Abstract
The biosynthesis of silver nanoparticles (AgNps) has a wide range of applications, and here we develop a rapid synthesis using the leaf extract of Ipomea carnea. We demonstrated that 100 mL of a 1 mM silver nitrate solution was reduced to AgNps by 500 µL of I. carnea extract in 5 min and that one or more of the chemical constituents present in the extract acted as the reducing agent. Surface plasmon resonance peaks were observed from 410 to 440 nm for AgNps synthesised using the plant extract, and the peaks showed a characteristic blue shift with variation of pH from 2 to 8. Particle size analysis revealed the size of the AgNps to be from 30 to 130 nm, which was also confirmed by dynamic light scattering, atomic force microscopy and transmission electron microscopy. Additionally, the antibacterial effects of the AgNps were evaluated against selected human pathogens such as Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Klebsiella pneumoniae, Aeromonas hydrophila, Salmonella typhi, Proteus vulgaris and Pseudomonas aeruginosa. Finally, the AgNps were impregnated with a cellulose acetate membrane to form an antimycobacterial membrane. Antimycobacterial activity against a non-pathogenic Mycobacterium smegmatis showed that the AgNp-embedded membrane system has a zone of inhibition of 14 mm.
1. Introduction
Nanoparticles are being studied for many applications, including biosensors, labels for cells and biomolecules, anti-microbial agents and cancer therapeutics Citation1–4. Silver nanoparticles have wide industrial applications in electrocatalysis, chemical sensors, catalysis and optical devices Citation5–8. Recently, the synthesis of silver nanoparticles has been reported using several methods: such as chemical, gamma-irradiation, thermal decomposition, photochemical and biosynthetic using microbes and plant resources Citation9–13.
Ipomea carnea is a fast-growing noxious weed of negligible economic value. However, it possesses polyphenols and alkaloids that can be utilised as reducing agents in the synthesis of nanoparticles. The antimicrobial properties of nanoparticles are well established, and a mechanism of inhibition has been proposed by several researchers Citation14–17. Silver is used traditionally as a disinfecting agent in medicine and as a culinary agent Citation18. AgNps are biosynthesised and widely used in medicine due to their remarkable physical, chemical and biological properties stemming from their large surface area and high reactivity. AgNps interact with a pathogen's nucleic material and hinder respiratory enzymes, thus eliminating pathogenicity Citation19.
This study describes the synthesis of silver nanoparticles using the weed I. carnea and their antibacterial activity against selected human pathogens (both Gram-positive and Gram-negative) and one non-pathogenic bacterium, Mycobacterium smegmatis. An attempt was made to fabricate a silver nanoparticle-impregnated cellulose acetate (CA) membrane, and the membrane was tested against M. smegmatis, which is analogous to M. tuberculosis. This membrane can be used as a mask to prevent infection among healthcare workers involved with tuberculosis (TB) patients.
2. Materials and methods
All chemicals were obtained from Sigma–Aldrich (USA) and used as received. A UV-vis spectrophotometer (JASCO V-650, Japan) containing double beams in identical compartments for reference and test solutions and fitted with a 1 cm path length quartz cuvette was used for this study.
The FT–IR spectra were recorded using a FT–IR (Perkin Elmer, USA) spectrophotometer. The particle size of synthesised AgNps was analysed using a particle size analyser (Nanotrac Ultra DLS, USA). The topography of the AgNps was analysed using an atomic force microscope (AFM; Veeco – di Innova, USA) with a silicon nitride tip and spring constant ranging from 0.01 to 0.1 N/m. The size and shape of the AgNps were analysed using a transmission electron microscope (TEM; JOEL JEM SX 100, Japan) operating at 100 keV. The I. carnea-stabilised AgNps were prepared for TEM measurement by placement of a drop of nanoparticles on carbon-coated copper grid followed by drying under vacuum.
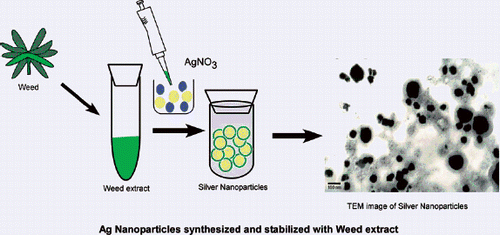
2.1. Preparation of plant extract and synthesis of nanoparticles
Fresh I. carnea leaves were cut and washed with Milli-Q water (18.2 MΩ cm−1 resistivity). The extraction procedure was as follows: 20 g of leaves was added to 100 mL of Milli-Q water and boiled for 60 min. The broth extract was decanted and kept at 4°C for further use. The leaf extract was then mixed with enough 1 mM silver nitrate to give the desired solution (0.5% v/v, 1% v/v, 2% v/v, 5% v/v and 10% v/v) and allowed to react at room temperature. The effect of pH on the resulting solution of AgNps and plant extract was also evaluated.
