Abstract
Chitosan–DNA nanoparticles employed in gene therapy protocols consist of a neutralised, stoichiometric core and a shell of the excess of chitosan which stabilises the particles against further coagulation. At low ionic strength, these nanoparticles possess a high stability; however, as the ionic strength increases, it weakens the electrostatic repulsion which can play a decisive part in the formation of highly aggregated particles. In this study, new results about the effect of ionic strength on the colloidal stability of chitosan–DNA nanoparticles were obtained by studying the interaction between chitosans of increasing molecular weights (5, 10, 16, 29, 57 and 150 kDa) and calf thymus DNA. The physicochemical properties of polyplexes were investigated by means of dynamic light scattering, static fluorescence spectroscopy, optic microscopy, transmission electronic microscopy and gel electrophoresis. After subsequent addition of salt to the nanoparticles solution, secondary aggregation increased the size of the polyplexes. The nanoparticles stability decreased drastically at the ionic strengths 150 and 500 mM, which caused the corresponding decrease in the thickness of the stabilising shell. The morphologies of chitosan/DNA nanoparticles at those ionic strengths were a mixture of large spherical aggregates, toroids and rods. The results indicated that to obtain stable chitosan–DNA nanoparticles, besides molecular weight and N/P ratio, it is quite important to control the ionic strength of the solution.
Key Words:
1. Introduction
Chitosan has been recognised in the past decade as a good candidate for gene delivery due to its properties as low toxicity, biodegradability and versatility as a carrier for intravenous and oral administration Citation1,Citation2. Also, the ability to condense efficiently with DNA forming tight polyplexes is important to avoid the degradation by DNAses Citation3. The chitosan–DNA interaction is driven mainly by the electrostatic interaction between the amino groups of chitosan and the charged phosphate groups of DNA Citation4,Citation5 and in a slightly acid medium, this interaction is fully favoured since the most of the amine groups will be charged. In this respect, many advances have been made on the effects of various parameters as molecular weight, charge (±) ratio, pH and particle size on the transfection efficiency. Many studies carried out at low pH showed that polyplexes made from mixing DNA with chitosan samples varying from 8 to 200 kDa yielded a blend of stable nanoparticles formed from toroids and rods Citation6,Citation7. The amount of chitosan required to fully compact DNA into well-defined toroidal and rod-like structures were found to be strongly dependent on the chitosan molecular weight, and thus its total charge. A higher charge ratio (±) was needed for the shorter chitosans, showing that an increased concentration of the low degree of polymerisation (DP) chitosan could compensate for the reduced interaction strength of the individual ligands with DNA Citation7. Well-defined oligomers of completely deacetylated chitosan ranging in molecular weight from 1.2 to 2.8 kDa were also able to form more stable nanoparticles at lower pH values. The longer chitosan oligomers formed soluble globules with DNA at lower charge ratios. As the chain length of chitosan increases, the binding with DNA may occur in a more cooperative manner, and soluble chitosan–DNA globules are formed at lower charge ratios Citation8,Citation9. Liu et al. Citation10 have showed that the charge density is important by studying the formation of polyplexes at different pH values. Chitosan–DNA nanoparticles formed with 5 kDa deacetylated chitosan indicated that upon interacting with DNA, the strength of binding was dependent on the pH of media. At pH 5.5, the charged chitosan had a strong binding affinity for DNA, whereas in pH 12.0 medium, only weak interactions existed. The authors also reported that no typical toroid patterns were observed, which was attributed to the compact form of DNA caused by highly charged chitosan.
Besides the progress about the DNA–chitosan interaction, much less attention has been paid on the stability of the nanoparticles as a function of ionic strength. This parameter is particularly important for transfection efficiency, since the particle size may be affected by ionic strength decreasing the blood circulation time and the cellular uptake Citation11–13. Jiang et al. have also shown that chitosan/DNA complexes challenged with foetal calf serum started to aggregate rapidly and complexes with a size above 1000 nm were detected after 10 min, which may affect the gene transfection Citation14. Koping-Hoggard et al. have shown that the molecular weights of the chitosans are not important for the transfection efficiency in the molecular weight interval 31–200 kDa Citation15. However, an increased ionic strength showed that complexes of T4 DNA with chitosan oligomers formed aggregates at NaCl concentrations smaller than 100 mM, and at higher NaCl concentrations (up to 100 mM), the aggregates dissociated and formed elongated coils, which were attributed to free T4 DNA. Therefore, the background of these previous studies clearly showed that the stability of the chitosan–DNA polyplexes is dependent on the molecular weight, ionic strength and charge ratio (N/P).
In this study, we provide new insights on the effects of ionic strength on the interaction of chitosan–DNA and the stability of the formed nanoparticles. We used purified deacetylated chitosan samples ranging in molecular weight from 5 to 150 kDa (5, 10, 15, 28, 50 and 150 kDa) and the light scattering technique to study the stability of complexes formed between chitosan and calf thymus DNA as a function of time. This technique allowed us to carefully evaluate how the stability of the polyplexes is affected by the solution ionic strength and its dependence on molecular weight and charge ratio (N/P). The shapes of the complexes were examined by transmission electronic microscopy (TEM) at varying salt concentrations and pH values, in order to evaluate if toroids could be formed at increasing salt conditions. The formation of soluble aggregates was also monitored by fluorescence microscopy using the rhodamine-labelled chitosans samples. Electrophoresis and the ethidium bromide assay of the polyplexes were also used to assess the effect of the added salt on the strength of interaction and dissociation of the complexes.
