Abstract
TiO2 nanotubes have been prepared by anodisation of titanium foil and their antibacterial activities have been tested against Gram-positive bacteria (Bacillus atrophaeus) while photocatalytic activity was tested for the degradation of the methyl orange dye. We found that the annealing temperature strongly affected antibacterial activity and photocatalytic dye degradation, as well as the production of reactive oxygen species under illumination. However, different trends were observed for dye degradation and antibacterial activity dependence on the annealing temperature. The relationship between annealing conditions, crystal structure, reactive oxygen species generation, dye degradation and antibacterial activity is discussed.
1. Introduction
Metal oxide materials have been attracting lots of attention in recent years due to their applications in photocatalytic degradation of pollutants Citation1–3 and as antibacterial materials for the prevention of the spread of infections Citation1–16. Photocatalytic degradation of pollutants is typically attributed to the reactions involving reactive oxygen species (ROS), although direct participation of photogenerated carriers in the photocatalytic reactions is also possible Citation1. Antibacterial activity of these materials is also commonly attributed to the production of ROS, which are non-selective microbicides Citation5. The ROS produced can include hydroxyl groups, hydrogen peroxide and superoxide anions Citation12,Citation15. Although some activity can occur even in dark conditions Citation7, it is generally accepted that UV illumination is needed for the photocatalytic generation of ROS and the efficient antibacterial action of TiO2 Citation5.
Illumination of TiO2 by UV light above the band gap results in the creation of electron–hole pairs Citation5,Citation12. These photogenerated carriers can then either recombine or participate in oxidation/reduction processes with adsorbates at the surface Citation5,Citation12, which can result in ROS generation by TiO2 photocatalysis Citation5.
Therefore, due to non-selective bactericidal action of ROS, there is considerable interest in developing TiO2 antibacterial coatings with altered properties and higher photocatalytic activity Citation5.
Although photocatalytic pollutant degradation and photocatalytic antibacterial activity are frequently attributed to the same mechanisms (ROS generation under illumination), it has been recently shown that antibacterial activity and photocatalytic dye degradation did not follow the same trends for ZnO and Ag:ZnO nanocrystals Citation3. Thus, comparative investigation of dye degradation and antibacterial activity and elucidating the reasons for the observed differences (if any) is of significant interest. While in many applications the removal of both bacterial and chemical contamination may be desirable, in other applications (e.g. antibacterial surface coatings in health care and food industry which should not cause discolouration of painted surfaces in contact with the coating) it may be preferable to decouple these two effects if possible.
Therefore, here we investigated the antibacterial and photocatalytic dye degradation activities of TiO2 nanomaterials as a function of their crystal structure. While there are many different methods for preparing TiO2 coatings, an ideal fabrication method should be simple, inexpensive and result in a high surface area TiO2 coating. One such method is the anodisation of titanium Citation16–23, which is also of interest due to the wide use of titanium and its alloys in biomedical applications Citation18. However, nanotubes produced by anodisation are typically amorphous, and they are annealed after anodisation to obtain crystalline nanotubes. Different annealing temperatures result in different crystal structures of the nanotubes Citation17,Citation21 and this can affect their photocatalytic and antibacterial activities. While the influence of annealing temperature on the photocatalytic properties of the nanotubes has been studied Citation19,Citation21, there were no studies on the effect of annealing temperature on their antibacterial activity. Therefore, here we studied antibacterial activity of TiO2 nanotubes against Gram-positive bacteria (Bacillus atrophaeus) as a function of annealing temperature. The photocatalytic activity of nanotubes annealed at different temperatures for the degradation of methyl orange has also been studied, and the trends of the two photocatalytic processes (dye degradation and antibacterial action) were compared and discussed.
