Abstract
A seeding growth approach to the preparation of silver nanoparticles with a controllable size was developed. It contained a two-step reaction: the first step was gold seed clusters quickly generated by a chemical reaction using sodium borohydride as a reducing reagent; the second one was controllable silver nanoparticles were grown at the mild condition by using the mixed reducing reagents (hydroxylamine hydrochloride and sodium hydroxide) to form a buffer system. The gold core was beneficial for the crystalline of silver cations to form the nanoparticles and the buffer system which was composed of hydroxylamine hydrochloride and sodium hydroxide, and was helpful for controlling the size and shape of the as-prepared silver nanoparticles. These as-prepared nanoparticles were characterised by X-ray powder diffraction, UV-Vis spectroscopy (UV-Vis) and transmission electron microscopy along with energy dispersive X-ray spectroscopy. The results indicated that the obtained silver nanoparticles are highly crystallised with an average diameter around 10 nm. The content of gold seeds and the mild reaction rate controlled by the buffer system were considered to be key factors in the control of silver nanoparticles’ morphology and size. A possible mechanism of the silver nanoparticles formed was also proposed.
1. Introduction
During the last few decades, silver nanoparticles have been investigated extensively because of their potential applications in electronics, photonics, surface-enhanced Raman scattering and excellent antibacterial activity Citation1–4. Thanks to the high reduction potential of silver cations, a large number of reductants have been developed to the preparation of silver nanocrystals, such as ascorbic acid Citation5, glucose Citation6, ethylene glycol (EG) Citation7, tri-sodium citrate Citation8,Citation9, etc. Nowadays, the preparation of silver nanoparticles with various morphologies ranging from spheres to wires, cubes, plates, prisms and bipyramids have been produced Citation9–16. Despite so much progress in the synthesis of silver nanoparticles Citation5–16, it is still a challenge to synthesise uniform and stable silver nanoparticles with a certain size.
The optical properties of silver nanoparticles depend greatly on the geometrical parameters such as shape and size Citation17; therefore it is crucial to be able to control these parameters. Until now, a large number of successful methods have been developed for the preparation of silver nanoparticles, for example, Henglein and coworkers investigated the particle growth of silver atoms and clusters of Ag+ ions reduction in aqueous solution by using pulse radiolysis technique for the first time and changed the shapes of silver plasmon absorption band in the presence of nucleophilic molecules which donates the electron density into the silver nanoparticles to shift the adsorption/desorption equilibrium Citation18–20. Morcillo et al. obtained silver colloidal nanoparticles with an average diameter of 50 nm by a chemical reduction method of silver nitrate with citrate Citation21. Also, spherical silver nanoparticles with various sizes were prepared by the reduction of silver nitrate with sodium borohydride following the Creighton way in an ice-cold bath Citation22. Apart from these, synthesis of silver nanostructures were uncovered with various morphologies through the polyol reduction of silver nitrate in the presence of poly(vinyl pyrrolidone), and EG served as both solvent and reducing agent Citation23,Citation24. Since Murphy and co-workers prepared silver nanorods of varied ratios using a seed-mediated growth approach in 2001 Citation5, tremendous research effort has been devoted to the synthesis of silver nanoparticles by this method. However, there have been only a handful of studies focusing on the shape-controlled synthesis of spherical silver nanoparticles. For example, Dadosh Citation25 and Mandal Citation26 prepared spherical silver nanoparticles with gold cores via a two-step reaction. Although silver nanoparticles of gold core with a narrow size distribution were achieved, the synthesis technique required to prolong heat treatment at relatively high temperature (∼100°C).
