion balance of PEI conjugated in vitro transfection systems was assessed earlier by Boussif et al. Citation13 and in our experiments also it was analysed that ideal cation/anion ratio is necessary for the stabilisation of conjugated nanosystems.
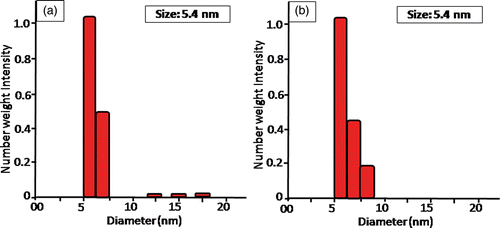
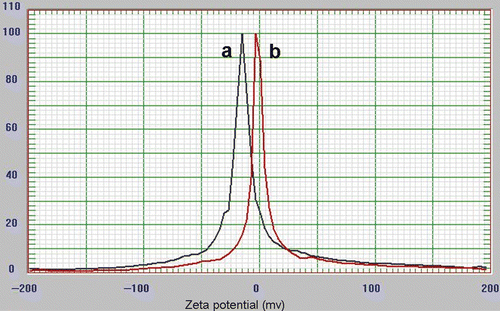
The distribution of GNP at the nano scale was substantially proved by DLS, as small intense peaks were obtained at 5–10 nm for GNP/citrate () and GNP/PEI (), denoting the formation of highly monodispersed and stable GNP. The stability of monodispersed colloidal systems was quantitatively analysed through zeta potential distribution (). The GNP/citrate is analysed to have a net zeta potential of −30 mV on its surface while the GNP/PEI has a net zeta potential of −10 mV.
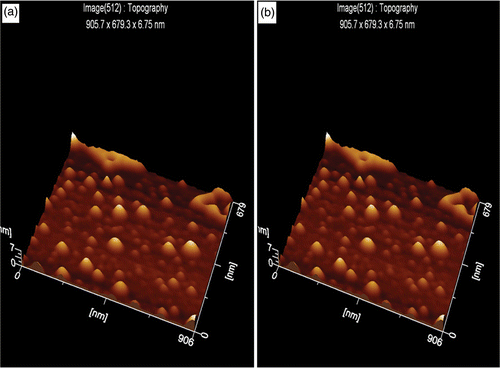
The topographic images of both GNP/citrate () and GNP/PEI () were obtained through AFM, and well-dispersed layer functionalised nanoparticles were identified. The cantilever tip of AFM is of silicon nitride; when brought into the proximity of the sample surface, forces between the tip and the sample lead to deflection of the cantilever, thus following the Hooke's law.
3.2. Cell viability assay
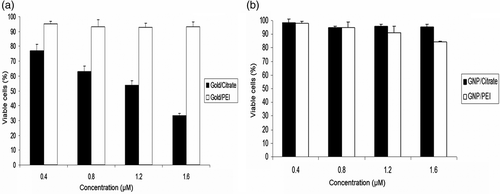
The cell viability assay performed by MTT clearly demonstrates that the A549 cells showed a rapid decrease in viability with respect to increase in concentration of GNP/citrate from 0.4 to 1.6 µM. However, the GNP/PEI did not show any reduction in the cell viability under the same concentrations (). The cell viability assay for MCF-7 showed no reduction in the number of viable cells () when treated with GNP/citrate and GNP/PEI.
Figure 6. (a) Cell viability assay by MTT shows reduction in viability of A549 cells with increase in concentrations of GNP/citrate nanoparticles. No significant effect on cellular viability was observed with GNP/PEI and (b) cell viability assays by MTT shows no significant effect on the viability of MCF-7. No significant reduction in viability observed in GNP/PEI and GNP/citrate on MCF-7.