2. Materials and methods
2.1. Sample preparation
Titanium foils (0.25 mm thickness) were cleaned by sonication in toluene, acetone, ethanol and deionised (DI) water and then dried with N2 gas. Then, the foils were immersed in the electrolyte consisting of 1.36 g ammonium fluoride (99+%, BDH) and 8 mL of DI water in 400 mL ethylene glycol (99.8%, Aldrich) for 2 h at 60 V, similar to previously reported procedures Citation22,Citation23. Annealing was performed in a tube furnace in air at a set temperature (450°C, 650°C and 850°C). Cooling and heating rate was 1°C/min. The morphology and structure of the prepared nanostructured films were examined by scanning electron microscopy (SEM) using a JEOL JSM-7001F field emission SEM and X-ray diffraction (XRD) using Bruker AXS SMART CCD diffractometer. Characterisation of ROS produced has been performed by electron paramagnetic resonance (EPR) measurements using a Bruker EMX EPR spectrometer at room temperature with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin trap. The solution for the measurement was prepared by placing 200 µL of distilled water and 50 µL of 0.1 M DMPO onto the nanotube substrates and illuminating with 365 nm UV (30 mW/cm2) for 2 min. Then, the solution was transferred into an EPR tube and the measurement was performed immediately. Instrument settings for the EPR measurement were 20 mW and 9.74 GHz for the microwave power and microwave frequency, respectively, with a modulation frequency of 100 kHz, a modulation amplitude of 2 G and a time-constant and a sweep time 655 ms and 84 s.
2.2. Tests of antibacterial activity
A Gram-positive bacterium B. atrophaeus ATCC 9372 was used for antibacterial activity testing. Luria–Bertani broth was used as the culture medium, while aqueous sodium chloride solution (0.9% w/v) was used as the suspension medium when doing the UV illumination since it was reported that Luria–Bertani broth is not a suitable medium for photocatalytic disinfection experiments Citation14. Bacillus atrophaeus was grown at 30°C. 10 µL of bacteria suspension (108 CFU/mL) was placed onto the coated plates and then 0.5 mL of pure aqueous sodium chloride was added to prevent the plates from drying up under UV illumination. The plates were then exposed to UV illumination (365 nm, Blak-Ray® B-100 AP Lamp, 102 mW/cm2 measured by an optical power meter) for 10 min. Serial dilution was then performed and 20 µL of dilution was pipetted onto a culture agar in triplicate to ensure reproducibility of the results. The samples were then kept at 30°C for 24 h and then the formation of colonies was determined by observation under an optical microscope.
2.3. Tests of photocatalytic activity
1 mg methyl orange dye was dissolved in 1000 mL of DI water. Ti foil or TiO2 nanotube samples (2.5 × 2.5 cm2) were placed into a Petri dish and covered with 5 mL of methyl orange solution and 15 mL of DI water. Exposure to UV illumination (365 nm, 70.7 mW/cm2) was performed under constant stirring of the solution with a magnetic stirrer. The absorption was measured immediately before the start of UV exposure. Then, at 15 min intervals, 2 mL of the solution was withdrawn for the absorption measurements.
3. Results and discussion
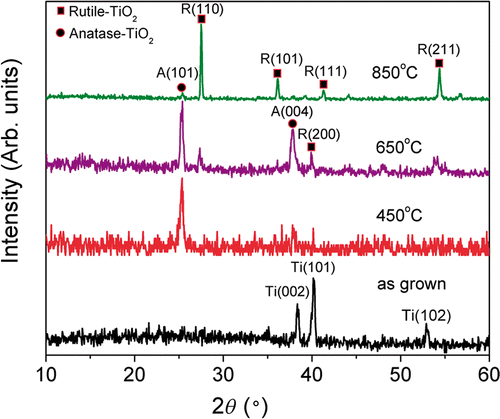
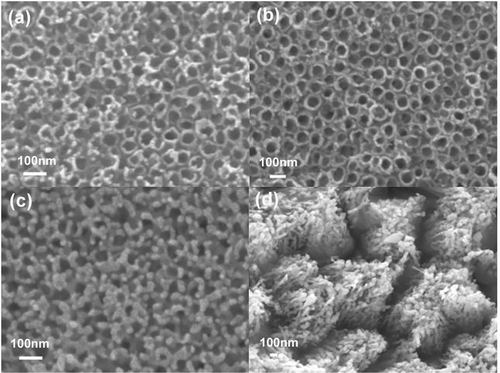
and show the top and the side views of the titania nanotubes for different annealing temperatures. It can be observed that there is a degradation of the morphology near the top of the nanotubes and breaking up of the top of the tube into porous structure after annealing 650°C, while after annealing at 850°C this process proceeds further along entire nanotube. From the XRD patterns shown in , we can observe that nanotubes not subjected to annealing are amorphous, in agreement with the literature, while after annealing at 450°C, anatase crystal structure is obtained Citation19,Citation20. With further increase in the temperature, rutile crystals start to form Citation17,Citation19–21, so that a change in the morphology and the appearance of rutile peaks can be observed after annealing at 650°C. Destruction of the tube array after annealing at or above 700°C has been reported previously Citation19,Citation20. For annealing temperature of 850°C, only rutile peaks are found in the XRD, while SEM images show porous crystallite assemblies instead of nanotube arrays.