Here, a chemical synthesis of silver nanoparticles was reported by a gold seed-mediated growth method. In this method, a certain amount of hydroxylamine hydrochloride containing some sodium hydroxide acted as mild reducing reagent, which was helpful for the solution to generate a buffer system. It is novel, although Leopold et al. reported a method to prepare silver nanoparticles using hydroxylamine hydrochloride as a reducing agent Citation27. In our study, silver nanoparticles were prepared by reduction of silver nitrate with the mixed reducing reagent in the presence of hexadecyl trimethyl ammonium bromide (CTAB) as a stabilising agent at room temperature. In the buffer system, the size of silver nanoparticles was controllable formed under mild conditions. This work provided a simple way to synthesise uniform and stable silver nanoparticles by a facile and simple synthetic route.
2. Experimental section
2.1. Materials
Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O), silver nitrate (AgNO3), sodium borohydride (NaBH4), hydroxylamine hydrochloride (NH2OH · HCl), sodium hydroxide (NaOH) and CTAB were obtained from Shanghai Chemical Reagent Co. Ltd. All the reagents used were of analytical grade as received. All the glass containers were cleaned with aqua regia (HCl : HNO3 = 3 : 1 (v/v)) and rinsed with deionised water (Caution! Aqua Regia is a very corrosive oxidising agent which should be handled with great care.).
2.2. Preparation of the gold seeds
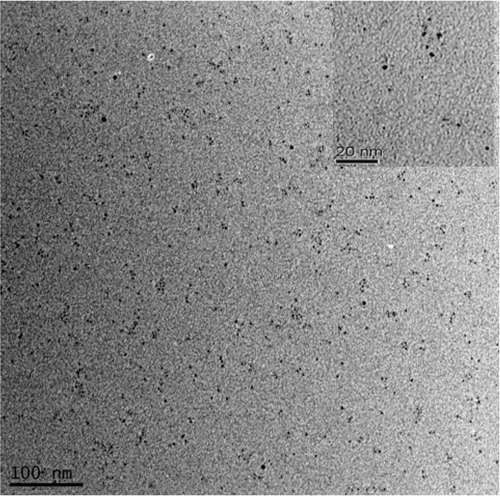
Briefly, CTAB solution (5 mL, 0.20 M) was mixed with 5.0 mL of M HAuCl4 · 3H2O Citation28. A quantity of 0.60 mL freshly prepared ice-cold 0.01 M NaBH4 was added to the stirring solution, which resulted in the formation of a brownish yellow solution. After vigorously stirring the solution for 2 min, the generated particles were used as seeds without aging. According to transmission electron microscopy (TEM), the size of these seed particles was less than 3 nm as shown in .
2.3. Preparation of silver nanoparticles
First, 6.4 mL of aqueous solution with a final concentration of 62.5 mM NH2OH · HCl was mixed with 100 mM NaOH to form the reducing reagent. Next, four sets of solutions were prepared containing 0.25 mL of 100 mM AgNO3, 1.6 mL of the mixed reductant, and 10 mL of 100 mM CTAB. Finally, a varied number of seed solutions (0.25 mL, 0.5 mL, 1 mL and 2 mL) were added respectively. After that, the solution was vigorously shaken for 2 min to mix uniformly and subsequent aging at room temperature. Within half an hour, a colour change occurred varying from light to dark red depending on the seed concentrations.
2.4. Characterisation
The as-prepared silver nanoparticles were characterised by XRD, UV-Vis spectroscopy, TEM and energy dispersive X-ray (EDX). The UV-Vis spectra of silver nanoparticles were measured in a Lambda 950 spectrophotometer using a 1 cm quartz cuvette. The morphology and microstructure of silver nanoparticles were characterised by TEM (Tecnai G2 F20, FEI Ltd., USA). TEM grids were prepared by placing 1 μL of the silver nanoparticles solution on a carbon-coated copper grid and drying at room temperature. The purity and crystal structure of the products were measured on a Bruker D8 Advance/Discover X-ray diffractometer (Bruker Co. Ltd., Germany) with a Cu-Kα radiation (λ = 1.5406 Å). The data were collected over a 2θ range of 30–90° at a scan rate of 0.02°/s. The XRD sample was prepared by depositing powdered nanocrystals on a Si (1 0 0) wafer.
