Abstract
Iron titanate nanopowders with a particle size range of 48–70 nm could be obtained after calcinations of the dried gel at 900°C for 2 h. Fe2TiO5 indicates a ferrimagnetic–paramagnetic behaviour, as evidenced by using vibrating sample magnetometer at room temperature. In the temperature range of 25–300°C the empirical equation of the heat capacity C p (J/mol K) = −692.328 + 1.39 T + 3.757 × 107/T 2 for Fe2TiO5 was determined from differential scanning calorimetry. Direct optical band gap of Fe2TiO5 was calculated using the Tauc model by UV-Vis diffuse reflectance spectroscopy. Band gap energy of Fe2TiO5 was determined as 1.95 eV.
1. Introduction
Titanium-substituted iron oxides are widespread in nature and represent an important mineral resource for the commercial extraction of both iron and titanium Citation1. Mixed metal oxides have been widely investigated since they have interesting catalytic properties Citation2. Chemical properties of the active sites can be significantly modified by mixing oxides, in the form of solid solutions, or by supporting the oxide catalyst on another oxide Citation3,Citation4. There has been growing interest in iron titanium oxide as a good candidate for new optical fibres Citation5–7, and as a sensor material for the detection of NO2 Citation8 and gas sensing applications Citation9. The polycrystalline ferrites have been studied for several years due to their wide use as magnetic materials for telecommunications audio and video power transformers and many other applications. The crystallographic, electrical and magnetic properties depend strongly on the stoichiometry, preparation conditions and particle size Citation10–21.
Fe-titanates exist principally as three minerals, namely, ilmenite (FeTiO3), pseudo-brookite (Fe2TiO5) and ulvöspinel (Fe2TiO4). These multifunctional oxides can be both ferrimagnetic and wide band gap semiconductors, and can be exploited in a variety of ways in radhard electronics, microelectronics and spintronics technologies Citation22.
The chemical synthesis of solid inorganic materials is making an increasingly important contribution to the development and manufacture of ceramic materials Citation23,Citation24. It also plays a vital role in the development of fabrication techniques for the successful exploitation of various useful properties of ceramics Citation25. There is a growing realisation that a single-source precursor with different metals assembled in one molecule in the desired metal ratios can lead to nanometre level defined morphology and interesting physical properties Citation26,Citation27.
In recent years, nano-structured iron–titanium mixed oxides with different Fe/Ti ratios were prepared by sol–gel methods under different preparative conditions with a mixture of iron–titanium oxides prepared in different calcination temperatures Citation28. Also polycrystalline Fe2TiO5 films were prepared on nesa silica glass substrates by the sol–gel method, and their photoanodic properties were measured in a three-electrode wet cell with an aqueous buffer solution of pH = 7 Citation29.
The sol–gel technique has many advantages over other fabrication techniques, such as homogeneity, stoichiometry control, purity, ease of processing and controlling the composition, and ability to coat large and complex area substrates Citation30.
In this study, we chose one typical wet-chemistry synthesis method, stearic acid gel, to prepare pure Fe2TiO5 nanopowders. The value of optical band gap, empirical equation of heat capacity and magnetisation of the iron titanate were determined.
2. Experimental
Fe2TiO5 powders were prepared along a synthetic procedure, as summarised in . Iron acetyl acetonate, tetra-n-butyl titanate and stearic acid were all analytical grade reagents. First, an appropriate amount of stearic acid was melted in a beaker at 73°C, and then a fixed amount of iron acetyl acetonate was added to the melted stearic acid and dissolved to form a transparent brown solution. Next, stoichiometric tetrabutyl titanate was added to the solution, stirring to form a homogeneous light brown solution, naturally cooling down to room temperature and drying in an oven for 12 h to obtain dried gel. Finally, the gel was calcined at 700°C, 800°C and 900°C temperatures in air to obtain nano-crystallites of Fe2TiO5.
The formation of process and structural characterisation of Fe2TiO5 phases have been investigated by XRD, Fourier transform infrared spectroscopy (FTIR), EDX, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) and the physical properties have been investigated by vibrating sample magnetometer (VSM), differential scanning calorimetry (DSC) and diffuse reflectance spectroscopy (DRS).