Figure 1. Side view SEM images of TiO2 nanotubes annealed at different temperatures: (a) no annealing, and annealed at (b) 450°C, (c) 650°C and (d) 850°C.
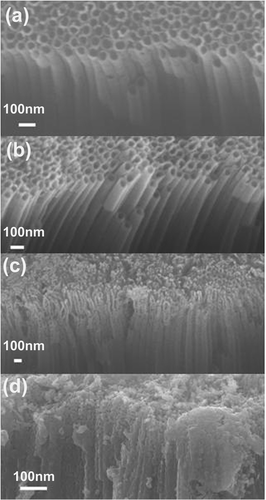
Figure 2. Top view SEM images of TiO2 nanotubes annealed at different temperatures: (a) no annealing, and annealed at (b) 450°C, (c) 650°C and (d) 850°C.
The results of antibacterial tests for B. atrophaeus are summarised in and photographs are shown in . The nanotube samples exhibited excellent antibacterial activity for all annealing temperatures except 850°C. Some degree of reduction in the number of bacteria colonies observed for the bare substrate (Ti foil) can be attributed to the effect of UV illumination. In agreement with the literature, more significant antibacterial effect is observed with the combination of TiO2 and UV illumination, compared to UV illumination only Citation14. It is generally accepted that antibacterial activity of metal oxides occurs mainly due to photocatalytic ROS generation Citation4,Citation7, although there can be additional contributing mechanisms accounting for the small activity observed in the absence of UV illumination Citation7. ROS generation after illumination of TiO2 can occur due to different chemical reactions Citation5. Both oxygen and water participate in these reactions, and consequently, interactions of water and oxygen with titania surfaces Citation24–27 and the photocatalytic processes in general have been extensively studied Citation19,Citation21,Citation28–32.
Figure 4. Photos of B. atrophaeus cultured after exposure to UV illumination on: (a) titanium foil, (b) titania tubes, no annealing; and titania tubes annealed at (c) 450°C, (d) 650°C and (e) 850°C.

Table 1. Number of bacterial colonies/antibacterial efficiency on different samples.
Photocatalytic degradation of methylene blue was reported to be strongly dependent on the annealing temperature, with the best performance achieved for 550°C, while further increase of temperature to 650°C resulted in worse performance Citation21. The worst performance was obtained for tubes without any annealing Citation21. Existence of an optimum annealing temperature for the highest photocatalytic activity was also observed in other studies Citation19, but the optimum temperature (400°C) was different Citation19. In general, some of the studies report a mixture of rutile and anatase Citation21 or even rutile, anatase and brookite Citation29 to be optimal for the highest photocatalytic activity, while in others the best results are obtained or pure anatase Citation28. Improved photocatalytic activity of mixed phase samples was attributed to lower recombination losses of photogenerated carriers due to improved separation of electrons and holes in mixed samples Citation29.
Obtained results for the photocatalytic degradation of methyl orange in our study are shown in . We can observe that the best photocatalytic activity is obtained for tubes annealed at 650°C, i.e. mixture of anatase and rutile, in agreement with the literature Citation21. Only slightly lower degradation was observed for pure anatase nanotubes, while no significant degradation was observed for rutile nanotubes in agreement with the majority of studies which found that pure rutile is inferior to mixed and anatase samples in terms of photocatalytic activity Citation19,Citation21,Citation28,Citation29,Citation32. However, unlike antibacterial performance, the nanotube samples without any annealing did not exhibit significant photocatalytic activity in degradation of methyl orange in agreement with the literature Citation21.