2.2. Fabrication of silver nanoparticle-impregnated membrane
CA was dissolved in dimethylformamide and continuously stirred with freeze-dried AgNps (5 µg/mL) for 4 h and casted as CA membrane (1 cm2) using the phase inversion technique. The AgNp-impregnated CA membrane was then periodically washed with Milli-Q water to remove trace solvents that also interfere with bacterial growth.
2.3. Antibacterial activity of biosynthesised silver nanoparticles
All the glassware, media and reagents used were sterilised in an autoclave at 121°C for 20 min. For the antibacterial assays, Bacillus cereus (MTCC 6840), Bacillus subtilis (MTCC 8114), Proteus vulgaris (MTCC 425), Stapyhlococcus aureus (MTCC 9542), Pseudomonas aeruginosa (MTCC 2488), Aeromonas hydrophila (MTCC 1739), Klebsiella pneumoniae (MTCC 4032) and Salmonella typhi (MTCC 734) strains were obtained from Institute of Microbial Technology (IMTECH), Chandigarh, India, and subcultures were prepared using reviving nutrient broth. The bacterial suspension was prepared by growing a single colony overnight in nutrient broth and adjusting the turbidity to 0.5 McFarland standards.
2.3.1. Growth curve studies
The bactericidal activity of the silver nanoparticles was tested by growth inhibition studies. Sterile Erlenmeyer flasks containing 100 mL nutrient broth and the desired amount of AgNps were inoculated with 1 mL of the freshly prepared bacterial suspension. The flasks were then incubated in a rotary shaker at 150 rpm at 37°C. Bacterial growth was monitored on an hourly basis for 24 h by measuring the absorbance at 600 nm using a UV-visible spectrophotometer. A control flask containing nutrient broth and bacteria but devoid of AgNps was also monitored.
2.3.2. Zone of inhibition studies – disc diffusion method
A disc diffusion method was used to evaluate the antibacterial activity of silver nanoparticles against selected human pathogens. A bacterial inoculum of 100 µL with 14 × 107 CFU (colony forming units) in their respective log phase were smeared on Mueller–Hinton agar plates. Various concentrations of AgNps (0, 2, 5 and 7 µg) suspended in DMSO were loaded on a 6 mm diameter disc. The discs were placed on the agar plates and incubated at 37°C for 18 h. The zone of inhibition (ZOI) was measured by standard methods. This assay was performed in triplicate and average results were tabulated.
2.3.3. Minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of AgNps was determined for each of the test organisms. The test organisms were inoculated in 5 mL of nutrient broth and incubated at 37°C for 3–4 h. Various concentrations of AgNps (0, 2, 5, 7 and 10 µg) were added to the tubes at the initial log phase of the organism to arrest the growth. Optical density at 620 nm was read for each of the test organisms inoculated with the nanoparticles prior to plating on nutrient agar. The plates were incubated at 37°C for 24 h and the total number of colonies was counted to determine the inhibitory efficacy of the AgNps.
2.3.4. Antimycobacterial studies of silver nanoparticle-impregnated membrane
The disc diffusion assay was carried out using a AgNp-impregnated CA membrane to determine the mycobactericidal activity of AgNps against the Gram-positive bacterium M. smegmatis.A bacterial inoculum of 100 µL with 14 × 107 CFU in their respective log phase were smeared on Mueller–Hinton agar plates. The AgNp-impregnated CA membrane was cut into a disc shape with 6 mm diameter and placed at the centre of the agar plate. A CA membrane without AgNps was used as a control. The plates were incubated at 37°C for 18 h. The ZOI was measured by standard methods.