2. Materials and methods
2.1. Materials
Chitosans (57 and 150 K) used as starting materials were purchased from Wako Chemical Co. Water was deionised using a Milli-Q water purification system (Millipore). Rhodamine isothiocyanate, sodium periodate, sodium hydroxide, sodium acetate, acetic acid, and sodium nitrite were purchased from Aldrich Chemical Co. All solvents were of reagent grade and used as received. Spectra/pore membranes (Spectrum) were employed for dialysis. The sodium salt of highly polymerised calf thymus DNA (10–15 k base pairs) was purchased from Sigma (St. Louis, USA) and used without further treatment. The protein contamination was determined to be negligible since A 260/A 280 = 1.90, an indication of the DNA purity Citation16.
2.2. Instrumentation
1H-NMR spectra were recorded on a Bruker ARX-400 400 MHz spectrometer. UV-vis spectra were measured with a Cary 100 spectrometer. Fluorescence measurements were obtained using the Fluorimeter Hitashi F4500.
2.3. Deacetylation of chitosan
Deacetylated chitosan was prepared as reported previously Citation17. Briefly, a solution of chitosan (CH) (4.0 g, 2.45 × 10−3 mol of monosaccharide units) in aqueous acetic acid (200 mL, 2 wt%) was added dropwise to aqueous NaOH (100 mL, 50 wt%) at room temperature under magnetic stirring in a nitrogen atmosphere. At the end of the addition, the suspension was refluxed for 1 h. It was poured into stirred water (4 L) preheated to 80°C. The precipitate was decanted, washed five times with water and separated by filtration. The solid was dissolved in acetic acid (200 mL, 2 wt%) and subjected to the same procedure once more to achieve a higher deacetylation degree. The resulting polymer was purified by dialysis against water for 3 days and isolated by lyophilisation.
2.4. Degradation of CH 57 kDa
First, 0.51 g of chitosan (CH) was dissolved in acetic acid solution (30 mL, 2%). After complete dissolution, dissolved oxygen was removed by bubbling N2 gas in the solution for 1 h. The mixture was then cooled to 4°C using a water-ice bath. A freshly prepared solution of NaNO2 (2 mL, 0.06 M) was poured into the solution and the reaction was allowed to continue in darkness, at 4°C for 24 h. The polymer was purified by dialysis (Mw 6000–8000 cut off, spectra/pore) against deionised water for 2 days, 1 day against NaOH 0.05 M and 2 days in water (water was changed three times a day), and isolated by lyophilisation. The reaction yielded 0.334 g of product. The same procedure was employed to obtain 5 kDa chitosan, increasing the NaNO2 concentration (2 mL, 0.18 M) and using an MWCO 3000 dialysis membrane.
2.5. Rhodamine-labelled chitosans
The labelled chitosans were prepared using the rhodamine isothiocyanate derivative. The procedure is illustrated below for the synthesis of rhodamine-labelled chitosan. Chitosan 57 kDa (218 mg) was dissolved overnight in acetic acid solution (20 mL, 0.02 M). Thereafter, the pH of the solution was adjusted to 6.0 by adding aqueous NaOH (0.05 M) and the solution degassed and kept under N2 atmosphere. Next, rhodamine isothiocyanate (12 mg) was dissolved in methanol (5 mL) and injected dropwise into the chitosan solution under stirring. The solution was protected from light throughout the experiment and the reaction time was 24 h. The reaction mixture was dialysed (membrane of MWCO 12000–14000 or MWCO 3000) first against water for 3 days, then against aqueous NaOH (0.05 M) for 1 day and finally against water for 3 days. To determine the labelling efficiency, solutions of the rhodamine-labelled chitosans were prepared in acetic acid buffer/methanol (v/v) at pH = 4.5 and the absorbance measured at 554 nm. A calibration curve was prepared with solutions of 3.5 × 10−6 to 2 × 10−5 mol/L using a stock solution of rhodamine derivative (0.93 mg/10 mL). The other rhodamine-labelled chitosans were prepared under identical conditions to yield from 0.8% to 1.45% substituted amino groups ().
Table 1. Properties of the deacetylated chitosans.
2.6. dn/dC and molecular weight measurements
Molecular weight characteristics were obtained using a GPC system consisting of an Agilent 1100 isocratic pump, a TSK-GELPW (Tosoh Biosep) column, a Dawn EOS multi-angle laser light scattering detector (Wyatt Technology Corp.) and an Optilab DSP interferometric refractometer (Wyatt Technology Corp.); injection volume: 100 µL; flow rate: 0.5 mL/min; eluent: acetic acid (0.3 M)/sodium acetate (0.2 M) buffer pH 4.5, 25.0°C. The samples were prepared by weighing the exact amount to obtain solutions varying from 0.5 to 1.0 mg/mL followed by stirring for 3 days and filtering (0.45 µm) immediately before the injection.
For dn/dC measurement experiments, a stock solution of each PC–CH was prepared by dissolution of 1.0 mg/mL in 5 mL of acetic acid (0.3 M)/sodium acetate (0.2 M) buffer at pH 4.5. Then, the stock solution was diluted in the buffer to yield five final polymer concentrations varying between 1.0 and 0.2 mg/mL. These solutions were stirred for 3 days. A part of each solution was filtered in a 0.45 µm filter, and the refractive index increments were measured consecutively using a differential Optilab DSP interferometric refractometer (Wyatt Technology Corp.). All data were acquired, stored and processed with the software Astra V 5.1.5.