Figure 5. Photocatalytic degradation of methyl orange by titania nanotubes annealed at different temperatures.
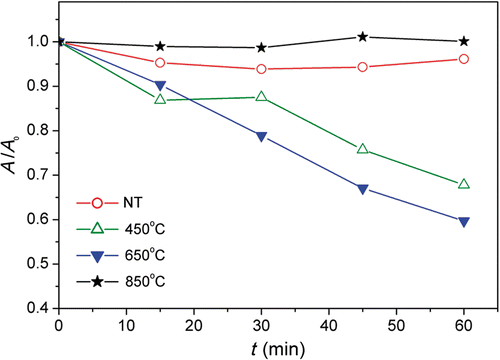
While the differences in native defects and crystal structure for samples annealed at different temperatures are expected to affect both antibacterial and photocatalytic dye degradation activities, the reasons for the observed differences in the two processes on nanotubes annealed at different temperatures are not very clear. Previous observation of different trends in photocatalytic degradation of different dyes and antibacterial activity was attributed to the nanoparticle aggregation in the solution Citation3. However, this cannot explain the results observed here, since the titania nanotubes are on a substrate, not dispersed in the solution. Also, while it was proposed that both photocatalytic degradation of organic molecules and antibacterial activity involve the production of ROS, it should be noted that photocatalytic degradation of organic molecules may involve adsorption of these molecules onto the catalyst surface Citation33, and adsorption of the reagent molecules is dependent on the existing surface adsorbates (hydroxyl groups Citation34). Thus, if the photocatalytic degradation of the dye mainly proceeds via direct oxidation by valence band holes rather than indirect oxidation by generated ROS, it would be expected that different trends can be observed for photocatalytic dye degradation and antibacterial activity. Furthermore, different ROS can be produced in photocatalytic processes, which can have different effects on dye degradation and bacteria survival. Even for the same type of bacteria, studies on the effect of TiO2 photocatalytic bactericidal effects reported different roles of various ROS, so that in one case linear correlation between OH√ radicals and antibacterial activity was observed Citation35, while in another case significant contribution of H2O2 in cooperation with other ROS was found Citation36. On the other hand, it was demonstrated that the quantum yield of the OH√ radicals on titania was low, and it was proposed that the direct oxidation by photogenerated holes plays a dominant role in photocatalytic reactions Citation37.
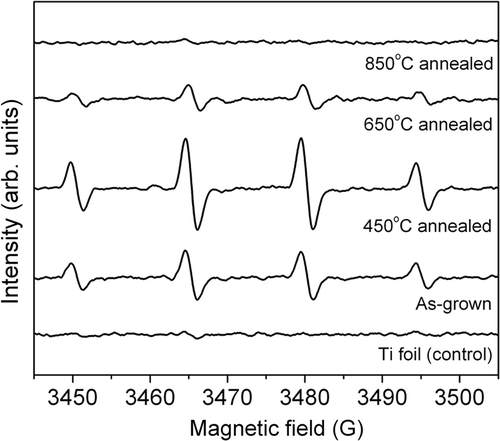
To clarify the difference between two processes, we have examined the ROS production on different titania samples using EPR spectroscopy. The obtained results are shown in . We can observe a clear characteristic signal from DMPO-OH√ adduct in as-grown nanotubes, as well as in nanotubes annealed at 450°C and 650°C. Thus, OH√ radicals are produced in all the samples which exhibit significant antibacterial activity. Thus, the obtained results are consistent with the assumption that OH√ radicals have a significant role in the antibacterial activity Citation35, while the dominant process in the photocatalytic dye degradation is the direct oxidation by the photogenerated holes Citation37. The latter process would be dependent on the energy level alignment of the dye and the photocatalyst, which could also explain previously reported substrate-specific photocatalytic activity of ZnO and Ag:ZnO Citation3. Furthermore, the OH√ radicals production is higher for annealing at 450°C compared to 650°C, while the opposite is observed for photocatalytic degradation of methyl orange, further supporting the hypothesis that OH√ radicals are not the dominant species responsible for dye degradation. However, further experiments are needed to fully clarify the role of other possible reactive species (superoxide ions, trapped electrons, singlet oxygen, etc.).