3. Results and discussion
3.1. UV-visible spectroscopy studies
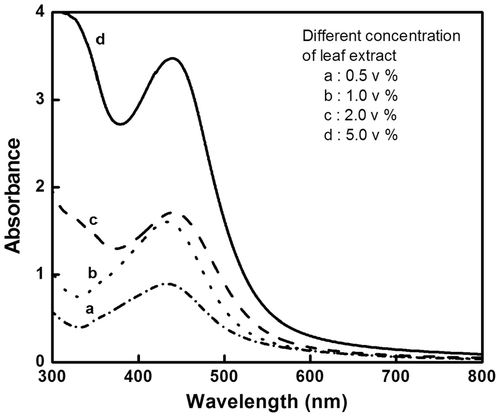
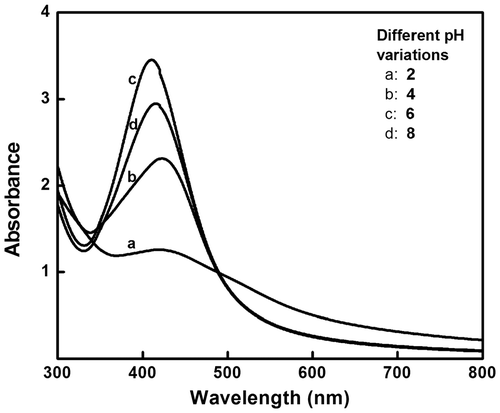
UV-visible absorption spectroscopy is one of the main tools to confirm the formation of nanoparticles in aqueous solutions. A plant extract of 0.5 mL causes a colour change from transparent (aqueous silver nitrate solution) to reddish brown within 5 min, indicating the formation of AgNps (). Noble metal nanoparticles exhibit a specific surface plasmon resonance (SPR) characteristic to each metal. The SPR peak obtained for AgNps varies from 410 to 440 nm, in the range of Mie scattering, which confirms the formation of AgNps Citation20. Increasing the volume ratio of I. carnea extract to silver nitrate solution increases the formation of AgNps, which is evident from the increase in intensity/absorbance of the SPR peak (). pH variation studies were conducted (pH 2, 4, 5, 6 and 8) with 1% v/v of leaf extract mediating AgNp synthesis. As the pH increased from 2 to 8, different SPR peaks appeared showing a characteristic blue shift, indirectly revealing that increasing pH reduces the size of the particle (), which was in accordance with an earlier report Citation21. At pH 5 with 1 mL of leaf extract, the desired size (60–70 nm) nanoparticle was obtained, and hence pH 5 was set as optimum for the synthesis of AgNps.
Figure 1. (Colour online) Colour change from transparent to orange and then to reddish brown when IC leaf extract is added to 1 mM silver nitrate solution.

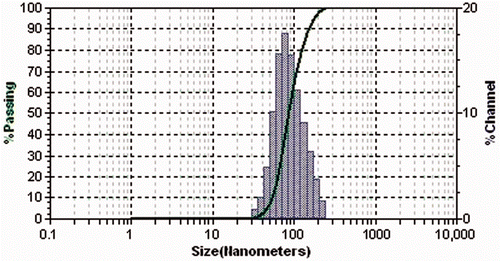
3.2. Particle size analysis
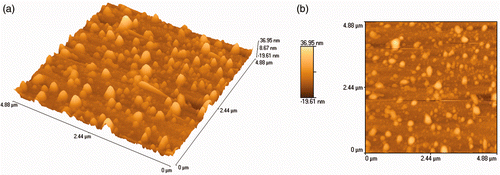
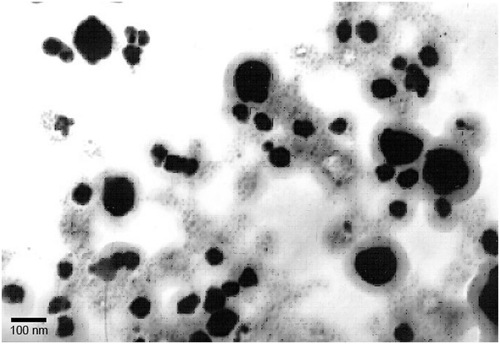
The particle size of the synthesised AgNps was analysed using a dynamic light scattering (DLS) particle size analyser, an AFM and a TEM. The DLS particle size analysis was carried out using a standard analysis time, and the size of AgNps was found to vary between 30 and 130 nm (). Topographic images from AFM analysis showed the presence of uniformly distributed AgNps with similar shape and size, between 25 and 150 nm, as seen in . The transmission electron micrograph () showed the nanoparticle size range to be between 20 and 140 nm, coinciding with the range obtained from both the DLS and AFM analyses. Thus, the DLS, AFM and TEM analyses gave similar results for the size range of the nanoparticles.
