Abstract
Copper sulphide (CuS) nanomaterials with interesting morphology were synthesised using copper nitrate trihydrate, thiourea and water as a solvent by a simple hydrothermal route. A systematic investigation was carried out to investigate the effect of reaction time (5, 16 and 24 h) at 150°C on the morphology of the materials. Without the use of any template or additives, shape controlled synthesis of CuS nanocrystallites were achieved. The possible mechanism for the formation of the various nanostructures of CuS in this system is discussed. The prepared materials were characterised by X-ray diffraction, field emission scanning electron microscopy (FE-SEM) and DRS-UV–Vis absorption analysis. The UV–Vis spectrum shows that it is the promising material which can absorb in the visible region and hence could be used for photocatalytic applications. In addition, the electrochemical characteristic of the synthesised material was investigated by cyclic voltammetric analysis, which shows that CuS could be used for electrocatalytic applications.
Keywords:
1. Introduction
Due to their small size, nanoparticles exhibit novel chemical, physical, magnetic, electronic and surface properties which drastically differ from the bulk material properties. In order to obtain the nanostructural materials, different techniques have been adopted to synthesise copper sulphide (CuS) nanoparticles such as microwave irradiation method Citation1, convenient solution process Citation2, molecular template method Citation3, hydrothermal or solvothermal method Citation4,Citation5, polyol route Citation6, one-step solid-state reaction Citation7, chemical vapour deposition Citation8, wet chemical Citation9, sonochemical method Citation10, etc. Among these above preparative methods, hydrothermal route is simple, efficient and low-cost method.
CuS is an important p-type semiconductor among the metal chalcogenides with its interesting morphology and has been extensively used in many applications like solar cells, catalysis, photocatalysis, chemical sensors, optical filters, surperionic materials, nanometer-scale switches, high-capacity cathode material in lithium secondary batteries, superconductor, thermoelectric cooling material and ideal characteristics for solar energy absorption Citation11–17. They are also widely applied in thin films and composite materials for their unique optical and electrical properties. In the last few years, the synthesis and characterisation of CuS has become an intriguing area of research because of its interesting morphologies like nanospheres, nanowires, nanoplates, hollow spheres, snowflakes, nanotubes, nanorods, flower-like structures, nanoribbons and especially some complex hierarchical micro/nanostructures Citation18–23.
In this work, CuS nanostructures with different morphologies were successfully synthesised using hydrothermal route. Here, copper nitrate is used as a copper source and thiourea as a sulphur source using water as a solvent without any external surfactant. The reaction was carried out at 150°C for different time periods of 5, 16 and 24 h, respectively. The effect of reaction time and formation of mechanism of its morphology has been discussed. These CuS nanomaterials may have great potential application in the field of catalysis and photovoltaics. Such methods of growing CuS nanostructures could be applied in industry owning to its simple approach, innocuous reagents, benign to environment, reproducibility and high yields.
2. Experimental method
2.1. Materials
Copper nitrate trihydrate Cu(NO3)2.3H2O and thiourea were all of analytical grade and used as received. All chemicals were used without any further purification.
2.2. Synthesis of CuS nanomaterials
In a typical synthesis, Cu(NO3)2 · 3H2O (1 mmol) was dissolved in 40 mL of water. A green solution was formed and immediately thiourea (3 mmol) was added to the above solution under vigorous stirring to ensure well dispersion of the reactants. A pale white colour solution was formed, which was then transferred into a 300 mL Teflon-lined autoclave. The autoclave was sealed into a stainless-steel tank and maintained at 150°C for 5 h. After cooling to room temperature naturally, the obtained blue-black products were filtered, washed with distilled water and absolute ethanol several times. Finally, the products were dried at 50°C for further characterisation. The synthesis is repeated with different reaction times like 16 and 24 h to know the effect of growth time on the morphology of the products.
2.3. Measurement and characterization
The particle size and morphology of the products was studied by field emission scanning electron microscopy (FE-SEM) (HITACHI SU6600 SEM). The phase and the crystallographic structure were identified by X-ray diffraction (XRD, Philips X’ Pert Pro, Cu-Kα: λ = 0.1540598 nm). Optical property of CuS nanostructures was characterised by UV–Vis spectrophotometer (HITACHI U-2800). The electrochemical property of the material was tested using a CHI 660C series electrochemical workstation.