4. Conclusion
We have studied the properties and the antibacterial activity of titania nanotubes prepared by anodisation and annealed at different temperatures. The antibacterial and photocatalytic activities of the nanotubes were strongly dependent on the annealing temperature. However, different trends were observed for antibacterial and photocatalytic activities for dye degradation. The as-grown nanotubes exhibited production of OH√ radicals under UV illumination and antibacterial activity, but no significant dye degradation for methyl orange dye. Thus, we can conclude that photocatalytic degradation of the dye likely involves direct charge transfer of photogenerated carriers, while antibacterial activity may occur due to the involvement of ROS produced.
Acknowledgements
This study is partly supported by the University Development Fund, Research Fund for the Control of Infectious Diseases grant (Ref. No. 07060602) funded by the Health, Welfare, and Food Bureau, Hong Kong Government and RGC CRF CityU6/CRF/08. The authors thank Prof. S.Y. Tong for useful discussions on the possible effects of different adsorbates and the defects on TiO2 surfaces.
This article was originally published with errors. This version has been corrected. P lease see Erratum (http://dx.doi.org/10.1080/17458080.2013.829704).
References
- Shang , L , Li , B J , Dong , W J , Chen , B Y , Li , C R , Tang , W H , Wamg , G , Wu , J and Ying , Y B . 2010 . Heteronanostructure of Ag particle on titanate nanowire membrane with enhanced photocatalytic properties and bactericidal activities . J. Hazard. Mater. , 178 : 1109 – 1114 . doi: 10.1016/j.jhazmat.2010.01.093
- Damodar , R A , You , S J and Chou , H H . 2009 . Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes . J. Hazard. Mater. , 172 : 1321 – 1328 . doi: 10.1016/j.jhazmat.2009.07.139
- Karunakaran , C , Rajeswari , V and Gomathisankar , P . 2010 . Antibacterial and photocatalytic activities of sonochemically prepared ZnO and Ag-ZnO . J. Alloys Compounds , 508 : 587 – 591 . doi: 10.1016/j.jallcom.2010.08.128
- Sayilkan , F , Asitürk , M , Kiraz , N , Burunkaya , E , Arpaç , E and Sayilkan , H . 2009 . Photocatalytic antibacterial performance of Sn4 +-doped TiO2 thin films on glass substrate . J. Hazard. Mater. , 162 : 1309 – 1316 . doi: 10.1016/j.jhazmat.2008.06.043
- Page , K , Wilson , M and Parkin , I P . 2009 . Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections . J. Mater. Chem. , 19 : 3819 – 3831 . doi: 10.1039/b818698g
- Tam , K H , Djurišić , A B , Chan , C MN , Xi , Y Y , Tse , C W , Leung , Y H , Chan , W K , Leung , F CC and Au , D TW . 2008 . Antibacterial activity of ZnO nanorods prepared by a hydrothermal method . Thin Solid Films , 516 : 6167 – 6174 . doi: 10.1016/j.tsf.2007.11.081
- Adams , L K , Lyon , D Y and Alvarez , P JJ . 2006 . Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions . Water Res. , 40 : 3527 – 3532 . doi: 10.1016/j.watres.2006.08.004
- Rincón , A G and Pulgarin , C . 2005 . Use of coaxial photocatalytic reactor (CAPHORE) in the TiO2 photo-assisted treatment of mixed E. coli and Bacillus sp. and bacterial community present in wastewater . Catal. Today , 101 : 331 – 344 . doi: 10.1016/j.cattod.2005.03.022
- Jones , N , Ray , B , Ranjit , K T and Manna , A C . 2008 . Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms . FEMS Microbiol. Lett. , 279 : 71 – 76 . doi: 10.1111/j.1574-6968.2007.01012.x