Powder X-ray diffraction patterns of the samples were recorded using X`pert pro Philips Diffractometer with Cu-Kα radiation (λ = 0.15418 nm) in the range from 10° to 80° (2θ) to examine the crystallisation and structural development of Fe2TiO5 powders.
The morphology of the product was studied by SEM(Philips XL30) and TEM (Zeiss EM900, 80 keV).
The FTIR analysis was performed using (Magna-IR 550 Nicolet) spectrometer by employing KBr pellet technique. The chemical composition of the product was tested by energy dispersive X-ray spectroscopy (EDS, Philips XL30). Magnetisation measurement is carried out using VSM (BHV-55, Riken, Japan) at room temperature.
The specific heat capacity was measured by scanning method and using a differential scanning calorimeter and DSC (PL DSC-polymer laboratory) in a pure nitrogen atmosphere. The sample (calcined at 900°C) was heated from 25°C to 300°C with a heating rate of 10°C min−1. The UV-Vis DRS was performed on an AVASPEC spectroscopy.
3. Results and discussion
3.1. X-ray diffraction patterns
shows the XRD patterns of the Fe2TiO5 powders after heat-treatment from 700°C to 900°C in air for 2 h. At 700°C, the crystallisation of orthorhombic iron titanate phase (▴ marked peaks) began along with traces of hematite phase (▪ marked peaks) (). Further, by increasing the calcination temperature to 800°C, the hematite phase was decreased with an increase in the intensity of Fe2TiO5 phase (). The nanopowders were obtained after calcination at 900°C ().
Figure 2. X-ray diffraction patterns of Fe2TiO5 powders calcined at (a) 700°C; (b) 800°C and (c) 900°C.
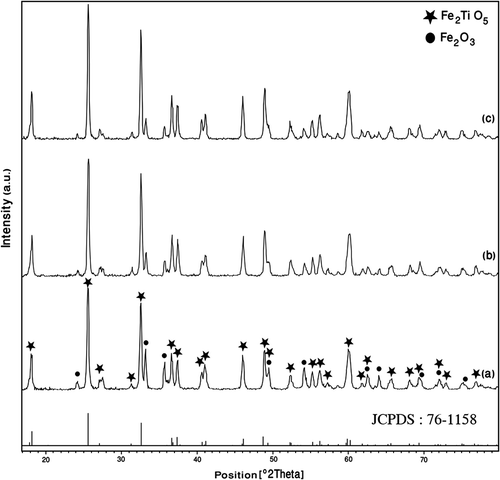
However, at this temperature, the nanopowders displayed sharp and intense peaks indicating fine crystalline orthorhombic Fe2TiO5 phase. All the peaks corresponding to orthorhombic phase were well matched with database in JCPDS (file number: 76–1158).
3.2. Morphology of samples
X-ray diffraction has been performed on powder and the particles size L has been estimated with the Scherrer formula Citation31:
The particle size was calculated by Scherrer's formula for different calcination temperatures. The crystallite size of the powders calcined at 700°C, 800°C and 900°C were about 34, 27 and 34 nm, respectively.
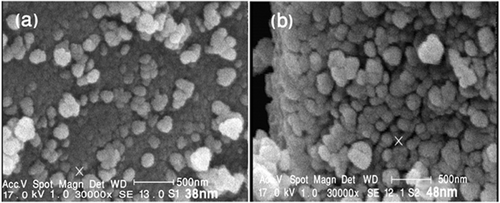
The surface morphology of the obtained Fe2TiO5 nanocrystals was investigated by SEM images at 800°C and 900°C. SEM images in revealed that the surface morphology of Fe2TiO5 particles was quasi-spheric. Images show that all powders have agglomerated graining structure.
is the TEM micrograph of Fe2TiO5 powders calcined at 900°C for 2 h. Image shows that all powders have agglomerated graining structure. The particle size was estimated in the ranges about 48–70 nm.