3.3. Flow-cytometric analysis for cellular apoptosis
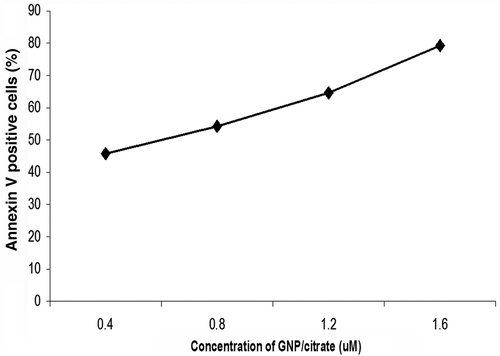
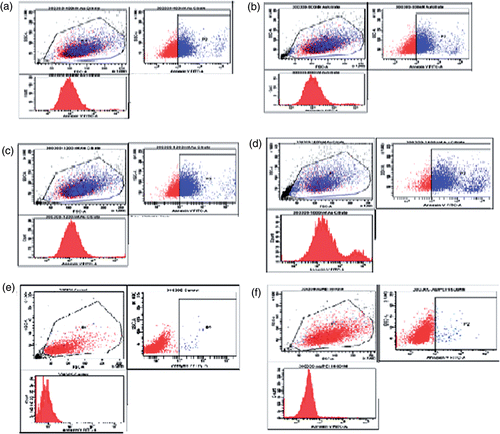
Cellular response to surface-functionalised GNP was evaluated by staining for phosphatidylserine (PS) molecules that are expressed on the surface of the apoptotic cells using Annexin V stain Citation14 after treatment with GNs for 48 h in different concentrations. Quantification of apoptosis was performed by flow cytometric analysis. The unstained cells were used for gating (P1 gating) the cell populations, and this setup was maintained throughout the experiment. The percentage of FITC–Annexin V-stained apoptotic cells were analysed through the respective dot plots of P2 population. The number of apoptotic cells increased proportionally with increase in concentration of GNP/citrate from 0.4 to 1.6 µM ( and ). The Annexin V staining of GNP/PEI-treated A-549 cell lines appeared to be almost identical to that of the control untreated cells (). From these data, it is clearly evident that the citrate-stabilised GNP, but not GNP/PEI, were able to induce apoptosis in a concentration-dependent manner.
Figure 7. (Colour online) Dot plots show that treatment of A549 cells with GNP/citrate leads to apoptosis in a concentration-dependent manner. (a) 0.4, (b) 0.8, (c) 1.2 and (d) 1.6 µM. The gradual shifting of the A549 cell population towards the P2 population with increase in the GNP/citrate concentration indicates increase in the percentage of apoptosis. (e) Dot plot for untreated cells used as control and (f) Dot plots show that treatment of A549 cells with GNP/PEI at 1.6 µM concentration.
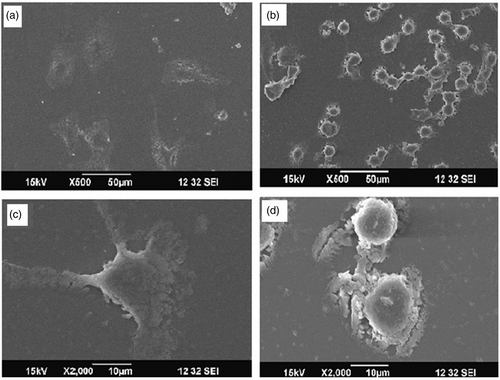
3.4. Analysis of cellular morphology change
The A549 cells treated with GNP/PEI showed a morphology similar to that of the control untreated cells, as they remained adhered on to the cover slip and showed a well-spread morphology with prominent podial expansion (). But in the case of the cells treated with GNP/citrate, the cells were circular and had minimal podial expansion (). It has been proved that the endocytosis of foreign nanoparticles may cause disruption of the cell membrane and disorganisation of cytoskeleton Citation15,Citation16. Hence, the change in morphology due to the shrinkage of cellular membrane of A549 cell lines treated with GNP/citrate can be attributed to the possible cytoskeletal disruption occurred during the endocytosis of GNP.