3. Results and discussion
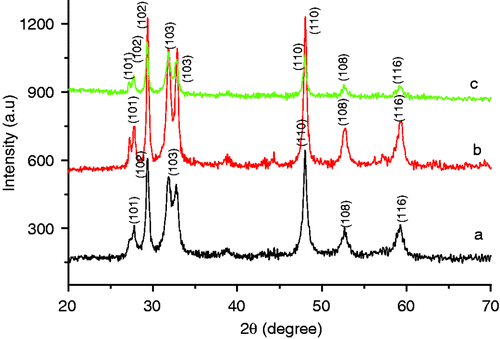
The XRD pattern of the CuS nanostructures is shown in . The major XRD peaks at 2θ = 27.78, 29.36, 31.87 and 47.96 can be attributed to (101), (102), (103) and (110) planes of the CuS, respectively, (JCPDS No. 06-0464). Except for these peaks, no characteristic peaks were detected for other impurities like Cu2S, copper oxide, etc. The strong and sharp diffraction peaks suggested that the as-obtained products are well crystalline. The diffraction peaks could be indexed to the hexagonal CuS crystal phase.
Figure 1. XRD of CuS nanostructure synthesised at 150°C for different reaction time (a) 5 h, (b) 16 h and (c) 24 h.
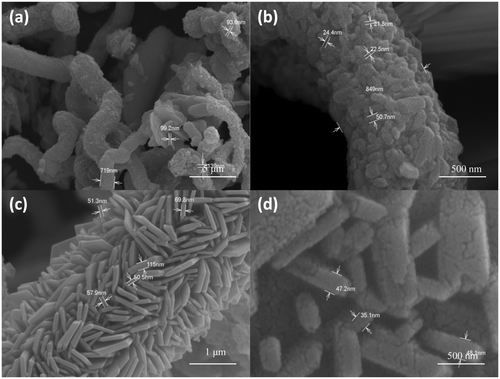
Figures show the SEM images of CuS nanostructures prepared at different reaction time. From the figure, it can be seen that nanostructures with different morphology is obtained by varying the reaction time. shows the SEM image of CuS nanostructures at 150°C for 5 h, which shows the formation tubular and spherical structures. A careful examination of these structures reveals the presence of nanorods (15–18 nm) which are self assembled to form large structures. Initially, when the reaction was carried out for 5 h, tubular structures and spheres were observed but there was not much regularity in the features. and ) shows the SEM image of CuS nanostructure at 150°C for 16 h, which shows the formation of self-assembled hexagons into tubules and a careful examination at the face of tubule contained flower-like arrangements with the thickness of flakes ranging between 90 and 125 nm. In , flower-like CuS crystal could be seen which consists of an aggregation of many flake-like microcrystals, and the petal of the flower-like structures is about 95 nm in thickness. At a higher reaction time, there were no spherical structure formations but instead the product was mainly tubular structured. shows the SEM image of CuS nanostructure at 150°C for 24 h, which shows the formation of nanoplates self assembled into cylindrical structures. The width of the plates is around 30–40 nm. Such formation of CuS nanoplates is a typical Ostwald ripening process. Initially, small crystalline nuclei are generated in a supersaturated solution, which is then followed by crystal growth. It is well known from homogeneous nucleation theory that the synthesis includes two steps, nucleation and growth phase. At the nucleating stage, the intrinsic crystal properties dominate the shape of the initial CuS seeds that is plate seeds. Later, these seeds get adsorbed to each planes and the growth starts at different rates along different planes resulting in the formation of cylinder-like structures. From our experimental results, it can be concluded that the growth of crystals has a lower orientation when the reaction time was lowered and has a higher orientation at higher reaction time.
Figure 2. FE-SEM images of CuS nanostructures synthesised at 150°C for a reaction time of 5 h at different magnifications.