TEM counting provides a larger average grain size than XRD. The reason for this apparent discrepancy is that the size provided by XRD corresponds to the average of the smallest undistorted regions in the material, whereas TEM counting is related to regions separated by more-or-less sharp contours in the TEM micrograph. Therefore, when dislocations are arranged in a configuration, which causes small orientation differences between two adjacent regions, the XRD size corresponds to the two separate regions, whereas in the TEM micrograph the two regions may seem to correspond to the same grain Citation32.
3.3. EDS and IR spectra
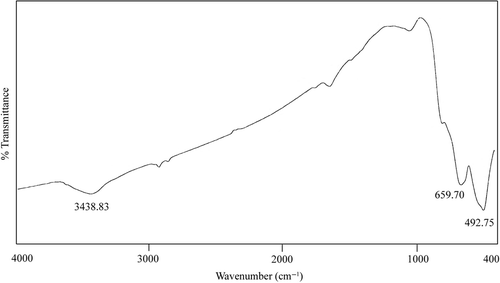
EDS spectrum of the as-synthesised nanopowder confirms that our product consists of iron and titanium elements. No other phase was shown to be present in the prepared iron titanate product. The formation of Fe2TiO5 is further supported by the FTIR spectra, as shown in . In this spectrum the Fe2TiO5 powders calcined at 900°C showed peak at 493 cm−1which is assigned to the Fe–O absorption band and the strong peak at around 660 cm−1 can be assigned to Fe2TiO5, corresponding to the formation of iron titanate Citation28. The broad absorption observed at 3439 cm−1 for Fe2TiO5 nanostructure was assigned to the stretching vibrations of –OH group of adsorbed water Citation33.
3.4. Magnetic properties
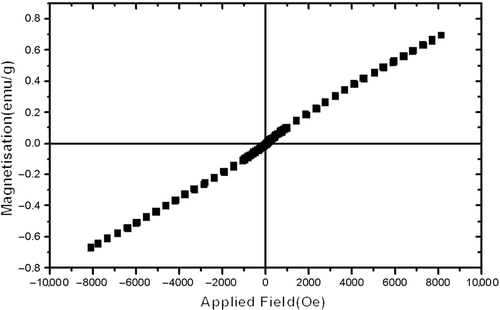
Iron titanates are reported as ferrimagnetic materials Citation22, but specifically an anti-ferromagnetic ordering with weak ferromagnetism has been predicted for Fe2TiO5 Citation34. Comparison of the hysteresis loops () measured at room temperature with typical curves obtained from mixed magnetic systems shows a ferrimagnetic–paramagnetic behaviour of the high-pure Fe2TiO5 nanopowders Citation35. On the other hand, XRD patterns show rhombohedral Fe2O3 (as impurity). The magnetic behaviour of α-Fe2O3 (rhombohedral) and γ-Fe2O3 (cubic) is obviously different. Single-crystal α-Fe2O3 is paramagnetic, whereas γ-Fe2O3 is ferrimagnetic at room temperature Citation36. Therefore the ferrimagnetic–paramagnetic behaviour of the sample originated from Pseudobrookite and hematite phases, respectively.