Figure 4. DLS particle size analysis of green-synthesised silver nanoparticles having a size range from 30 to 130 nm.
3.3. FT–IR studies and reducing mechanism
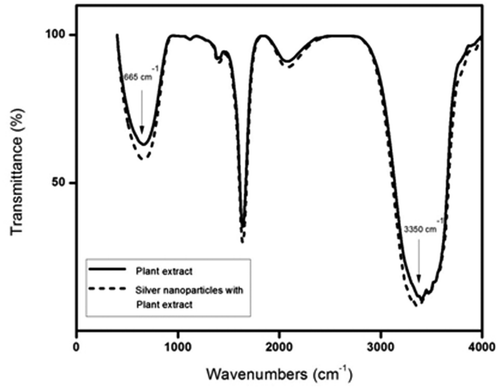
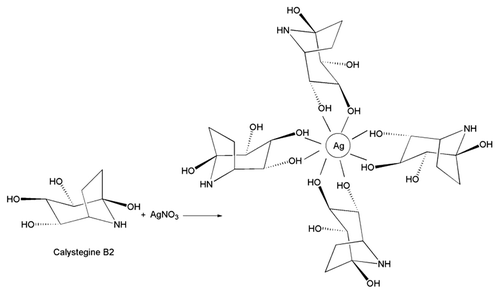
FT–IR analysis was carried out to evaluate functional group changes in the constituents of I. carnea extract before and after the addition of silver nitrate solution (). There was a significant change in the wave number of free hydroxyl groups and phenolic groups (3350 cm−1), suggesting that alkaloids with polyphenols may be involved in the reduction of silver nitrate and further stabilisation of AgNps. Interestingly, there was also a significant change in the wave number corresponding to sulphonic groups (650 cm−1). Earlier work using GC–MS to reveal the phytochemistry of I. carnea reported the presence of calystegine B2, calystegine C1 and swainsonine alkaloids as major chemical constituents Citation22. Thus, polyphenols may be involved in reducing silver nitrate to AgNps ().
3.4. Antibacterial activity
3.4.1. Pattern of bacterial growth inhibition
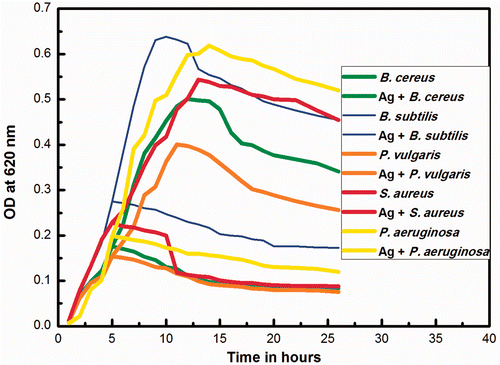
The growth kinetics of the bacteria is shown in . Growth was inhibited in the initial stages, and in media containing AgNps, there was no obvious visible growth after the fourth hour. In the control experiment, we observed typical lag, log and death phases at the 4th, 13th and 26th hour, respectively. All organisms showed a typical inhibitory point after the addition of silver nanoparticles. The growth curves of B. cereus, B. subtilis, S. aureus, K. pneumoniae, A. hydrophila, P. vulgaris, S. typhi and P. aeruginosa showed similar results. There was an increase in growth from the lag to log phase and a constant pattern of growth in the succeeding stationary phase.
3.4.2. Disc diffusion method
The antibacterial activity of the synthesised silver nanoparticles was studied by a qualitative disc diffusion assay on both Gram-positive and Gram-negative bacteria. shows the diameter of the ZOI for each organism. After 18 h of incubation at 37°C, the nutrient agar plates containing various concentrations of nanoparticles exhibited a ZOI of around 11–15 mm diameter (). Control plates devoid of nanoparticles did not exhibit any inhibition zones. Bacterial inhibition may be due to the release of free radical from the surface of the AgNp that attacks the membrane lipids and then lead to a breakdown of membrane function Citation23. Previous studies, using a 6 mg/mL nanoparticle concentration, have found the ZOI to be between 12 and 13.6 mm for both ciprofloxacin-resistant and non-resistant strains of P. aeruginosa Citation24, but our results, using around 7 µg of nanoparticles, gave a much larger ZOI of about 15 mm.
Figure 10. (Colour online) Antibacterial tests: ZOI seen around different concentrations of green-synthesised silver nanoparticles present wells against different microorganisms and inset showing positive control of ethambutol.
