Abstract
Thin films of pure and Ni-doped TiO2 were prepared on a glass substrate by sol–gel and spin-coating process from specially formulated ethanol sols. The morphologies of the films surface were observed with atomic force microscope (AFM). The tribological properties of the obtained thin films sliding against steel ball were evaluated on a one-way reciprocating friction tester. AFM results show that by the addition of the Ni in TiO2, smooth surfaces were obtained. As a result, the Ni-doped TiO2 films exhibit better wear protection properties than pure TiO2. The best protection was observed for 5% Ni-doped TiO2 films in this study.
Key Words:
1. Introduction
Titania (TiO2) has received a lot of attention in the past two decades, due to many advanced applications of the material in photocatalysis, solar energy cells, gas sensors Citation1–3, etc. Research study has been carried out on the mechanical properties of the TiO2 thin film. Jämting et al. Citation4,Citation5 reported that TiO2 thin films have higher mechanical strength. Zhang et al. Citation6,Citation7 found that they have excellent wear resistance capability under lower load.
Several methods have been employed to fabricate TiO2 thin films, including e-beam evaporation Citation8, sputtering Citation9, chemical vapour deposition Citation10 and sol–gel process Citation11. Due to several advantages such as low processing temperature, low equipment cost and good homogeneity, the sol–gel process is one of the most appropriate technologies to prepare thin films.
In addition to pure titania, the interest has been steadily grown to study metal ion-doped titania. The doping modifies the electronic structure of the material, which can cause more effective electron–hole pair generation that is especially important for applications that are based on light-induced phenomena. Sol–gel method is also used for the preparation of doped TiO2 films Citation12,Citation13.
Nickel-doped TiO2 has shown several interesting properties like photo-catalytic activity and UV light-induced hydrophilicity Citation14–18. However, information regarding the mechanical and tribological performance of Ni-doped TiO2 is not available, though this is a crucial problem faced during the engineering application of films. In this article, we present the results of the systematic study of the tribological properties of nickel-doped TiO2 films prepared by sol–gel method.
2. Experimental section
2.1. Preparation of TiO2 thin film
Nickel-doped titania thin films on glass substrate were prepared by sol–gel method using commercially available tetrabutyl titanate and nickel nitrate as the precursor materials and triethanolamine as the stabilising agent in anhydrous ethanol. The chemical reagents are all AR grade and used without further purification. First, tetrabutyl titanate was added to an anhydrous ethanol solution. The mixture was stirred at room temperature for 15 min and then triethanolamine dissolved in the mixture. After stirring for 5 min, distilled water was slowly dripped into the solution under continuous vigorous stirring. The volume ratio of the composition in mixed solution is Ti(OBu)4:EtOH:H2O:N(C2H5OH)3 = 3:12:1:1. Then, nickel nitrate was dissolved into some distilled water. The solution was incorporated into the TiO2 sol. The mixed solution was stirred for 1 h and then aged overnight at room temperature. A clear, transparent and light yellow solution was ready for film preparation. The precursor sol will not precipitate even after several months.
Glass plates with size of 25 × 76 × 1 mm3 were pretreated in piranha solution (in which the volume ratio of 98% H2SO4 and 30% H2O2 was 7:3) at 90°C for 30 min. After being rinsed with distilled water, these plates were blow dried by nitrogen. TiO2 thin films were prepared on these plates by spin-coating method with speed 2000 cycle/s and time 30 s. The films were dried at 80°C for 15 min, and then sintered at 480°C for 60 min in an atmospheric oven. These films were cooled to room temperature in the oven. Finally, uniform and transparent TiO2 thin films were obtained. The TiO2 films containing Ni molar concentrations 2%, 5% and 10% were denoted as 2%, 5% and 10% Ni–TiO2, respectively.
2.2. Characterisation of films
Because the thickness of films is too thin, the crystal structures of pure and doped TiO2 powders, prepared after employing the same annealing process used for thin films, were analysed using a SHIMADZU Rigaku D/max-rB X-ray diffractometer. Cu-Kα radiation was produced using the tube's 40 kV and 20 mA regime.
The morphological properties of the samples’ surfaces were performed with a Shimadzu SPM-9500 Atomic Force Microscope (AFM). Typically, a tapping mode was utilised, which is known to provide optimal performance in such cases.