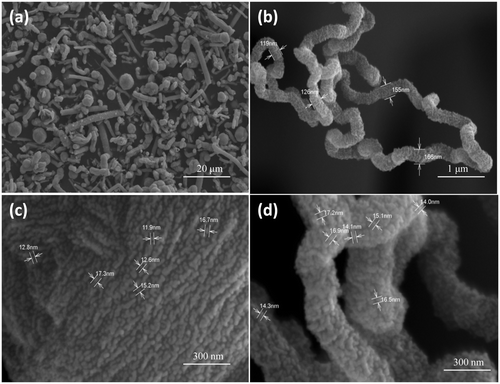
Figure 3. FE-SEM images of CuS nanostructures synthesised at 150°C for a reaction time of 16 h at different magnifications.
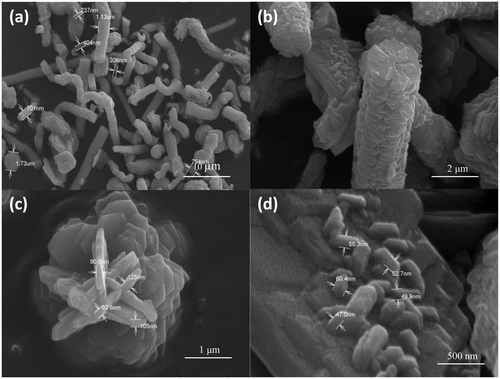
Figure 4. FE-SEM images of CuS nanostructures synthesised at 150°C for a reaction time of 24 h at different magnifications.
Based on the above results, the below mechanism is postulated. Copper ions, thiourea in solvent forms a complex, which then under solvothermal treatment, CuS nanomaterials are formed. Initially during nucleation phase, aggregation of nuclei takes place and finally these building blocks build into CuS nanostructures due to Ostwald ripening with diverse morphology.
It was speculated that the formation of CuS nanostructures might follow these three steps: first, when the copper-thiourea complexes were solvothermally treated in solvents, a large amount of CuS primary particles were produced and then the primary particles aggregate together and finally, the particles assemble preferentially into larger diverse structures. Also, a high temperature of 150°C facilitates the homogeneous formation of nanostructures and due to the high temperature, both nucleation and growth phase takes place with the formation of CuS nanostructures with well-defined morphology. In hydrothermal reaction conditions, when the reaction time was made longer, the primary nanostructures grew after the nucleation stage to secondary structures and oriented itself homogenously to form well-defined stable structures. The crystallographic phase of the seed formed during nucleation process is the main criteria deciding the final morphology of the products. Initially, covellite CuS nuclei were formed which later grew preferentially in the same direction to form CuS nanostructures. The different morphology at various reaction times was due to the long phase of nucleation or seed period where CuS seeds took different shapes, depending on its surface energies of the initial seeds. At a longer time, these seeds grew uniformly and self assembled to form homogeneous structures. This can be seen from , where a uniform self-assembly of CuS hexagonal plates stacked into cylindrical structures is obtained.
The stability of nanomaterials is an important factor to be taken into account during its synthesis. It is well known that strong intermolecular forces, such as Vander Waals attraction, π–π interaction, etc., contribute to the aggregation of nanoparticles. Thus, it is a challenge to obtain stable dispersion. To achieve this, stabilisers are normally used during the synthesis of nanoparticles. But the CuS nanomaterials reported in this work was stable at room temperature with no sign of precipitation without the need of any stabilisers.