3. Results and discussion
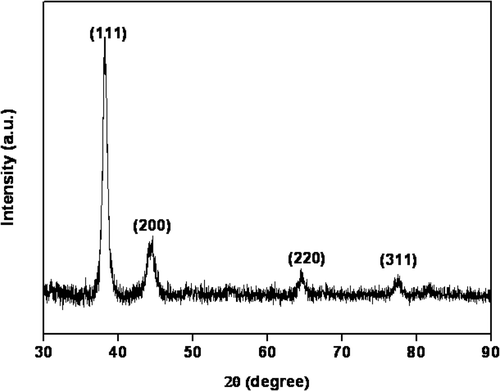
The XRD pattern of the obtained silver nanoparticles was shown in . It is no doubt that the four peaks appeared in 38.12°, 44.30°, 64.44° and 77.40° ascribed to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of a face-centred cubic (fcc) lattice of silver crystals (JCPDS file No. 04-0783), respectively. The relatively low-diffraction intensity of the (2 0 0), (2 2 0) and (3 1 1) planes compared with that of the (1 1 1) plane suggested that the (1 1 1) plane was the predominant orientation, as confirmed by high-resolution TEM measurements. The average size of silver nanoparticles, determined by the Scherrer equation, was estimated to be 11.2 nm.
Figure 2. XRD pattern of the as-prepared silver nanoparticles (the addition of gold seeds solution is 1.0 mL).
The UV–Vis spectra of the silver nanoparticles synthesised in the presence of different volume of gold seeds was shown in , and photographs of the silver colloids shown in the inset, which were labelled according to the ordering of the spectra. The change of peak position and the shape of the absorption spectra were obvious, shown in . For all the samples, with the increase of gold seeds addition in the reaction vial, the appearance of plasmon absorption peak λ max blue shifted from 416 to 402 nm. Compared with the absorption of silver nanoparticles, the blue shift of the absorption band might be attributed to the size effect of the silver nanoparticles, The wavelength of absorption peaks of silver nanoparticles decreased as the particle size decreased, however, the homogeneity of sample c played an important role in the absorption as its concentration of silver nanoparticles might be higher than sample b, which was consistent with the TEM images, shown in .
Figure 3. UV-Vis spectra of silver nanoparticles obtained from various gold seeds addition. (a) 0.25 mL, (b) 0.5 mL, (c) 1.0 mL and (d) 2.0 mL.
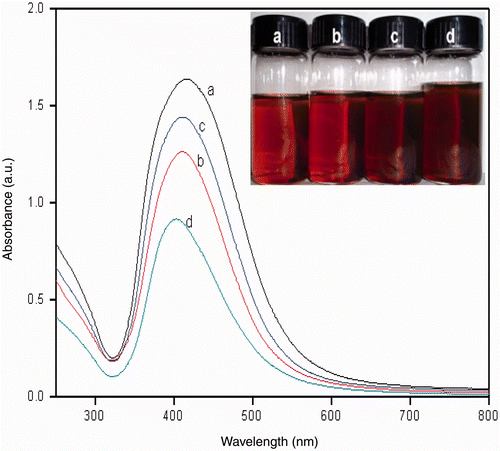
Figure 4. TEM images of silver nanoparticles synthesised by various gold seeds addition. (a) 0.25 mL, (b) 0.5 mL, (c) 1.0 mL and (d) 2.0 mL.