2.7. Chitosan/DNA nanoparticle formation for dynamic light scattering as a function of time
Prior to preparation, the milli-Q water and all buffers were filtered through 5 µm membrane, the test tubes having previously been washed with milli-Q water. CH samples were dissolved in the appropriate buffers (acid acetic/acetate, pH 4.0 and phosphate pH 6.3) at room temperature. A stock solution of calf thymus DNA was added to test tubes containing 1 mL of the appropriate buffer to adjust the concentration of phosphate groups to 2.5 × 10−5 M as determined by the UV absorption at 260 nm, using a molar extinction coefficient, 6600 M−1cm−1. Stock solutions of the CH samples were used to titrate DNA solutions, which were vortexed briefly (30 seg.) to obtain different N/P (nitrogen/phosphate) ratios. Nanoparticles for light scattering measurements were prepared at ionic strengths 10, 150 and 500 mM. Nanoparticles were prepared inside the cuvettes by titration of ct-DNA solution with stock solutions of the chitosan samples to obtain increasing N/P ratios. Dynamic light scattering (DLS) experiments were performed on a Zetasizer nano-ZS90, a He–Ne laser (λ = 633 nm) and 90° scattering angle. The temperature was 25°C unless otherwise stated. The measurements were prepared in triplicate and the average sizes used.
2.8. Ethidium bromide displacement assay
A stock solution of ethidium bromide (EtBr, 2.5 × 10−3 M) was prepared in water and this solution (2.5 µL) was added to the cuvettes containing the appropriate buffers (2 mL of ionic strength 10, 150 and 500 mM at pH 4.0 and 6.3) and mixed by gentle agitation. A stock solution of calf thymus DNA was added to the solution to obtain a concentration of 1.6 × 10−5 M of phosphate. The solution was left to rest for 24 h. Then, solutions containing the DNA–ethidium bromide (DNA–EtBr) complex were titrated into the cells to reach a predetermined monomer:nucleotide molar ratio (N/P ratio) using micropipette. After each addition, the solution was briefly stirred prior to measurement of the fluorescence (three readings). The excitation wavelength (λ ex) was set at 560 nm and the emission wavelength (λ em) at 605 nm. Steady-state fluorescence spectra were recorded on a Hitashi 4500 spectrofluorimeter. The temperature control of the samples was achieved using a water-jacketed cell holder connected to a Fisher circulating water bath. All measurements were carried out at 25.0 ± 0.1°C unless otherwise stated and the slit settings were 5 nm for both excitation and emission. The total chitosan solution added to the DNA solution did not exceed 5% of the total volume of the solution, and hence no correction was made for sample dilution. In the experiments performed at higher pH values, samples of calf thymus DNA solutions were prepared using 10 mM phosphate (7.0, 7.4) buffers. The stock chitosan solutions were kept at pH 6.3 to avoid precipitation and the absence of phase separations in these dilute mixtures was confirmed by visual inspection of the titrated solutions.
2.9. Gel electrophoresis
Gel electrophoresis was performed with a 0.75% agarose gel in 40 mM Tris-acetate-EDTA (TAE) buffer at pH 7.4. The ct-DNA-chitosan nanoparticles of N/P ratios 1–12 were prepared at a constant DNA concentration and incubated for 120 min at room temperature. Next, complex solution (8 µl, 30 µg/mL of DNA) was loaded into the agarose gel wells. After electrophoresis (90 V, 1.17 h), the DNA was stained by incubating the gels in TAE buffer of pH 7.4 containing 15 µL EtBr (1.0 mg/mL) for 30 min.
2.10. Photomicroscopy of the chitosan–DNA polyplexes
The polyplexes were prepared at an N/P of 5 and final ct-DNA concentration of 25 µg/mL in 5 mM phosphate of pH 6.3, with ionic strengths 10, 150 and 500 mM. All measurements were carried out at 25°C. Photomicrographs of the chitosan–DNA nanoparticles were performed on a Zeiss-Jenaval (Jena, Germany) fluorescence photomicroscope equipped with a fluorescence filter for rhodamine.
2.11. Transmission electronic microscopy
The nanoparticles were prepared with 70 µM of phosphate groups of DNA and chitosans CH 5 kDa and CH 150 kDa at an N/P ratio of 10 in 10 mM phosphate buffer at pH 6.3 and different ionic strengths (10, 150 and 500 mM). The samples were incubated at room temperature for 4 h. One drop of the complex solution was deposited on the copper grid (carbon-coated copper grid, 200 mesh). The grids were allowed to dry for 30 min, post-stained with uranyl acetate and then images recorded using a LEO–Zeiss 906 Transmission Electron Microscope operating at 80 kV.
3. Results and discussion
3.1. Dynamic light scattering
The molecular characteristics of the all chitosans samples used for the preparation of CH–DNA nanoparticles are presented in . The chitosan samples employed (Mw 5, 10, 16, 28, 57 and 150 kDa) were deacetylated efficiently and had similar deacetylation degrees (98–99%) determined from potentiometric titration and HNMR. The chromatograms for chitosan samples having different molecular weights exhibited a highly distinct Gaussian shape for all traces (data not shown).
Initially, DLS measurements were performed to evaluate the effect of the ionic strength on the sizes and stability of the nanoparticles formed by interaction between DNA and chitosans of different molecular weights. As expected, in general, when pH and ionic strength were kept low (pH 4.0, ionic strength 10 mM) nanoparticle sizes remained smaller than 250 nm as seen from , independent of the chitosan molecular weight and N/P ratio. However, the lower molecular weight sample (5 kDa) formed the smaller nanoparticles with diameters ranging from 120 to 140 nm and the higher molecular chitosan (CH 150 kDa) exhibited nanoparticle sizes ranging from 200 to 240 nm, which is in agreement with results previously published on calf thymus DNA using the same range of chitosan molecular weight Citation18.
Table 2. Hydrodynamic diameters (Dh) of the complexes prepared from chitosan with calf thymus DNA at pH 4.0 and ionic strength 10 mM.