3.5. Analysis of nuclear morphology change
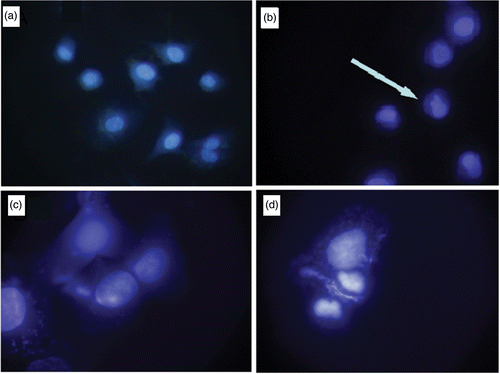
Nuclear condensation due to the induced stress is regarded as one of the signature events of apoptosis Citation17. Significant amount (79%) of A549 cell lines treated with GNP/citrate exhibited nuclear morphology changes which were visualised through DAPI staining (). This can be inferred due to the high level of apoptosis induced by GNP/citrate.
3.6. DNA fragmentation assay
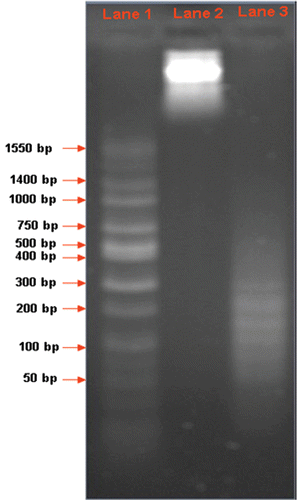
DNA fragmentation assay was performed by isolating total genomic DNA from 1 × 106 A549 cells treated with 1.6 µM GNP/citrate and visualised by agarose gel electrophoresis Citation18. The DNA from the untreated A549 cells was used as the control. Thick band of total genomic DNA can be visualised in an approximate size range of 2.5 Kb, clearly indicating that the DNA from untreated cells are not fragmented. The total DNA isolated from GNP/citrate-treated A549 cells appeared only as a fragmented smear, which directly indicates the possible interruption occurred on lysosome buffering and the subsequent nuclear damage underwent during cellular apoptosis. The isolated DNA fragments were compared with the wide range DNA ladder sequence loaded in lane 1 ().
4. Conclusions
The GNP functionalised with citrate evoke a cell-specific death response in the A549 cell lines. But the same concentration of GNP functionalised with PEI did not show any cytotoxic response to the same cell line. Moreover, GNP functionalised with citrate and PEI failed to induce any death response in MCF-7 cell lines. The negative response obtained among MCF-7 cells can be comparable to the prior report on leukaemia cells as well as cells like BHK21 or HepG2 cell line Citation19. The induction of apoptosis in the human lung carcinoma cell line A549 has been shown to involve concentration dependence of the nanoform. The possibility of GNP/citrate that evokes an apoptotic response in the A549 cell lines can be attributed to the kosmotropic property of citrate as reported by Zhao et al. Citation20. High concentrations of kosmotropic salts have long been a tool of researchers to study protein–protein interactions, as they are capable of salting out proteins from solution, creating local areas of high protein concentrations Citation21–23. This could mean that the GNP/citrate internalised can activate the caspase molecule because of the kosmotropic property of the citrate salt. But the exact reason for the failure to evoke any apoptotic response in the MCF-7 cell line remains unclear at this stage. From our preliminary experimental results, it is evident that unravelling the mechanism of such specific cell responses would definitely enhance the efforts to develop nanoconjugate-based therapeutic strategies and also to analyse the toxicity of nanomaterials.
Acknowledgements
This study was supported by the Department of Science and Technology, Government of India, under the NanoScience and Nanotechnology Initiative motivated by Prof. C.N.R. Rao.
References
- Kam , NWS , Jessop , TC , Wender , PA and Dai , HJ . 2004 . Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells . J. Am. Chem. Soc. , 126 : 6850 – 6851 .
- Colvin , VL . 2003 . The potential environmental impact of engineered nanomaterials . Nat. Biotech. , 21 : 1166 – 1170 .
- Wu. , X , Liu , H , Liu , J , Haley , KN , Treadway , JA , Larson , JP , Ge , N , Peale , F and Bruchez , MP . 2003 . Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots . Nat. Biotech. , 21 : 41 – 46 .
- Derfus , AM , Chan , WC and Bhatia , S . 2004 . Probing the cytotoxicity of semiconductor quantum dots . Nano Lett. , 4 : 11 – 18 .