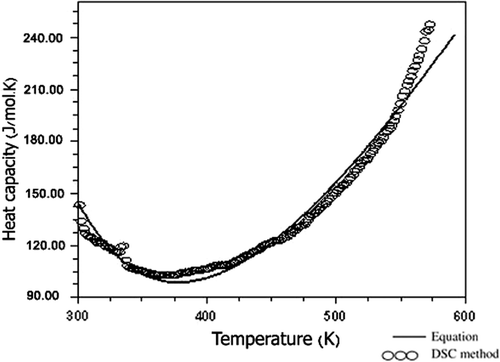
3.5. Heat capacity
The titanium-based oxides are well-known owing to their excellent dielectric, piezoelectric, pyroelectric and photostrictive properties Citation37,Citation38. Fe2TiO5 is a ferroelectromagnetic material in which both ferroelectric and magnetic ordering co-exist simultaneously Citation39. Piezoelectric and pyroelectric phenomena coexist in ferroelectric materials Citation40 whereas pyroelectric and piezoelectric properties are related to heat capacity Citation41. The temperature dependence of the heat capacity of Fe2TiO5 determined by the scanning method is shown in . In the temperature range between 25°C and 300°C, the empirical equation for the Cp of Fe2TiO5 is determined from the experimental values as follows:
3.6. Optical properties
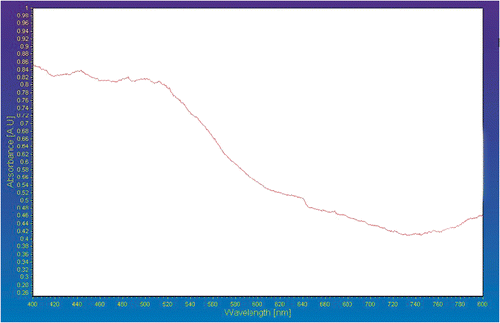
The absorption coefficient and optical band gap of a material are two important parameters by which the optical characteristics and its practical applications in various fields are judged. displays absorption versus wavelength behaviour of iron titanate calcined at 900°C by UV-Vis DRS.
The nature of the optical inter-band transition and value of the energy gap Eg can be determined using the Tauc model:
Figure 9. Tauc plot exhibiting (αhν)2 versus photon energy (hν) for the calculation of direct band gap of Fe2TiO5 by extrapolating on hν axis α = 0.
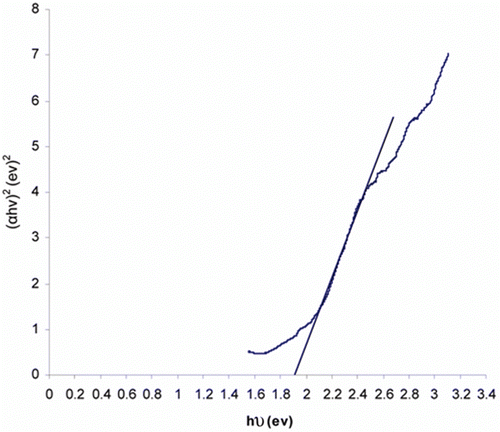
The value of direct band gap for iron titanate came out to be 1.95 eV.
4. Conclusion
This study has demonstrated the feasibility of the synthesis of pure Fe2TiO5 powders using a wet-chemistry synthesis method using stearic acid gel. Well-crystallised Fe2TiO5 nanopowders could be synthesised at 900°C for 2 h. In addition, the microstructures of Fe2TiO5 powders have been evaluated using TEM and the grain sizes are shown to vary between 48 and 70 nm. This study also showed that the synthesised Fe2TiO5 has indicated a ferrimagnetic–paramagnetic behaviour, as evidenced by using VSM at room temperature. Moreover, the empirical equation for the C p of Fe2TiO5 has been determined from DSC. The band gap energy of Fe2TiO5 was determined by DRS too.
Acknowledgements
The authors thank the Iranian Nanotechnology Initiative for supporting this work.
References
- Taylor , R W . 1964 . Phase equilibria in the system FeO–Fe2O3–TiO2, at 1300°C . Am. Mineral. , 49 : 1016 – 1030 .
- Seiyama , T . 1978 . Metal Oxides and Their Catalytic Actions , Tokyo : Kodansha Scientific Books .
- Wachs , I E . 2005 . Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials . Catal. Today , 100 : 79 – 94 .
- Wachs , I E and Segawa , K . 1992 . “ Supported metal oxides ” . In Characterization of Catalytic Materials , Edited by: Wachs , IE . 69 – 88 . Boston , MA : Butterworth-Heinemann .
- Fades , B D , Zelinski , B J and Uhlmann , D R . 1993 . “ Sol-gel derived ceramic coatings ” . In Ceramic Films and Coatings , Edited by: Watchmann , J and Haber , JRA . 224 – 283 . Park Ridge , NJ : Noyes Publications .
- Dislich , H . 1993 . “ Thin films from the sol-gel process ” . In Sol–gel Technology for Thin Films, Fibers, Performs, Electronics, and Speciality Shapes , Edited by: Klein , LC . 50 – 79 . Park Ridge , NJ : Noyes Publications .