The effect of an increasing ionic strength on the nanoparticles properties may appear in two ways; first, the screening effect of the added salt can induce nanoparticles aggregation and second, dissociation of the complexes may take place due to the decreased electrostatic interaction between DNA and chitosan. In general, at pH 4.0, the results showed that the nanoparticle sizes remained constant during weeks independent of the chitosan molecular weight; however, the diameters would be expected to increase with ionic strength, since even at low pH, the screening effect of the added salt could influence the nanoparticle sizes, and this in fact happened but no large aggregates were observed. This was tested at 10–500 mM of ionic strength with nanoparticles prepared with CH 57 kDa at N/P ratio 5.0 and the sizes increased from 150 to 250 nm (data not shown).
At higher pH values, the ionisation degree of chitosan is decreased and the screening effect of the added salt was more pronounced. shows the nanoparticle sizes vs. time prepared at pH 6.3 and ionic strength of 10 mM. At ionic strength 10 mM and N/P ratios lower than 7.0, the nanoparticles synthesised with chitosan CH 5 kDa, CH 16 kDa, CH 29 kDa and CH 150 kDa samples exhibited increasing sizes as a function of time, except those prepared with CH 150 kDa, which exhibited a constant size around 250 nm for N/P 5.0 (). For instance, at N/P ratios smaller than 5.0 hydrodynamic diameters (Dhs) of nanoparticles prepared with CH 5 kDa and CH 29 kDa increased from values around 200 nm to 1000–1500 nm after 3 h.
Figure 1. Nanoparticle sizes as a function of time for complexes of ct-DNA with (a) CH 5 kDa, (b) CH 16 kDa, (c) CH 29 kDa and (d) CH 150 kDa at pH = 6.3 and ionic strength 10 mM at varied N/P ratios.
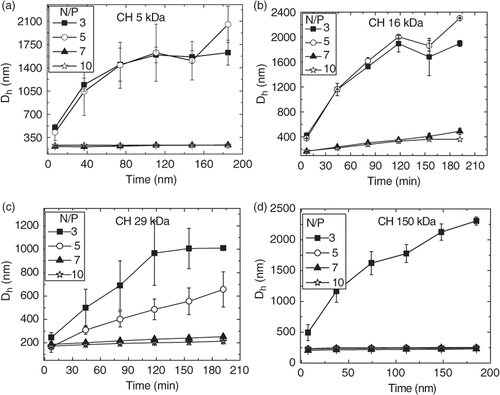
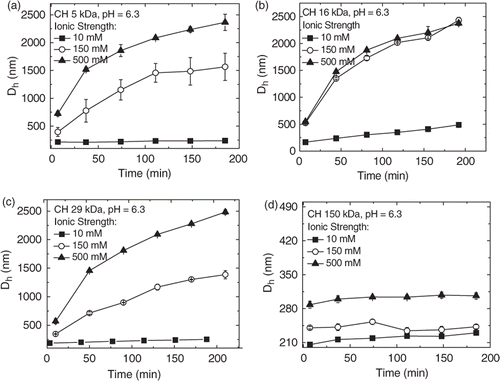
Generally, the dimensions of complexes prepared with linear DNA are higher than those observed with smaller plasmids Citation7. Although these large structures could at least partly arise from the polydispersity of the employed linear DNA, it has been recently reported that, even at low pH, large plasmids (8–10 kbp) may lead to the formation of chitosan–DNA nanoparticles varying from 250 to 2500 nm for N/P ratios smaller than 10 Citation19. However, our measurements show that at N/P ratio 7.0 and pH 6.3, the Dhs remained constant for all nanoparticles. Aiming to evaluate the effect of the added salt on the stability of the nanoparticles at this ratio (7.0), Dh was monitored during 3 h in solutions having ionic strengths 10, 150 and 500 mM. As the ionic strength was increased from 10 to 150 mM, the hydrodynamic diameters (Dhs) increased continuously for nanoparticles prepared with the lower molecular weight chitosans (CH 5 kDa and CH 16 kDa) (). As seen from , a similar increase was observed for 29 kDa, which is in agreement with those obtained by Jiang et al. for nanoparticles prepared with deacetylated chitosan 47 kDa and a similar aggregation was observed even in a 25 mM at pH 5.5 acetate buffer Citation14. Strand et al. also recently reported aggregation behaviour working on deacetylated chitosans varying from 4.7 to 33 kDa. The osmolality of the formulations was adjusted to 300 mosM/kg by dilution with a hypertonic medium and all polyplexes aggregated rapidly forming structures with sizes higher than 1 µm. The authors concluded that the degree of aggregation increased slightly with Mw Citation20. On the other hand, nanoparticles prepared with CH 150 kDa, the hydrodynamic diameters increased from 210 to 280 nm from 10 to 500 mM of ionic strength but their sizes remained constant as a function of time (). These results clearly show that nanoparticles prepared at N/P ratios lower than 7.0 achieve stable sizes only after a period of time, which can vary from 2 to 3 h after preparation and are dependent on the molecular weight, pH and ionic strength.
Figure 2. Nanoparticle sizes as a function of time for complexes of ct-DNA with (a) CH 5 kDa, (b) CH 16 kDa, (c) CH 29 kDa and (d) CH 150 kDa at pH = 6.3 and increasing ionic strengths at N/P ratio 7.0.