- Connor , EE , Mwamuka , J , Gole , A , Murphy , CJ and Wyatt , MD . 2005 . Gold nanoparticles are taken up by human cells but do not cause cytotoxicity . Small , 1 : 325 – 327 .
- Pernodet , N , Fang , X , Sun , Y , Bakhtina , A , Ramakrishnan , A , Sokolov , J , Ulman , A and Rafailovich , M . 2006 . Adverse effects of citrate/GNPs on human dermal fibroblasts . Small , 2 : 766 – 773 .
- Mfhlen , KH and Beller , FK . 1979 . Use of radioactive gold in the treatment of pleural effusions caused by metastatic cancer . J. Cancer Res. Clin. Oncol. , 94 : 81 – 85 .
- Glomm , WR . 2005 . Functionalized gold nanoparticles for applications in bionanotechnology . J. Dispers. Sci. Technol. , 26 : 389 – 414 .
- Moghimi , SM , Hunter , AC and Murray , JC . 2005 . Nanomedicine: current status and future prospects . FASEB J. , 19 : 311 – 330 .
- Hu , J , Wang , Z and Li , J . 2007 . Gold nanoparticles with special shapes: controlled synthesis, surface- enhanced Raman scattering and the application in biodetection . Sensors , 7 : 3299 – 3311 .
- Zou , X , Ying , E and Dong , S . 2006 . Seed-mediated synthesis of branched gold nanoparticles with the assistance of citrate and their surface-enhanced Raman scattering properties . Nanotechnology , 17 : 4758 – 4764 .
- Kim , BJ , Kim , SJ and Park , SY . 1998 . Thermally stable nonlinear optical polymer from Rosin derivative . J. Indus. Eng. Chem. , 4 : 238 – 244 .
- Boussif , O , Lezoualc’h , F , Zanta , MA , Mergny , MD , Scherman , D , Demeneix , B and Behr , JP . 1995 . A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine . PNAS , 92 : 7297 – 7301 .
- Nabhan , C , Gajria , D , Krett , NL , Gandhi , V , Ghias , K and Rosen , ST . 2002 . Caspase activation is required for Gemcitabine activity in multiple Myeloma cell lines . Mol. Cancer Ther. , 1 : 1221 – 1227 .
- Gupta , AK and Curtis , ASG . 2004 . Surface modified superparamagnetic nanoparticles for drug delivery: interaction studies with human fibroblasts in culture . J. Mater. Sci.: Mater. Med. , 15 : 493 – 496 .
- Zhengwei , M , Wang , B , Lie , M , Changyou , G and Jiacong , S . 2007 . The influence of polycaprolactone coating on the internalization and cytotoxicity of gold nanoparticles . Nanomed. Nanotech. Biol. Med. , 3 : 215 – 223 .
- Jurisicova , A , Varmuza , S and Casper , RF . 1996 . Programmed cell death and human embryo fragmentation . Mol. Hum. Reprod. , 2 : 93 – 98 .
- Park , HJ , Lyons , JC , Ohtsubo , T and Song , CW . 2000 . Cell cycle progression and apoptosis after irradiation in an acidic environment . Cell Death Differ. , 7 : 729 – 738 .
- Patra , HK , Banerjee , S and Chaudhuri , U . 2007 . Cell selective response to gold nanoparticles . Nanomedicine , 3 : 111 – 119 .
- Zhao , H , Olubajo , O , Song , Z , Sims , AL , Person , TE , Lawal , RA and Holley , LA . 2006 . Effect of kosmotropicity of ionic liquids on the enzyme stability in aqueous solutions . Bioorg. Chem. , 34 : 15 – 25 .
- Schmidt , U and Darke , PL . 1997 . Dimerization and activation of the herpes simplex virus type 1 protease . J. Biol. Chem. , 272 : 7732 – 7735 .
- Stennicke , HR and Salvesen , GS . 2000 . Caspase assays . Methods Enzymol. , 322 : 91 – 100 .
- Donepudi , M , Mac Sweeney , A , Briand , C and Gruetter , MG . 2003 . Insights into the regulatory mechanism for caspase-8 activation . Mol. Cell. , 11 : 543 – 549 .