- Pettit , R B , Ashley , C S , Readand , S T and Brinker , C J . 1993 . “ Antireflective films from the sol-gel process ” . In Sol–gel Technology for Thin Films Fibers Performs Electronics and Speciality Shapes , Edited by: Klein , LC . 80 Park Ridge , NJ : Noyes Publications .
- Bein , T , Brown , K , Fye , G C and Brinker , C J . 1989 . Molecular sieve sensors for selective detection at the nanogram level . J. Am. Cer. Soc. , 111 : 7640 – 7641 .
- Yan , Y and Bein , T . 1994 . “ Design of thin films with nanometer porosity for molecular recognition ” . In Interfacial Design and Chemical Sensing , Edited by: Mallouk , TE and Harrison , DJ . 16 – 26 . Washington , DC : American Chemical Society .
- Randhawa , B S , Kaur , S and Bassi , P S . 2000 . Calcium ferrite formation from the thermolysis of calcium tris (maleato) ferrate(III) . Bull. Mater. Sci. , 23 : 305 – 307 .
- Dong , L , Liu , Z , Hu , Y , Xu , B and Chen , Y . 1998 . Dispersion and reduction behavior of CuO/α-Fe2O3 systems . J. Chem. Soc. Faraday Trans. , 94 : 3033 – 3038 .
- Khedr , M H , Omar , A A and Abdel-Moaty , S A . 2006 . Magnetic nanocomposites: Preparation and characterization of Co-ferrite nanoparticles . Colloids Surf A Physicochem. Eng. Aspects , 281 : 8 – 14 .
- Lahiri , P and Sengupta , S . 1995 . Physico-chemical properties and catalytic activities of the spinel series MnxFe3–xO4 towards peroxide decomposition . J. Chem. Soc. Faraday Trans. , 91 : 3489 – 3494 .
- Singh , N K , Tiwari , S K , Anitha , F KI and Singh , R N . 1996 . Synthesis of (La, Sr)CoO3 perovskite films through a sol–gel route and their physicochemical and electrolysis . J. Chem. Soc. Faraday Trans. , 92 : 2397 – 2400 .
- Khedr , M H , Omar , A A , Nasr , M I and Sedeek , E K . 2006 . Effect of firing temperature on microstructure and magnetic properties of nanocrystalline Ni0.5Zn0.5Fe2O4 prepared by wet and dry methods . J. Anal. Appl. Pyrolysis , 76 : 203 – 208 .
- Ramoukatty , C G and Sugunan , S . 2001 . Surface properties and catalytic activity of ferrospinels of nickel, cobalt and copper, prepared by soft chemical methods . J. Appl. Catal. A Gen. , 218 : 39 – 51 .
- Jebarthinan , N J and Krishnaswamy , V . 1995 . “ Catalysts ” . In Catalysis – Present and Future , Edited by: Kanta Rao , P , Tagore , S and Benwal , BS . 228 New Delhi : Wiley .
- Radwan , N RE and El-Shobaky , H G . 2000 . Solid–solid interactions between ferric and cobalt oxides as influenced by Al2O3-doping . Thermochim. Acta , 360 : 147 – 156 .
- El-Shobaky , G A , Radwan , N RE and Radwan , E M . 2001 . Investigation of solid–solid interactions between pure and LiO2 doped magnesium and ferric oxide . Thermochim. Acta , 380 : 27 – 35 .
- Bujoreanu , V M and Segal , E . 2000 . On the kinetics of manganese ferrite formation from aqueous solution of MnO2 and FeSO4·7H2O . J. Therm. Anal. Calorim. , 61 : 967 – 977 .
- Enhessari , M , Kargar-Razi , M , Moarefi , P and Parviz , A . Synthesis, Characterization and photocatalytic properties of MnTiO3-Zeolite-Y nanocomposites, J. Nanostruct. 1 (2012), pp. 119–125
- Ishikawa , Y . 1957 . Magnetic properties of the FeTiO3–Fe2O3 solid solution series . J. Phys. Soc. Jpn. , 12 ( 10 ) : 1083 – 1098 .