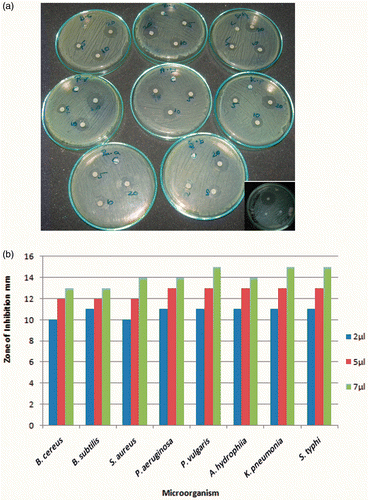
Table 1. ZOI (mm) against different bacterial strains and at different concentrations of silver nanoparticles.
Bacillus cereus, B. subtilis, S. typhi, P. aeruginosa and A. hydrophila showed a ZOI of 13 mm when 7.0 µg of silver nanoparticles were loaded onto the disc. Staphylococcus aureus showed a ZOI of 14 mm. Klebsiella pneumoniae and P. vulgaris showed a ZOI of 15 mm (). When silver nanoparticles with an average size of 22.5 nm prepared by bacterial reduction were employed, Escherichia coli showed an inhibition zone of around 9 mm (10 µg nanoparticle per disc) Citation25. In a different antimicrobial study using chemically synthesised, uncapped silver nanoparticles of 3 nm average size, a ZOI of around 14–15 mm was reported for E. coli strains Citation26. However, this study also used discs with higher nanoparticle loading (100 µg) and fewer bacteria (103–104 CFU). In this study, the average particle size was about 50 nm, and we observed an enhanced antibacterial activity even at a higher number of bacterial colonies (107 CFU) at a much lesser nanoparticle concentration (7 µg per disc). Thus, the effective inhibition observed in our studies is in agreement with earlier reports Citation25,Citation26.
3.4.3. Minimum inhibitory concentration
The MIC of nanoparticles was evaluated to determine the toxicity of silver. This concentration is reached when there is a decrease in bacterial growth but no accompanying toxicity is observed. For all organisms, 20 µL aqueous AgNps having 10 µg AgNp gave a significant decrease in CFUs (). With 40 µL (20 µg AgNps), many fewer colonies were formed. At 100 µL (50 µg AgNps), even fewer CFUs were observed. However, as this last concentration may be toxic to humans, the optimum MIC was determined to be 20 µg.
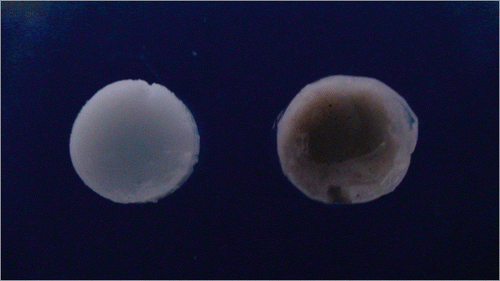
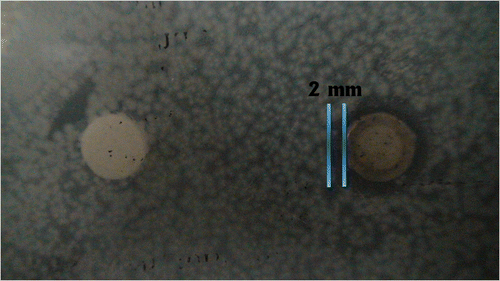
3.4.4. Antimycobacterial membrane studies
The colour of pure CA membrane was significantly different from the colour of AgNp-impregnated CA membrane (). It was found that the silver nanoparticle-impregnated CA membrane exhibited an inhibition zone of 14 mm. No inhibition zone was observed with the pure CA membrane (). This clearly demonstrates that the antimycobacterial activity is due to the silver nanoparticles embedded in the membrane and not due to the CA itself. The development of low fouling membranes or antifouling membranes is an intense area of research in membrane separation technology Citation27,Citation28. The use of nanoparticles in the manufacture of membranes allows for both a high degree of control over membrane fouling and the ability to produce desired structures Citation29–31. In particular, AgNp-impregnated nanofibres and nanofibrillar aerogels can be used for antimicrobial membrane synthesis. The membrane is immersed in silver nitrate and then irradiated with UV light or exposed to sodium borohydride to reduce the silver nitrate, producing a CA membrane with embedded silver nanoparticles. The AgNps synthesised in this article showed better results than previous reports when used to impregnate a CA membrane. Biofouling can be avoided using the bactericidal properties of silver nanoparticles, and indeed, silver is a typical example of a bactericide that is used for fouling mitigation in polymeric membranes. Escherichia coli and Staphylococcus aureus have typically been used as model organisms to evaluate the antimicrobial properties of AgNp-impregnated CA membranes Citation32. However, in this study, we evaluated the antimicrobial activity of AgNps against M. smegmatis.