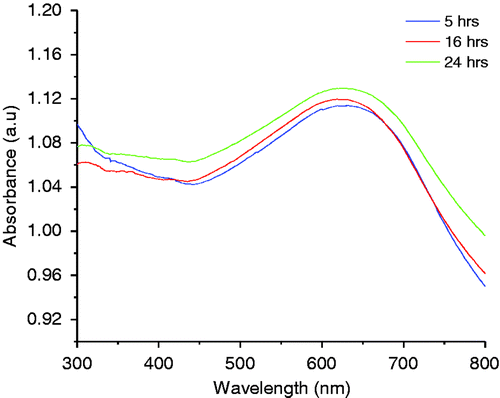
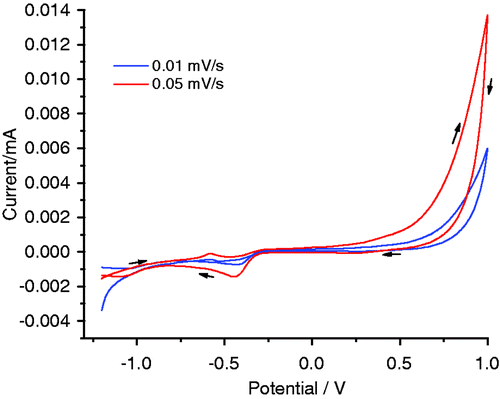
The optical absorbance spectra of CuS nanostructures showed a broad absorption in the visible region around 600 nm (). The spectrum is much broader in the visible region and it exhibits peaks at about 620 nm. It is clear from the graph that the material can absorb the visible light, and hence CuS is a promising photocatalytic material for the efficient use of light, especially sunlight. To know the electrochemical characteristics of the synthesised material, cyclic voltammograms were recorded (). The corresponding CV curves of the as-prepared CuS nanomaterials in 1 M KOH electrolyte at two different scan rates are shown in . The sample in alkaline solution displays a characteristic redox peak between −0.4 and −0.7 V at the electrodes in alkaline solutions, which can be clearly seen at a higher scan rate. Thus, CuS samples exhibit good electrochemical reversibility in this potential range and scan rate and such materials could be used as electrocatalysts.
4. Conclusion
In summary, different morphology of CuS nanostructures were successfully prepared by a simple way using hydrothermal route using copper nitrate trihydrate, thiourea and water as a solvent without the need of any external stabilisers. CuS nanostructures with different self-assembled morphology are obtained at 150°C for 5, 16 and 24 h and the results show that uniform and well self-assembled structures could be obtained at longer reaction time periods. Further XRD results indicated the presence of pure crystalline phase CuS without any impurities. A possible mechanism for the formation of CuS nanoarchitectures is proposed, and an optical study has also been carried out. The UV–Vis spectrum shows that it is the efficient material for the large-scale environmental applications for dye degradation in the visible light region.
Acknowledgements
I thank VIT University, Vellore for supporting this work under the research associate fellowship.
References
- Cheng , FM , Qi , ZY , Xiao , FQ , Gen , TZ , Mao , LL and Sheng , QF . 2010 . Controlled synthesis of various hierarchical nanostructures of copper sulfide by a facile microwave irradiation method . Colloids Surf. , A 371 : 14 – 21 .
- Yanshan , L , Donghuan , Q , Li , W and Yong , C . 2007 . A facile solution route to CuS hexagonal Nanoplatelets . Mater. Chem. Phys. , 102 : 201 – 206 . doi: 10.1016/j.matchemphys.2006.12.004
- Guangzhao , M , Wenfei , D , Dirk , GK and Helmuth , M . 2004 . Synthesis of copper sulfide nanorod arrays on molecular templates . Nano Lett. , 4 : 249 – 252 . doi: 10.1021/nl034966v
- Qingyi , L , Feng , G and Dongyuan , Z . 2002 . One-step synthesis and assembly of copper sulfide nanoparticles to nanowires, nanotubes, and nanovesicles by a simple organic amine-assisted hydrothermal process . Nano Lett. , 4 : 725 – 728 .