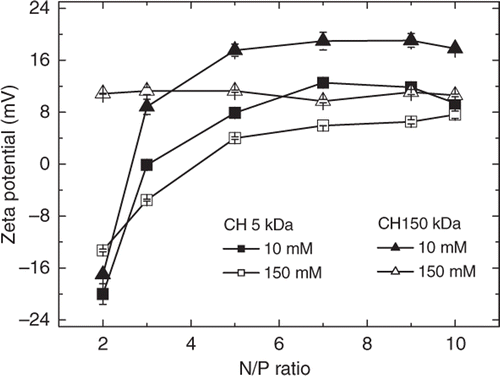
It has been shown that the pH and increasing ionic strengths can change the surface charge and accordingly the sizes of organic Citation21 and inorganic Citation22 nanoparticles. On increasing the ionic strength from submillimolar range to that of the physiological environment, the charge surface may affect the cellular uptake of nanoparticles in vitro and in vivo Citation23. Aiming to verify if increasing ionic strengths could affect the surface charge of the chitosan–DNA nanoparticles, the zeta potentials were measured for nanoparticles prepared with 5 and 150 kDA chitosans at ionic strengths 10 and 150 mM. The aggregation verified in the light scattering measurements is closely related to the zeta potential measurements obtained at pH 6.3 (). At low ionic strength (10 mM), nanoparticles synthesised with CH 5 kDa reached positive zeta potential values only for N/P ratios higher than 5.0 (+4.0 mV) while those synthesised with CH 150 kDa exhibited positive potentials at N/P 3.0 (+8 mV). At ionic strength 150 mM, the zeta potentials of nanoparticles obtained with both chitosans, CH 5 kDa and CH 150 kDa, were greatly decreased and at N/P ratio 7.0 these values were around +4 and +10 mV, respectively. In general, at N/P ratios higher than 5.0–6.0, the zeta potential reached a plateau which implies that the added chitosan does not participate in complexation remaining dissolved in solution as free polymer chains. This proposition has been recently supported by the analysis of nanoparticles using the asymmetrical flow field fractionation (AF4) technique, which revealed that for nanoparticles prepared with 6.4 kb EGFPLuc plasmid at N/P ratio 5.0, about 73% of the chitosan remained free in the dispersion and that the DNA/chitosan complexes had a broad size distribution ranging from 20 to 160 nm in hydrodynamic radius Citation24.
3.2. Photomicroscopy of the rhodamine-labelled chitosan–DNA polyplexes
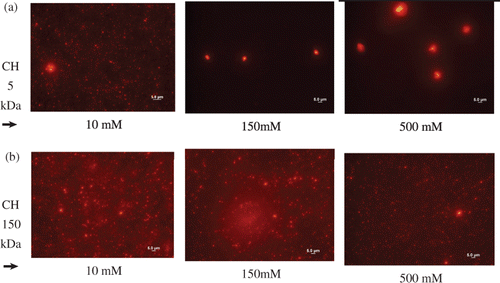
The nanoparticles sizes were qualitatively assessed by fluorescence microscopy using the rhodamine-labelled chitosan derivatives for the preparation of the nanoparticles. Measurements were taken after 3 h at N/P ratio 5.0 and pH 6.3. The imaging observation correlated well with the data obtained from light scattering measurements. shows the photomicrographies taken from nanoparticles prepared with rhodamine-labelled chitosans 5 and 150 kDa at pH 6.3 and increasing ionic strengths. At lower ionic strength, 10 mM, the photomicrographies from nanoparticles synthesised with CH 5 kDa and CH 150 kDa were similar and sizes smaller than 1000 nm were mainly observed as red dots (). As seen from , photomicrographies from nanoparticles formed with CH 150 kDa showed no aggregation at increasing ionic strengths, which is in agreement with results observed in the light scattering measurements (). However, in buffered higher ionic strength solutions, 150 and 500 mM, large aggregates were observed for nanoparticles prepared with CH 5 kDa, whose sizes varied from 3 to 5 µm (, 150 and 500 mM).
3.3. Morphology of chitosan–DNA nanoparticles by TEM
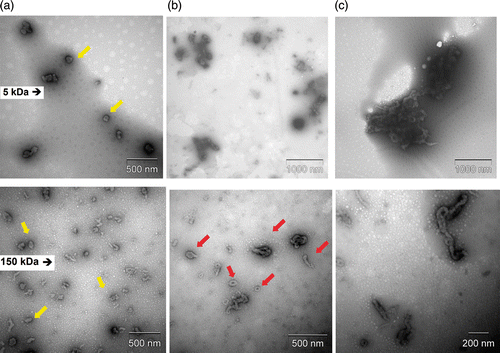
Aiming to determine if ionic strength could affect the shapes and stability of the nanoparticles, TEM was employed. shows TEM images taken after 3 h of preparation of the chitosan–DNA nanoparticles synthesised with CH 5 kDa and CH 150 kDa, at pH 6.3, N/P ratio 7.0 in different ionic strengths. At 10 mM of ionic strength, the nanoparticles prepared with CH 5 kDa and CH 150 kDa appeared mainly as spheroids or rods (arrows). However, a mix of toroids, spheroids and rod-like structures were observed for nanoparticles prepared with 150 kDa at 150 mM of ionic strength (arrows, ). At 500 mM of ionic strength, the images of the particles showed a clear increase in size mainly for nanoparticles prepared with CH 5 kDa. The obsverved aggregates in are an indication that dissociation of the complexes did not occur after 3 h even for the highest ionic strength studied.