Friction and wear tests were carried out on a UMT-3 tester (CETR Corporation Ltd, USA) under reciprocating distance of 5 mm per pass. The counterpart was GCr15 steel ball with a diameter of 4 mm. All the tests were conducted under the following conditions: room temperature, relative humidity about 40%, applied load 0.5 N with sliding speed 10 mm/s. The friction coefficient was recorded automatically by a computer. During the whole friction process, friction coefficient remained stable for a particular period and then abruptly rose. This period was regarded as the wear life of the films. Three replicated tests were carried out for each specimen and the average friction coefficient and wear life are used in this article.
3. Results and discussion
3.1. Characterisation of the films using AFM and XRD
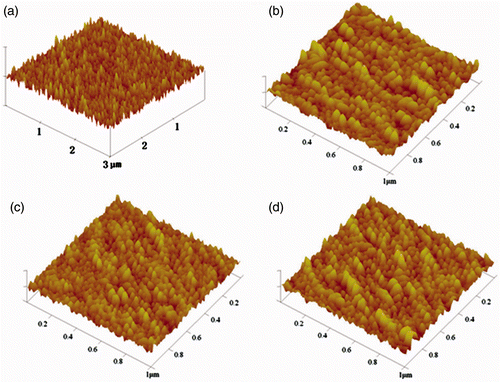
AFM was used to characterise the uniformity and particle size of pure and doped films. The results are shown in . Compared with the AFM image of pure TiO2 film that we reported before Citation19, Ni-doped TiO2 films exhibit similar surface morphology with no crack, however with a smoother surface. Roughness average (Ra), values are 4.24, 2.74, 2.34 and 2.71 nm for pure and 2%, 5% and 10% Ni-doped TiO2 films, respectively. It indicates the reduction in particle size in the films because of Ni doping. The smoothest surface is observed for 5% Ni-doped TiO2 films. Further increase in Ni concentration to 10% will damage the surface and increase Ra value. The similar results were observed for Cu-doped TiO2 films prepared by sol–gel technology Citation19,Citation20.
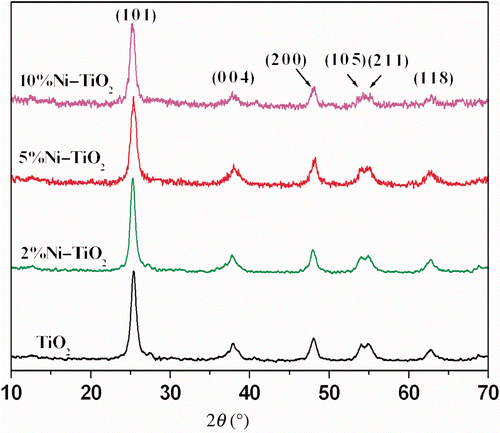
shows the XRD patterns of pure and Ni-doped TiO2 powders annealed at 480°C. For TiO2 powder, typical peaks in XRD pattern are observed at 2θ values 25.3°, 38.1°, 48.0° and 55.0°, assigned to (1 0 1), (1 1 2), (2 0 0) and (2 1 1) planes, respectively. Calculations show that the observed crystalline peaks correspond to anatase TiO2 phase. There is no indication of rutile phase formation up to a sintering temperature of 480°C.
In the case of Ni-doped TiO2 films, similar XRD patterns were observed. Crystalline phases of Ni ion on these films have not been detected in the XRD pattern of doped film up to 10 mol% concentration. However, significant change in the XRD peak broadening has been observed. The peak broadening of XRD spectra show the decrease in the average crystal size of TiO2 particles. According to Scherrer's formula Citation21
3.2. Tribological properties of films
Tribological properties of the TiO2 films were tested on a UMT-3 friction and wear tester. and present the tribological performance of the TiO2 and Ni-doped TiO2 films at a normal load of 0.5 N.
Figure 3. (Colour online) Friction coefficients of pure and doped TiO2 as a function of sliding time.
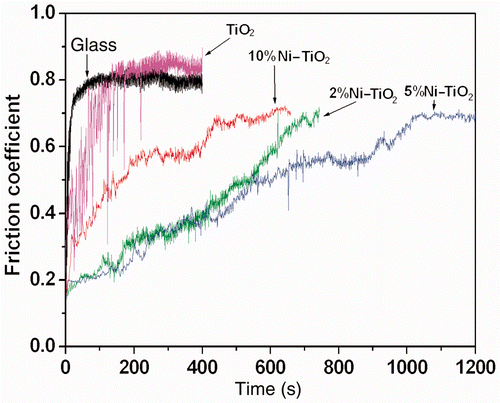
Table 1. Tribological properties of the films.