4. Conclusion
We have demonstrated a simple, fast and green method to synthesise AgNps using I. carnea. The synthesised AgNps were confirmed by UV-visible spectrophotometer. The size, topography and shape of AgNps were measured using a DLS particle size analyzer, AFM and TEM, respectively. The reducing mechanism may involve the hydroxyl groups whose presence in the alkaloids and polyphenols of the extract was confirmed by FT–IR. We also fabricated a CA membrane impregnated with biosynthesised AgNps and tested it against M. smegmatis. The results suggest that the weed extract-based synthesis of silver nanoparticles is very efficient against selected human pathogens and can be used in the fabrication of hospital clothes, gloves and masks to avoid the spread of infection among healthcare workers.
Acknowledgements
Authors S.C.G. Kiruba Daniel and B. Nazeema Banu have equally contributed to this study. We are very much thankful to Anna University of Technology Tiruchirappalli for the funding of Instrument facilities. S.K. sincerely acknowledge the financial assistance from DST Nanomission infrastructure project # SR/NM/PG-05/2008 and Periyar-TBI for instruments facility. B.N.B. acknowledges CSIR for providing Senior Research Fellowship (09/960(002)/2K9-EMR-I).
References
- Nam , J M , Thaxton , C S and Mirkin , C A . 2003 . Nanoparticle-based bio-barcodes for the ultrasensitive detection of proteins . Science , 301 : 1884 – 1886 .
- Tkachenko , A G , Xie , H , Coleman , D , Glomm , W , Ryan , J , Anderson , M F , Franzen , S and Feldheim , D L . 2003 . Multifunctional gold nanoparticle-peptide complexes for nuclear targeting . J. Am. Chem. Soc. , 125 : 4700 – 4701 .
- Sharma , V K , Yngard , R A and Lin , Y . 2009 . Silver nanoparticles: Green synthesis and their antimicrobial properties . Adv. Colloid Interface Sci. , 145 : 83 – 96 .
- Hirsch , L R , Stafford , R J , Bankson , J A , Sershen , S R , Rivera , B , Price , R E , Hazle , J D , Halas , N J and West , J L . 2003 . Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance . Proc. Nat. Acad. Sci. USA , 100 : 13549 – 13554 .
- Sandra , R and Alberto , V . 2003 . Electrocatalysis on silver and silver alloys for dichloromethane and trichloromethane dehalogenation . Electrochim. Acta , 49 : 4035 – 4046 .
- Joyce , T , Jose , H , Arben , M , Salvador , A and Fortunato , S . 2004 . Oil dispersion of AgI/Ag2S salts as a new electroactive material for potentiometric sensing of iodide and cyanide . Sens. Actuators, B , 101 : 57 – 62 .
- Adhyapak , P V , Karandikar , P , Vijaya Mohanan , K , Athawale , A A and Chandwadkar , A J . 2004 . Synthesis of silver nanowires inside mesoporous MCM-41 host . Mater. Lett. , 58 : 1168 – 1171 .
- Robert , L . 2004 . The Telecommunications Act of 1996: Residential rates and competition . Utilities Policy , 12 : 139 – 152 .
- Kim , Y H , Lee , D K and Kang , Y S . 2005 . Synthesis and characterization of Ag and Ag-SiO2 nanoparticles . Colloids Surf., A , 257–258 : 273 – 276 .
- Lee , G , Shin , S , Kim , Y and Oh , S G . 2004 . Preparation of silver nanorods through the control of temperature and pH of reaction medium . Mater. Chem. Phys. , 84 : 197 – 204 .
- Subrata , K , Madhuri , M , Sujit , K G and Tarasankar , P . 2004 . Photochemical deposition of SERS active silver nanoparticles on silica gel . J. Photochem. Photobiol., A , 162 : 625 – 632 .
- Boldyrev , V V . 2002 . Thermal decomposition of silver oxalate . Thermochim. Acta , 388 : 63 – 90 .