3.4. Ethidium bromide assay
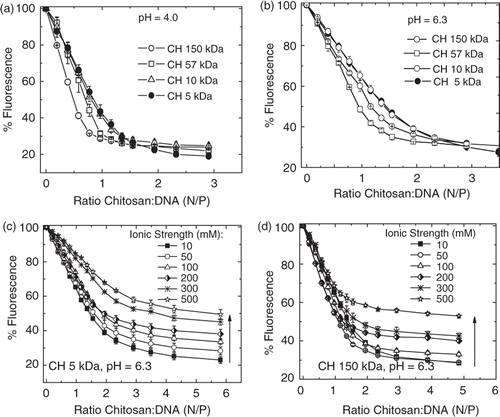
The interaction of CH with calf thymus DNA was also monitored by the fluorescence quenching ethidium bromide (EtBr) assay to evaluate the effect of ionic strength and molecular weight on the strength of interaction. The fluorescence quantum yield (Φf) of EtBr in aqueous solution is negligible, whereas in the presence of native double-stranded DNA, Φf increases due to the intercalation of the dye between base pairs and the formation of DNA–EtBr complexes. Addition of polycations to aqueous solutions of the DNA–EtBr complex results in a pronounced decrease of fluorescence intensity due to the competitive displacement of the dye by chitosan to form a complex with DNA. Titrations of DNA–EtBr aqueous solutions with chitosan solutions were carried out at pH values 4.0 and 6.3. The data are presented in as relative fluorescence intensity values of EtBr vs. nitrogen/phosphate (N/P) ratio. A decrease of ethidium fluorescence was observed for all mixtures, indicating that DNA–chitosan complexes were formed at both pH values. The quenching was more pronounced for solutions of lower pH and ionic strength values (). The effect of molecular weight of CH on chitosan–DNA interaction is clearly noted only for N/P ratios below 2.0. This effect is discernable in , where we compare titrations of the DNA–EtBr complex at pH = 4.0 with four deacetylated chitosans (150, 57, 10 and 5 kDa) () Citation17. The chitosans 57 and 150 kDa displaced EtBr more efficiently, thus indicating that the chitosan–DNA interaction is dependent on molecular weight. A comparison between 5 and 10 kDa chitosans showed no difference within the experimental error. For N/P ratios higher than 2, the levelling off of EtBr fluorescence was the same for all tested chitosan samples, i.e., for 5 and 10 kDa chitosans a higher concentration was needed to displace the same amount of EtBr from ct-DNA–EtBr complex. Increasing the pH from 4 to 6.3 resulted in a decreased interaction between chitosan and DNA since the ionisation degree was decreased from 1.0 to 0.5 (). However, at pH 6.3, the same quenching efficiency at higher N/P ratios was not reached, i.e. the plateau of 20%, even at N/P 6.0. The screening effect from the added salt affected the interaction of DNA with both chitosans, CH 5 kDa and CH 150 kDa; however, it can be seen that the fluorescence quenching is more efficient for higher Mw, CH 150 kDa (). However, it is worth mentioning that even at higher ionic strengths, the interaction is strong enough to decrease almost 50% of the EtBr fluorescence, indicating that the screening effect of the added salt is not able to hinder the complex formation. The same experiments carried out at pH 7.0 also showed that chitosan–DNA interaction take place even at pH 7.0 (data not shown). This can be attributed to unprotonated, neutral chitosan units abstracting protons from the buffer in order to interact with the anionic phosphate groups of the DNA, resulting in proton exchange between the buffer and chitosan Citation25. The interaction between DNA and chitosan is exothermic in sodium phosphate buffer Citation26 and the polyanionic nature of DNA increases the ionisation of chitosan by reducing its surface potential and thereby increasing the polycation pKa Citation27.
Figure 6. (a–d) Titration of ct-DNA–EtBr solution by deacetylated chitosan at different pH values (a, b) and different ionic strength in phosphate buffer pH 6.3 (c, d). The fluorescence intensity relative to the fluorescence of ct-DNA–EtBr in the absence of polycation is plotted as a function of nitrogen/phosphate (N/P) ratio. Data points represent the mean (SD of two samples, with at least three readings of each). (a, b) Molecular effect of chitosan on ct-DNA–EtBr fluorescence and the following buffers were used: (a) HAc/NaAc pH 4.0 and (b) phosphate pH 6.3, ionic strength 10 mM. (c, d) Ionic strength effect on the chitosan–DNA interaction (c) CH 5 kDa and (d) CH 150 kDa and ct-DNA–EtBr.
3.5. Gel electrophoresis
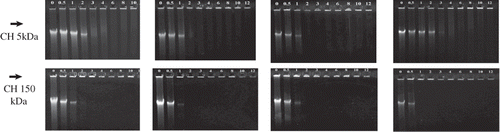
The stabilities of the complexes formed between DNA and chitosans of different molecular weights were assessed by gel electrophoresis aiming to evaluate if polyplexes could dissociate more easily when submitted to a high ionic strength. At pH 4.0, gel electrophoresis from nanoparticles formed with CH 10 kDa, CH 16 kDa, CH 29 kDa and CH 57 kDa exhibited no DNA release at N/P ratios higher than 2.0 (data not shown). shows the electrophoresis of polyplexes formed with CH 5 kDa and CH 150 kDa at increasing N/P ratios. Uncomplexed ct-DNA is loaded in the first lane on the left, while ct-DNA–CH complexes prepared at pH 6.3 and different ionic strengths with 5 and 150 kDa of increasing N/P ratios were loaded in the other lanes. As expected, the ct-DNA shows a long trail due to the high size polydispersity of the DNA sample. Polyplexes formed at 10 mM of ionic strength with CH 5 kDa exhibited no DNA release when the N/P ratios were higher than 2.0, and for CH 150 kDa, no DNA release was observed at N/P ratios higher than 1.0. This result agrees with those obtained from the ethidium bromide assay, which showed that at N/P ratio 1.0, the fluorescence quenching of EtBr–DNA complex by CH 5 kDa reached only 50%, indicating a weaker interaction with DNA. Increasing the ionic strength of the solution from 10 to 150 mM, the DNA release was not significantly affected for polyplexes prepared with CH 5 kDA and only at 1 M the DNA release was increased again. This result can be attributed to the weakening of the interaction between DNA and CH 5 kDa, which indicates that although aggregation may be induced by increasing ionic strengths, as detected in the light scattering and microscopy measurements, the strength of the interaction is significantly affected but only at 1.0 M of added salt. On the other hand, for polyplexes formed with CH 150 kDa, the screening effect of higher ionic strength is not enough to break the complex, which may be responsible for the decreasing DNA release, as shown in . However, for nanoparticles prepared with CH 150 kDa, big aggregates are formed and the stronger CH–DNA interaction makes the releasing of DNA from the polyplexes more difficult. These results indicate that DNA cannot be released from polyplexes only by increasing the ionic strength. The nanoparticles stability is a required property to obtain high transfection levels and also to protect DNA from degradation pathways; therefore, besides parameters such as N/P ratio, molecular weight and pH, the ionic strength of the media must be carefully controlled during preparation of the nanoparticles to avoid the formation of large aggregates.