In this experiment, it was found that the glass substrate is easily worn off when sliding against the GCr15 steel ball. The initial friction coefficient is 0.24 and this remains for only few seconds and then sharply increases to 0.81. Pure TiO2 thin films prepared in the experiment can protect the glass substrate from severe wear. Before the film is worn out, the friction coefficient of the films sliding against GCr15 steel ball remains stable for a longer period of time (∼150 s). All three Ni-doped TiO2 films show better wear protection performance than pure TiO2 film. However, the improvement in wear protection for Ni-doped TiO2 films depends on the concentration of Ni in TiO2 films. The performance of Ni-doped TiO2 films improved with increase in the Ni concentration until 5%. Afterwards, the further increase in of Ni concentration damage the performance.
The tribological performance of pure and Ni-doped TiO2 films can be explained by AFM observation for films. The improvement in tribological performance for Ni-doped TiO2 films can be explained by the change in film surface when Ni is doped into TiO2 films. It has been observed in AFM and XRD experiments that Ni doping results in the smaller average size of TiO2 particles and smoother films surface. It is well known that the roughness plays a great role in determining the tribological performance of surface. The Ni-doped TiO2 films are more smooth and therefore have better friction-reducing and wear protection performance than pure TiO2 films. However, the performances depend on the concentration of Ni dopant. When Ni is at 5% concentration in TiO2 films, the smallest Ra value is obtained, whereas best friction-reducing and wear protection behaviour is observed. Further increase in Ni concentration to 10% greatly damages the uniform surface. It inevitably affects the tribological performance of Ni-doped TiO2 films.
4. Conclusions
Pure and Ni-doped TiO2 films have been successfully obtained by sol–gel and spin-coating process. Results show that the addition of inorganic Ni salts to the starting sol of TiO2 can improve the tribological properties of the corresponding films. However, the wear life of Ni-doped films does not increase monotonically with increasing Ni concentration in TiO2 matrix. The 5% Ni-doped TiO2 shows the best wear protection in this study.
Acknowledgements
This study was supported by the Natural Science Foundation of Shandong Province (Y2008F05) and Applied and Basic Research Foundation of Qingdao City (09-1-3-35-jch).
This article was originally published with errors. This version has been corrected. P lease see Erratum (http://dx.doi.org/10.1080/17458080.2013.829704).
References
- Zayim , EO . 2005 . Effect of calcination and pH value on the structural and optical properties of titanium oxide thin films . J. Mater. Sci. , 40 : 1345 – 1352 . doi: 10.1007/s10853-005-0563-5
- Koo , H-J , Kim , YJ , Lee , YH , Lee , WI , Kim , K and Park , N-G . 2008 . Nano-embossed hollow spherical TiO2 as bifunctional material for high-efficiency dye-sensitized solar cells . Adv. Mater. , 20 : 195 – 199 . doi: 10.1002/adma.200700840
- Tang , H , Prasad , K , Sanjinés , R and Lévy , F . 1995 . TiO2 anatase thin films as gas sensors . Sens. Actuators, B , 71 : 26 – 27 .