- Sawai , J . 2003 . Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay . J. Microbiol. Methods , 54 : 177 – 182 . doi: 10.1016/S0167-7012(03)00037-X
- Yamamoto , O , Nakakoshi , K , Sasamoto , T , Nakagawa , H and Miura , K . 2001 . Adsorption and growth inhibition of bacteria on carbon materials containing zinc oxide . Carbon , 39 : 1643 – 1651 . doi: 10.1016/S0008-6223(00)00289-X
- Caballero , I , Whitehead , K A , Allen , N S and Verrai , J . 2009 . Inactivation of Escherichia coli on immobilized TiO2 using fluorescent light . J. Photochem. Photobiol. A , 202 : 92 – 98 . doi: 10.1016/j.jphotochem.2008.11.005
- Văcăroiu , C , Enache , M , Gartner , M , Popescu , G , Anastasescu , M , Brezeanu , A , Todorova , N , Giannakopoulou , T , Trapalis , C and Dumitru , L . 2009 . The effect of thermal treatment on antibacterial properties of nanostructured TiO2(N) films illuminated with visible light . World J. Microbiol. Biotechnol. , 25 : 27 – 31 . doi: 10.1007/s11274-008-9856-6
- Cushnie , T PT , Robertson , P KJ , Officer , S , Pollard , P M , McCullagh , C and Robertson , J MC . 2009 . Variables to be considered when assessing the photocatalytic destruction of bacterial pathogens . Chemosphere , 74 : 1374 – 1378 . doi: 10.1016/j.chemosphere.2008.11.012
- Wong , M S , Chu , W C , Sun , D S , Huang , H S , Chen , J H , Tsai , P J , Lin , N T , Yu , M S , Hsu , S F , Wang , S L and Chang , H H . 2006 . Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens . Appl. Environ. Microbiol. , 72 : 6111 – 6116 . doi: 10.1128/AEM.02580-05
- Shankar , K , Basham , J I , Allam , N K , Varghese , O K , Mor , G K , Feng , X J , Paulose , M , Seabold , J A , Choi , K -S and Grimes , C A . 2009 . Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry . J. Phys. Chem. C , 113 : 6327 – 6359 . doi: 10.1021/jp809385x
- Macak , J M , Tsuchiya , H , Ghicov , A , Yasuda , K , Hahn , R , Bauer , S and Schmuki , P . 2007 . TiO2 nanotubes: Self-organized electrochemical formation, properties and applications . Curr. Opin. Solid State Mater. Sci. , 11 : 3 – 18 . doi: 10.1016/j.cossms.2007.08.004
- Ghicov , A and Schmuki , P . 2009 . Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures . Chem. Commun. , : 2791 – 2808 . doi: 10.1039/b822726h
- Sreekantan , S , Hazan , R and Lockman , Z . 2009 . Photoactivity of anatase–rutile TiO2 nanotubes formed by anodization method . Thin Solid Films , 518 : 16 – 21 . doi: 10.1016/j.tsf.2009.06.002
- Li , G , Liu , Z -Q , Lu , J , Wang , L and Wang , Z . 2009 . Effect of calcination temperature on the morphology and surface properties of TiO2 nanotube arrays . Appl. Surf. Sci. , 255 : 7323 – 7328 . doi: 10.1016/j.apsusc.2009.03.097
- Mohammadpour , B , Irajizad , A , Ahadian , M M , Taghavinia , N and Dolati , A . 2009 . Comparison of various anodization and annealing conditions of titanium dioxide nanotubular film on MB degradation . Eur. Phys. J. Appl. Phys. , 47 10601 (pp. 1–7) doi: 10.1051/epjap/2009070
- Raja , K S , Gandhi , T and Misra , M . 2007 . Effect of water content of ethylene glycol as electrolyte for synthesis of ordered titania nanotubes . Electrochem. Commun. , 9 : 1069 – 1076 . doi: 10.1016/j.elecom.2006.12.024
- Shankar , K , Mor , G K , Praksam , H E , Yoriya , S , Paulose , M , Varghese , O K and Grimes , C A . 2007 . Highly-ordered TiO2 nanotube arrays up to 220 μm in length: Use in water photoelectrolysis and dye-sensitized solar cells . Nanotechnology , 18 065707 (pp. 1–11) doi: 10.1088/0957-4484/18/6/065707