4. Conclusions
Our data show that polydisperse deacetylated chitosans from 5 to 150 kDa form nanoparticles with DNA whose sizes and stabilities depend on molecular weight, pH, charge ratio and ionic strength. When pH is kept low, all deacetylated chitosans bind efficiently to DNA, and nanoparticle sizes slightly increase in the ionic strength range from 10 to 150 mM. At low ionic strength, the nanoparticles stability is provided at N/P ratios higher than 2.0 by the electrostatic repulsion between the charged chitosan chains adsorbed on the nanoparticles surface. The interaction of deacetylated chitosans with DNA at pH 6.3 and low ionic strength leads to formation of stable nanoparticles only at N/P ratio 7.0. Nanoparticles prepared at N/P ratio 7.0 with lower molecular weights (57, 27, 16 and 5 kDa) aggregates in a period of time of 3 h when challenged by added salt even at 150 mM of ionic strength. Only chitosan 150 kDa formed nanoparticles with stable sizes at 10 and 150 mM of ionic strength. Evidences from optic microscopy and TEM images also pointed out to the formation of larger aggregates. The fluorescence and electrophoresis measurements showed that at increasing salt concentration, the strength of interaction decreases. Therefore, nanoparticles prepared at elevated ionic strengths may have limited stability at low charge ratios and this study contributes to the optimisation of protocols for the preparation of stable chitosan–DNA nanoparticles.
Acknowledgements
This study was supported by a grant (2007/00339-7) from Fapesp (Fundação de Amparo à Pesquisa do Estado de São Paulo) to M.J.T, by a PhD scholarship from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) program to A.H.C., and by a scholarship from Fapesp to I.P.D.P.
This article was originally published with errors. This version has been corrected. P lease see Erratum (http://dx.doi.org/10.1080/17458080.2013.829704).
References
- Kim , T-H , Jiang , H-L , Jere , D , Park , I-K , Cho , M-H , Nah , J-W , Choi , Y-J , Akaike , T and Cho , C-S . 2007 . Chemical modification of chitosan as a gene carrier in vitro and in vivo . Prog. Polym. Sci. , 32 : 726 – 753 . doi: 10.1016/j.progpolymsci.2007.05.001
- Zheng , F , Shib , X-W , Yang , G-F , Gong , L-L , Yuan , H-Y , Cui , Y-J , Wang , Y , Du , Y-M and Li , Y . 2007 . Chitosan nanoparticle as gene therapy vector via gastrointestinal mucosa administration: Results of an in vitro and in vivo study . Life Sci. , 80 : 388 – 396 . doi: 10.1016/j.lfs.2006.09.040
- Richardson , SCW , Kolbe , HVJ and Duncan , R . 1999 . Potential of low molecular mass chitosan as a DNA delivery system: Biocompatibility, body distribution and ability to complex and protect DNA . Int. J. Pharm. , 178 : 231 – 243 . doi: 10.1016/S0378-5173(98)00378-0
- Liu , W , Sun , S , Cao , Z , Zhang , X , Yao , K , Lu , WW and Luk , KDK . 2005 . An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes . Biomaterials , 26 : 2705 – 2711 . doi: 10.1016/j.biomaterials.2004.07.038
- Kiang , T , Wen , J , Lim , HW and Leong , KW . 2004 . The effect of the degree of chitosan deacetylation on the efficiency of gene transfection . Biomaterials , 25 : 5293 – 5301 . doi: 10.1016/j.biomaterials.2003.12.036
- MacLaughlin , FR , Mumper , J , Wang , J , Tagliaferri , JM , Gill , I , Hinchcliffe , M and Rolland , AP . 1998 . Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery . J. Controlled Release , 56 : 259 – 272 . doi: 10.1016/S0168-3659(98)00097-2
- Danielsen , S , Varum , KM and Stokke , BT . 2004 . Structural analysis of chitosan mediated DNA condensation by AFM: Influence of chitosan molecular parameters . Biomacromolecules , 5 : 928 – 936 . doi: 10.1021/bm034502r
- Koping-Hoggard , M , Melnikova , YS , Varum , KM , Lindman , B and Artursson , P . 2003 . Relationship between the physical shape and the efficiency of oligomeric chitosan as a gene delivery system in vitro and in vivo . J. Gene Med. , 5 : 130 – 141 . doi: 10.1002/jgm.327
- Koping-Hoggard , M , Varum , KM , Issa , M , Danielsen , S , Christensen , BE , Stroke , BT and Artusson , P . 2004 . Improved chitosan-mediated gene delivery based on easily dissociated chitosan polyplexes of highly defined chitosan oligomers . Gene Ther. , 11 : 1441 – 1452 . doi: 10.1038/sj.gt.3302312
- Liu , W , Sun , S , Cao , Z , Zhang , X , Yao , K , Lu , WW and Luk , KDK . 2005 . An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes . Biomaterials , 26 : 2705 – 2711 . doi: 10.1016/j.biomaterials.2004.07.038
- Mao , H-Q , Roy , K , Troung-Le , VL , Janes , KA , Lin , KY , Wang , Y , August , JT and Leong , KW . 2001 . Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency . J. Controlled Release , 70 : 399 – 421 . doi: 10.1016/S0168-3659(00)00361-8
- Wolfert , MA and Seymour , LW . 1996 . Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block co-polymers . Human Gene Ther. , 3 : 269 – 273 .