- Jämting , ÅK , Bell , JM , Swain , MV , Wielunski , LS and Clissold , R . 1998 . Measurement of the micro mechanical properties of sol-gel TiO2 films . Thin Solid Films , 332 : 189 – 194 . doi: 10.1016/S0040-6090(98)01102-X
- Olofinjana , AO , Bell , JM and Jämting , ÅK . 2000 . Evaluation of the mechanical properties of sol-gel-deposited titania films using ultra-micro-indentation method . Wear , 241 : 174 – 179 . doi: 10.1016/S0043-1648(00)00372-0
- Zhang , W , Liu , W and Wang , C . 2002 . Tribological behavior of sol-gel TiO2 films on glass . Wear , 253 : 377 – 384 . doi: 10.1016/S0043-1648(02)00139-4
- Zhang , W , Liu , W , Li , B and Mai , G . 2002 . Characterization and tribological investigation of sol–gel titania and doped titania thin films . J. Am. Ceram. Soc. , 85 : 1770 – 1776 . doi: 10.1111/j.1151-2916.2002.tb00351.x
- Barros , AD , Albertin , KF , Miyoshi , J , Doi , I and Diniz , JA . 2010 . Thin titanium oxide films deposited by e-beam evaporation with additional rapid thermal oxidation and annealing for ISFET app lications . Microelectron. Eng. , 87 : 443 – 446 . doi: 10.1016/j.mee.2009.06.020
- Takeda , S , Suzuki , S , Odaka , H and Hosono , H . 2001 . Photocatalytic TiO2 thin film deposited onto glass by DC magnetron sputtering . Thin Solid Films , 392 : 338 – 344 . doi: 10.1016/S0040-6090(01)01054-9
- Zhang , JY and Ian Boyd , B . 2002 . Structural and electrical properties of tantalum oxide films grown by photo-assisted pulsed laser deposition . Appl. Surf. Sci. , 186 : 40 – 46 . doi: 10.1016/S0169-4332(01)00752-8
- Mechiakh , R , Ben Sedrine , N , Chtourou , R and Bensaha , R . 2010 . Correlation between microstructure and optical properties of nano-crystalline TiO2 thin films prepared by sol–gel dip coating . Appl. Surf. Sci. , 257 : 670 – 676 . doi: 10.1016/j.apsusc.2010.08.008
- Zhang , Y , Huang , Y , Wang , Y , Ji , X , Shih , SJ and Jia , B . 2009 . Study of Nano-Ag Particles Doped TiO2 Prepared by Photocatalysis . J. Nanosci. Nanotechnol. , 9 : 3904 – 3908 . doi: 10.1166/jnn.2009.NS87
- Sonawane , RS and Dongare , MK . 2006 . Sol-gel synthesis of Au/TiO2 thin films for photocatalytic degradation of phenol in sunlight . J. Mol. Catal. A: Chem. , 243 : 68 – 76 . doi: 10.1016/j.molcata.2005.07.043
- Ramírez-Meneses , E , García-Murillo , A , Carrillo-Romo , Fde J , García-Alamilla , R , Del Angel-Vicente , P , Ramírez-Salgado , J and Bartolo Pérez , P . 2009 . Preparation and photocatalytic activity of TiO2 films with Ni nanoparticles . J. Sol-Gel Sci. Technol. , 52 : 267 – 275 . doi: 10.1007/s10971-009-2015-1
- Kisand , V , Joosta , U , Reedo , V , Pärna , R , Tätte , T , Shulga , J , Saar , A , Matisen , L , Kikas , A and Kink , I . 2010 . Influence of the heating temperature on the properties of nickel doped TiO2 films prepared by sol-gel method . Appl. Surf. Sci. , 256 : 4538 – 4542 . doi: 10.1016/j.apsusc.2010.02.043
- Sharma , SD , Singh , D , Saini , KK , Kant , C , Sharma , V , Jain , SC and Sharma , CP . 2006 . Sol-gel-derived super-hydrophilic nickel doped TiO2 film as active photo-catalyst . Appl. Catal., A , 314 : 40 – 46 . doi: 10.1016/j.apcata.2006.07.029
- Tseng , HH , Wei , MC , Hsiung , SF and Chiou , CW . 2009 . Degradation of xylene vapor over Ni-doped TiO2 photocatalysts prepared by polyol-mediated synthesis . Chem. Eng. J. , 150 : 160 – 167 . doi: 10.1016/j.cej.2008.12.015
- Dhayal , M , Sharma , AD , Kant , C , Saini , KK and Jain , SC . 2008 . Role of Ni doping in surface carbon removal and photo catalytic activity of nano-structured TiO2 film . Surf. Sci. , 602 : 1149 – 1154 . doi: 10.1016/j.susc.2007.12.044
- Chao , W-L , Wan , Y , Wang , Y-X and Liu , C-S . 2010 . Tribological Properties of Cu-Doped TiO2 Films . Acta Phys. Chim. Sin. , 26 : 2317 – 2322 .
- Celik , E , Gokcen , Z , Ak Azem , NF , Tanoglu , M and Emrullahoglu , OF . 2006 . Processing, characterization and photocatalytic properties of Cu doped TiO2 thin films on glass substrate by sol–gel technique . Mater. Sci. Eng., B , 132 : 258 – 265 . doi: 10.1016/j.mseb.2006.03.038
- Cullity , BD . 1978 . Elements of X-ray Diffraction , 2nd , Reading , MA : Addison-Wesley .