- Rao , C NR and Gopalakrishnan , J . 1997 . New Directions in Solid State Chemistry , 2nd , 1 – 535 . UK : Cambridge University Press .
- Segal , D . 1997 . Chemical synthesis of ceramic materials . J. Mater. Chem. , 7 : 1297 – 1305 .
- Yoshimura , M and Livage , J . (Guest eds.), Soft processing for advanced inorganic materials, MRS Bull. 25 (2000), pp. 12–13
- Hubert-Pfalzgraf , L G . 2004 . To what extent can design of molecular precursors control the preparation of high tech oxides . J. Mater. Chem. , 14 : 3113 – 3123 .
- Kesler , V G . 2003 . Molecular structure design and synthetic approaches to the heterometallic alkoxide complexes (soft chemistry approach to inorganic materials by the eyes of a crystallographer) . Chem. Commun. , 1 : 1213 – 1222 .
- Khaleel , A . 2009 . Sol–gel synthesis, characterization, and catalytic activity of Fe(III) titanates . Colloids Surf. A Physicochem. Eng. Aspects , 346 : 130 – 137 .
- Kozuka , H and Kajimura , M . 2001 . Sol–gel preparation and hotoelectrochemical properties of Fe2TiO5 thin films . JSST , 22 : 125 – 132 .
- Dislich , H and Glaswerke , S . 1986 . Sol-gel: Science, processes and products . J. Non-Crystalline Solids , 80 : 115 – 1210 .
- Guinier , A . 1957 . Théorie ET technique de la radiocristallographie, éd dunod . Acta Cryst. , 10 : 386 – 387 .
- Ungár , T . 2007 . Characterization of nanocrystalline materials by X-ray line profile analysis . J. Mater. Sci. , 42 : 1584 – 1593 .
- Li , L , Pan , Y , Chen , L and Li , G . 2007 . One-dimensional α-MnO2: Trapping chemistry of tunnel structures, structural stability, and magnetic transitions . J. Solid State Chem. , 180 : 2896 – 2904 .
- Hanel , D and Sevek , F . 1984 . Characterization of monoclinic Fe2TiO5 by Mössbauer spectroscopy and magnetic susceptibility . Mater. Res. Bull. , 19 : 35 – 39 .
- Roberts , A P , Cui , Y and Verosub , K L . 1995 . L Wasp-waisted hysteresis loops: Mineral magnetic characteristics and discrimination of components in mixed magnetic systems . J. Geophys. Res. , 100 : 17909 – 17924 .
- Wang , T , Jiang , X and Cong Huang , H C . 2010 . Influence of different preparation methods on crystallization and morphology of Fe2O3 nanoparticles . Micro Nano Lett. , 5 : 196 – 199 .
- Enhessari , M , Parviz , A , Ozaee , K and Karamali , E . 2010 . Magnetic properties and heat capacity of CoTiO3 nanopowders prepared by stearic acid gel method . J. Exp. Nanosci. , 5 : 61 – 68 .
- Enhessari , M , Parviz , A , Karamali , E and Ozaee , K . 2010 . Synthesis, characterisation and optical properties of MnTiO3 nanopowders . J. Exp. Nanosci. , DOI: 10.1080/17458080.2010.529173 (in press)
- Prasad , N V , Srinivas , K , Kumar , G S and James , A R . 2001 . Impedance measurements on TiO2–Fe2O3 thin films . Appl. Phys. A Mater. Sci. Process , 72 : 341 – 345 .
- Lang , S B . 1974 . Sourcebook of Pyroelectricity , London, New York : Gordon and Breach Science Publishers .
- Yan , K R . Piezo-pyroelectric energy converter and method, Kucherov R. Yan (United States), inventors, Thermodyne Technologies, (United States), Canadian Patent, A 223877130508, 1997
- Khan , W S and Cao , C . 2010 . Synthesis, growth mechanism and optical characterization of zinc nitride hollow structures . J. Cryst. Growth , 312 : 1838 – 1843 .