- Erbacher , P , Zou , S , Bettinger , T , Steffan , A-M and Remy , J-S . 1998 . Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability . Pharm. Res. , 15 : 1332 – 1339 . doi: 10.1023/A:1011981000671
- Jiang , X , Dai , H , Leong , KW , Goh , S-H , Mao , H-Q and Yang , Y-Y . 2006 . Chitosan-g-PEG/DNA complexes deliver gene to the rat liver via intrabiliary and intraportal infusions . J. Gene Med. , 8 : 477 – 487 . doi: 10.1002/jgm.868
- Koping-Hoggard , M , Tubulekas , I , Guan , H , Edwards , K , Nilsson , M , Varum , KM and Artursson , P . 2001 . Chitosan as a nonviral gene delivery system. Structure-property relationships and characteristics compared with polyethyleneimine in vitro and after lung administration in vivo . Gene Ther. , 8 : 1108 – 1121 . doi: 10.1038/sj.gt.3301492
- Vijayanathan , V , Thomas , T , Shirahata , A and Thomas , TJ . 2001 . DNA condensation by polyamines: A laser light scattering study of structural effects . Biochemistry , 40 : 13644 – 13651 . doi: 10.1021/bi010993t
- Casé , AH , Picola , IPD , Zaniquelli , MED , Fernandes , JC , Taboga , SR , Winnike , FM and Tiera , MJ . 2009 . Physicochemical characterization of nanoparticles formed between DNA and phosphorylcholine substituted chitosans . J. Colloid Interface Sci. , 336 : 125 – 133 . doi: 10.1016/j.jcis.2009.02.069
- Strand , SP , Danielsen , S , Christensen , BE and Vårum , KM . 2005 . Influence of chitosan structure on the formation and stability of DNA-chitosan polyelectrolyte complexes . Biomacromolecules , 6 : 3357 – 3366 . doi: 10.1021/bm0503726
- Xu , X , Capito , RM and Spector , M . 2008 . Plasmid size influences chitosan nanoparticle mediated gene transfer to chondrocytes . J. Biomed. Mater. Res. Part A, , 84A : 1038 – 1048 . doi: 10.1002/jbm.a.31479
- Strand , SP , Lelu , S , Reitan , NK , Davies , CL , Artursson , P and Vårum , KM . 2010 . Molecular design of chitosan gene delivery systems with an optimized balance between polyplex stability and polyplex unpacking . Biomaterials , 31 : 975 – 987 . doi: 10.1016/j.biomaterials.2009.09.102
- Park , K-H , Song , H-C , Na , K , Bom , H-S , Lee , KH , Kim , S , Kang , D and Lee , DH . 2007 . Ionic strength-sensitive pullulan acetate nanoparticles (PAN) for intratumoral administration of radioisotope: Ionic strength-dependent aggregation behavior and 99mTechnetium retention property . Colloids Surf. B , 59 : 16 – 23 . doi: 10.1016/j.colsurfb.2007.04.010
- Elbadawy , A , Luxton , TP , Silva , RG , Scheckel , KG , Suidan , MT and Tolaymat , TM . 2010 . Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions . Environ. Sci. Technol. , 44 : 1260 – 1266 . doi: 10.1021/es902240k
- Rajapaksa , TE , Bennett , KM , Hamer , M , Lytle , C , Rodgers , VGJ and Lo , DD . 2010 . Intranasal M cell uptake of nanoparticles is independently influenced by targeting ligands and buffer ionic strength . J. Biol. Chem. , 285 : 23739 – 23746 . doi: 10.1074/jbc.M110.126359
- Ma , PL , Buschmann , MD and Winnik , FM . 2010 . One-step analysis of DNA/chitosan complexes by field-flow fractionation reveals particle size and free chitosan content . Biomacromolecules, , 11 : 549 – 554 . doi: 10.1021/bm901345q
- Rungsardthong , U , Ehtezazi , T , Bailey , L , Armes , SP , Garnett , MC and Stolnik , S . 2003 . Effect of polymer ionization on the interaction with DNA in nonviral gene delivery systems . Biomacromolecules , 4 : 683 – 690 . doi: 10.1021/bm025736y
- Ma , PL , Lavertu , M , Winnik , FM and Buschmann , MD . 2009 . New insights into chitosan-DNA interactions using isothermal titration microcalorimetry . Biomacromolecules , 10 : 1490 – 1499 . doi: 10.1021/bm900097s
- Zelikin , AN , Trukhanova , ES , Putnam , D , Izumrudov , VA and Litmanovich , AA . 2003 . Competitive reactions in solutions of poly-L-histidine, calf thymus DNA, and synthetic polyanions: Determining the binding constants of polyelectrolytes . J. Am. Chem. Soc. , 125 : 13693 – 13699 . doi: 10.1021/ja036387y