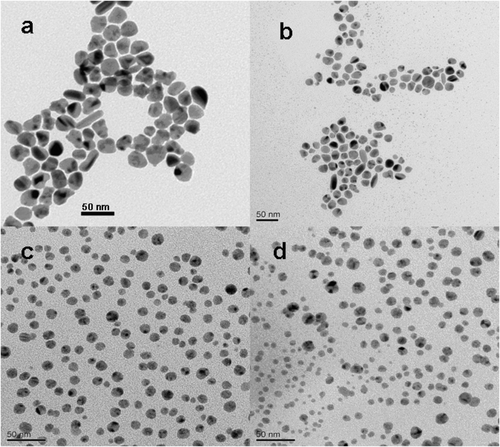
Investigation of the morphologies of the as-prepared silver nanoparticles was performed using TEM as shown in . It can be seen that the homogeneous silver nanoparticles formed when the addition of gold seeds was 1.0 mL (shown in ). It was indicated that the sample consisted of large amounts of small particulates with a size around 10 nm, which agreed well with the XRD results calculated by the Scherrer equation. Moreover, TEM images in parts a, b and d in showed different situations of silver nanocrystals with the addition of different dosages of gold seeds (in the volume of gold seeds at 0.25 mL, 0.50 mL and 2.0 mL). Therefore, the amount of gold seeds in the solution played a key role in controlling the morphologies of silver nanostructures along with the buffer system. It also can be seen that a few silver nanoparticles exhibited core–shell structure, which was attributed to the growth of silver nanoparticles attached from the gold seeds, as it can be seen more clearly in .
Figure 5. TEM images of the as-prepared silver nanoparticles (the addition of gold seeds solution is 1.0 mL) (a) high-resolution TEM micrograph of a single silver nanocrystal and (b) SAED pattern.
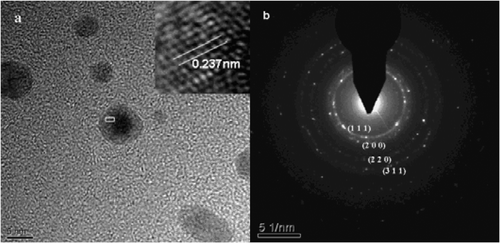
A typical high-resolution TEM image clearly exhibited the equally spaced lattice fringes shown in . The calculated fringe separation was 0.237 nm, which corresponds to the d-spacing of (1 1 1) plane of bulk silver (0.2359 nm). Moreover, showed the selected area electron diffraction (SAED) pattern of silver nanoparticles. The clear lattice fringes in high-resolution TEM image and the typical SAED pattern with bright circular rings corresponding to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes were confirmed by XRD that the silver nanoparticles are fcc structure.
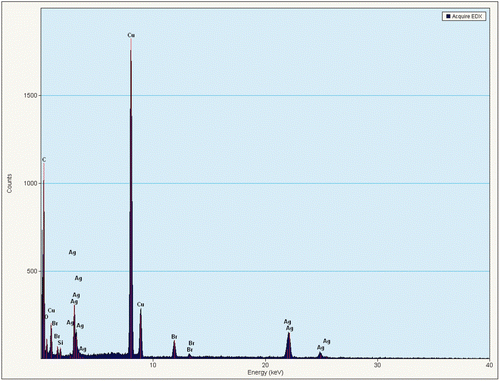
The EDX spectral result () confirmed that the particles were made up of metallic silver, and the peaks of Cu and Br were attributed to the copper grid substrate and remnants of the surfactant.
It can be seen that the morphology of the final products was greatly dependent on the addition of gold seeds. Considering the above results, a possible growth mechanism of silver nanoparticles was proposed. In aqueous solutions, the mixed reducing reagent (NH2OH · HCl and NaOH) can function as a buffer system to control the size of silver nanoparticles formed at mild conditions. The stable colloid of uniform silver nanoparticles was prepared by adding a certain amount of gold seeds under mild reaction conditions. On the basis of above results, the formation mechanism of the silver nanoparticles in aqueous solutions could be expressed by the following schemes:
In this process, a chemical reaction occurs that Ag+ was reduced to Ago under the effect of CTAB on the gold seed surface mostly depending on hydroxylamine. Moreover, the hydrogen ions and residual hydroxyl ions combined to form water that would promote the reaction. It made the formation of uniform silver nanoparticles as shown in . By adjusting the content of gold seeds, the enhanced reduction rate favoured the addition of more nuclei in a short period of time, which resulted in the formation of small silver particles and other shapes (shown in ).