- Gorai , S , Ganguli , D and Chaudhuri , S . 2005 . Shape selective solvothermal synthesis of copper sulphides: Role of ethylenediamine–water solvent system . Mater. Sci. Eng. , B 116 : 221 – 225 . doi: 10.1016/j.mseb.2004.10.003
- Guozhen , S , Di , C , Kaibin , T , Xianming , L , Liying , H and Yitai , Q . 2003 . General synthesis of metal sulfides nanocrystallines via a simple polyol route . J. Solid State Chem. , 173 : 232 – 235 . doi: 10.1016/S0022-4596(03)00031-8
- Xinbo , W , Changqi , X and Zhicheng , Z . 2006 . Synthesis of CuS nanorods by one-step reaction . Mater. Lett. , 60 : 345 – 348 . doi: 10.1016/j.matlet.2005.08.048
- Reijnen , L , Meester , B , Lange , FD , Schoonman , J and Goossens , A . 2005 . Comparison of CuxS films grown by atomic layer deposition and chemical vapor deposition . Chem. Mater. , 17 : 2724 – 2728 . doi: 10.1021/cm035238b
- Roy , P and Srivastava , SK . 2007 . Low-temperature synthesis of CuS nanorods by simple wet chemical method . Mater. Lett. , 61 : 1693 – 1697 . doi: 10.1016/j.matlet.2006.07.101
- Yan , H , Yaping , W , Wenhong , G , Yijing , W , Lifang , J , Huatang , Y and Shuangxi , L . 2011 . Synthesis of novel CuS with hierarchical structures and its application in lithium-ion batteries . Powder Technol. , 212 : 64 – 68 . doi: 10.1016/j.powtec.2011.04.028
- Wu , Y , Wadia , C , Ma , W , Sadtler , B and Alivisatos , AP . 2008 . Synthesis and photovoltaic application of copper (I) sulfide nanocrystals . Nano Lett. , 8 : 2551 – 2555 . doi: 10.1021/n1801817d
- Xu , H , Wang , W and Zhu , W . 2006 . Sonochemical synthesis of crystalline CuS nanoplates via an in situ template route . Mater. Lett. , 60 : 2203 – 2206 . doi: 10.1016/j.matlet.2005.12.098
- Qing , LH , Hu , C , Yong , CZ and Chang , LW . 2011 . CuS nanostructures prepared by a hydrothermal method . J. Alloys Compd. , 509 : 6382 – 6389 . doi: 10.1016/j.jallcom.2011.02.167
- Pei , LZ , Wang , JF , Tao , XX , Wang , SB , Dong , YP , Fan , CG and Qian , FZ . 2011 . Synthesis of CuS and Cu1.1Fe1.1S2 crystals and their electrochemical properties . Mater. Charact. , 62 : 354 – 359 . doi: 10.1016/j.matchar.2011.01.001
- Zhen , HW , Dian , YG , Ya , JZ and Zhi , DZ . 2010 . CuS: Ni flowerlike morphologies synthesized by the solvothermal route . Mater. Chem. Phys. , 122 : 241 – 245 . doi: 10.1016/j.matchemphys.2010.02.042
- Shuang , X , Qun , W , Ji , HC , Qing , HM and Yang , J . 2010 . Preparation and characteristics of porous CuS microspheres consisted of polycrystalline nanoslices . Powder Technol. , 199 : 139 – 143 . doi: 10.1016/j.powtec.2009.12.013
- Minmin , L , Qingsheng , W and Jianlin , S . 2010 . A simple route to synthesize CuS framework with porosity . J. Alloys Compd. , 489 : 343 – 347 . doi: 10.1016/j.jallcom.2009.09.129
- Solanki , N , Sengupta , R and Murthy , ZVP . 2010 . Synthesis of copper sulphide and copper nanoparticles with microemulsion method . Solid State Sci. , 12 : 1560 – 1566 . doi: 10.1016/j.solidstatesciences.2010.06.021
- Jun , L and Dongfeng , X . 2010 . Solvothermal synthesis of copper sulfide semiconductor micro/nanostructures . Mater. Res. Bull. , 45 : 309 – 313 . doi: 10.1016/j.materresbull.2009.12.013
- Zhiguo , C , Shaozhen , W , Dajie , S and Baoyou , G . 2010 . Controlled synthesis of copper sulfide 3D nanoarchitectures through a facile hydrothermal route . J. Alloys Compd. , 492 : L44 – L49 . doi: 10.1016/j.jallcom.2009.11.132
- Poulomi , R and Sunil Kumar , S . 2007 . Low-temperature synthesis of CuS nanorods by simple wet chemical method . Mater. Lett. , 61 : 1693 – 1697 . doi: 10.1016/j.matlet.2006.07.101
- Jing , Z , Jianxue , Z , Baohua , Z , Pingtang , Z and KaiXun , H . 2007 . Low-temperature synthesis of copper sulfide nano-crystals of novel morphologies by hydrothermal process . Mater. Lett. , 61 : 5029 – 5032 . doi: 10.1016/j.matlet.2007.03.111
- Dutta , A and Dolui , SK . 2008 . Preparation of colloidal dispersion of CuS nanoparticles stabilized by SDS . Mater. Chem. Phys. , 112 : 448 – 452 . doi: 10.1016/j.matchemphys.2008.05.072