- Zhang , Z R , Du , Y G , Petrik , N G , Kimmel , G A , Lyubinetsky , I and Dohnálek , Z . 2009 . Water as a catalyst: Imaging reactions of O2 with partially and fully hydroxylated TiO2(110) surfaces . J. Phys. Chem. C , 113 : 1908 – 1916 . doi: 10.1021/jp809001x
- Petrik , N G , Zhang , Z R , Du , Y G , Dohnálek , Z , Lyubinetsky , I and Kimmel , G A . 2009 . Chemical reactivity of reduced TiO2(110): The dominant role of surface defects in oxygen chemisorption . J. Phys. Chem. C , 113 : 12407 – 12411 . doi: 10.1021/jp901989x
- Soria , J , Sanz , J , Sobrados , I , Coronado , J M , Maria , A J , Hernández-Alonso , M D and Fresno , F . 2009 . FTIR and NMR study of the adsorbed water on nanocrystalline anatase . J. Phys. Chem. C , 111 : 10590 – 10596 . doi: 10.1021/jp071440g
- Diebold , U . 2003 . The surface science of titanium dioxide . Surf. Sci. Rep. , 48 : 53 – 229 . doi: 10.1016/S0167-5729(02)00100-0
- Zhang , J L , Xu , H S , Chen , H J and Anpo , M . 2003 . Study on the formation of H2O2 on TiO2 photocatalysts and their activity for the photocatalytic degradation of X-GL dye . Res. Chem. Intermed. , 29 : 839 – 848 . doi: 10.1163/156856703322601843
- Di Paola , A , Bellardita , M , Ceccato , R , Palmisano , L and Parrino , F . 2009 . Highly active photocatalytic TiO2 powders obtained by thermohydrolysis of TiCl4 in water . J. Phys. Chem. C , 113 : 15166 – 15174 . doi: 10.1021/jp904673e
- Thompson , T L and Yates , J T Jr . 2005 . TiO2-based photocatalysis: Surface defects, oxygen and charge transfer . Top. Catal. , 35 : 197 – 210 . doi: 10.1007/s11244-005-3825-1
- Daimon , T , Hirakawa , T , Kitazawa , M , Suetake , J and Nosaka , Y . 2008 . Formation of singlet molecular oxygen associated with the formation of superoxide radicals in aqueous suspensions of TiO2 photocatalysts . Appl. Catal. A , 340 : 169 – 175 . doi: 10.1016/j.apcata.2008.02.012
- Augustynski , J . 1993 . The role of the surface intermediates in the photoelectrochemical behaviour of anatase and rutile TiO2 . Electrochim. Acta , 38 : 43 – 46 . doi: 10.1016/0013-4686(93)80008-N
- Wang , X J , Liu , Y F , Hu , Z H , Chen , Y J , Liu , W and Zhao , G H . 2009 . Degradation of methyl orange by composite photocatalysts nano-TiO2 immobilized on activated carbons of different porosities . J. Hazard. Mater. , 169 : 1061 – 1067 . doi: 10.1016/j.jhazmat.2009.04.058
- Hernández-Alonso , M D , Fresno , F , Suárez , S and Coronado , J M . 2009 . Development of alternative photocatalysts to TiO2: Challenges and opportunities . Energy Environ. Sci. , 2 : 1231 – 1257 . doi: 10.1039/b907933e
- Cho , M , Chung , H , Choi , W and Yoon , J . 2004 . Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection . Water Res. , 38 : 1069 – 1077 . doi: 10.1016/j.watres.2003.10.029
- Kikuchi , Y , Sunada , K , Iyoda , T , Hashimoto , K and Fujishima , A . 1997 . Photocatalytic bactericidal effect of TiO2 thin films: Dynamic view of the active oxygen species responsible for the effect . J. Photochem. Photobiol. A , 106 : 51 – 56 . doi: 10.1016/S1010-6030(97)00038-5
- Ishibashi , K , Fujishima , A , Watanabe , T and Hashimoto , K . 2000 . Quantum yields of active oxidative species formed on TiO2 photocatalyst . J. Photochem. Photobiol. A , 134 : 139 – 142 . doi: 10.1016/S1010-6030(00)00264-1