- Mohanpuria , P , Rana , N K and Yadav , S K . 2008 . Biosynthesis of nanoparticles: Technological concepts and future applications . J. Nanopart. Res. , 10 : 507 – 517 .
- Sondi , I and Salopek-Sondi , B . 2004 . Silver nanoparticles as antimicrobial agent: A case study of E. coli as a model for gram-negative bacteria . J. Colloid Interface Sci. , 275 : 177 – 182 .
- Sivakumar , V , Nagaraja , B M , Shashikala , V , Padmasri , A H , Madhavendra , S S and Raju , B D . 2004 . Role of hydrotalcite precursors as supports for Pd catalysts in hydrodechlorination of CCl2F2 . J. Mol. Catal. A: Chem. , 223 : 313 – 319 .
- Jain , P and Pradeep , T . 2005 . Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter . Biotechnol. Bioeng. , 90 : 59 – 63 .
- Cho , K , Park , J , Osaka , T and Park , S . 2005 . The study of antimicrobial activity and preservative effects of nanosilver ingredient . Electrochim. Acta , 51 : 956 – 960 .
- Holladay , R , Moeller , W , Mehta , D , Brooks , J , Roy , R and Mortenson , M . Silver/water, silver gels and silver-based compositions, and methods for making and using the same, Application Number WO2005US47699 20051230, European Patent Office, 2006
- Rai , M , Yadav , A and Gade , A . 2009 . Silver nanoparticles as a new generation of antimicrobials . Biotechnol. Adv. , 27 : 76 – 83 .
- Kleemann , W . 1993 . Random-field induced antiferromagnetic, ferromagnetic and structural domain states . Int. J. Mod. Phys. B , 7 : 2469 – 2507 .
- Dubey , S P , Lahtinen , M and Sillanpaa , M . 2010 . Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa . Colloid Surf., A , 364 : 34 – 41 .
- De Balogh , K KIM , Dimande , A P , Lugt , J J , Molyneux , R J , Naude , T W and Welman , W G . 1999 . A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique . J. Vet. Diagn. Invest. , 11 : 266 – 273 .
- Mendis , E , Rajapakse , N , Byun , H G and Kim , S K . 2005 . Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects . Life Sci. , 77 : 2166 – 2178 .
- Noghabi , K A , Zahiri , H S and Yoon , S C . 2007 . The production of a cold-induced extracellular biopolymer by Pseudomonas fluorescens BM07 under various growth conditions and its role in heavy metals absorption . Process Biochem. , 42 : 847 – 855 .
- Shahverdi , A R , Fakhimi , A , Shahverdi , H R and Minaian , S . 2007 . Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli . Nanomed. Nanotechnol. Biol. Med. , 3 : 168 – 171 .
- Ruparelia , J P , Chatterjee , A K , Duttagupta , S P and Mukherji , S . 2008 . Strain specificity in antimicrobial activity of silver and copper nanoparticles . Acta Biomater. , 4 : 707 – 716 .
- Hua , H , Li , N , Wu , L , Zhong , H , Wu , G , Yuan , Z , Lin , X and Tang , L . 2008 . Anti-fouling ultrafiltration prepared from polysulfone-graft-methyl acrylate compolyers by UV-induced grafting method . J. Environ. Sci. , 20 : 565 – 570 .
- Su , Y , Cheng , W , Chao , L and Jiang , Z . 2009 . Preparation of antifouling ultrafiltration membranes with poly(ethylene glucose)-graft copolymers . J. Membr. Sci. , 329 : 246 – 252 .
- Li , J H , Xu , Y Y , Zhu , L P , Wang , J H and Du , C H . 2008 . Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance . J. Membr. Sci. , 326 : 659 – 666 .
- Cortalezzi , M M , Rose , J , Barron , A R and Wiesner , M R . 2002 . Characteristics of ultrafiltration ceramic membranes derived from alumoxane nanoparticles . J. Membr. Sci. , 205 : 33 – 43 .
- Cortalezzi , M M , Rose , J , Wells , G F , Bottero , J Y , Barron , A R and Wiesner , M R . 2003 . Ceramic membranes derived from ferroxane nanoparticles: A new route for the fabrication of iron oxide ultrafiltration membranes . J. Membr. Sci. , 227 : 207 – 217 .
- Chou , W L , Yu , D G and Yang , M C . 2005 . The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment . Polym. Adv. Technol. , 16 : 600 – 607 .