Moreover, the silver colloids were very stable which could be preserved easily for at least 1 month at room temperature.
4. Conclusions
In summary, preparation and characterisation of uniform spherical silver nanoparticles with an average size of around 10 nm were successfully synthesised by a seed-mediated growth method. Gold cores are beneficial to the crystalline of silver cations to form the nanoparticles and the buffer system which was composed of NH2OH · HCl and NaOH, was helpful for controlling the size and shape of the as-prepared silver nanoparticles. This method demonstrated a methodology for the preparation of functional materials, such as silver, in a buffer system to control its size and shape.
Acknowledgements
This work was supported by Natural Science Foundation of China (Grants Nos. 51102251 and 31170964), Ningbo Science and Technology Bureau (Grants Nos 2009B21005, 2010A610159 and 2011C50009), Postdoctoral Science Foundation of China (Grant No. 20100480072), Hundred Talents and Academy-Locality Cooperation Programs of Chinese Academy of Sciences, Zhejiang Provincial Natural Science Foundation of China (Grant No. R5110230) and the CAS/SAFEA International Partnership Program for Creative Research Teams.
References
- El-sayed , MA . 2001 . Some interesting properties of metals confined in time and nanometer space of different shapes . Acc. Chem. Res. , 34 : 257 – 264 . doi: 10.1021/ar960016n
- Hermanson , KD , Lumsdon , SO , Williams , JP , Kaler , EW and Velev , OD . 2001 . Dielectrophoretic assembly of electrically functional microwires from nanoparticle suspensions . Science , 294 : 1082 – 1086 . doi: 10.1126/science.1063821
- Zou , XQ , Ying , EB and Dong , SJ . 2007 . Preparation of novel silver–gold bimetallic nanostructures by seeding with silver nanoplates and application in surface-enhanced Raman scattering . J. Colloid Interf. Sci. , 306 : 307 – 315 . doi: 10.1016/j.jcis.2006.10.084
- Mohan , YM , Lee , K , Premkumar , T and Geckeler , KE . 2007 . Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications . Polymer , 48 : 158 – 164 . doi: 10.1016/j.polymer.2006.10.045
- Jana , NR , Gearheart , L and Murphy , CJ . 2001 . Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio . Chem. Commun. , : 617 – 618 . doi: 10.1039/b100521i
- Raveendran , P , Fu , J and Wallen , SL . 2006 . A simple and ‘‘green’’ method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles . Green Chem. , 8 : 34 – 38 . doi: 10.1039/b512540e
- Wiley , B , Sun , Y and Xia , Y . 2007 . Synthesis of silver nanostructures with controlled shapes and properties . Acc. Chem. Res. , 40 : 1067 – 1076 . doi: 10.1021/ar7000974
- Hu , J , Chen , Q , Xie , Z and Tian , Z . 2004 . A simple and effective route for the synthesis of crystalline silver nanorods and nanowires . Adv. Funct. Mater. , 14 : 183 – 189 . doi: 10.1002/adfm.200304421
- Caswell , KK , Bender , CM and Murphy , CJ . 2003 . Seedless, surfactantless wet chemical synthesis of silver nanowires . Nano Lett. , 3 : 667 – 669 . doi: 10.1021/nl0341178
- Hiramatsu , H and Osterloh , FE . 2004 . A simple large-scale synthesis of nearly monodisperse gold and silver nanoparticles with adjustable sizes and with exchangeable surfactants . Chem. Mater. , 16 : 2509 – 2511 . doi: 10.1021/cm049532v
- Chen , S and Carroll , DL . 2002 . Synthesis and characterization of truncated triangular silver nanoplates . Nano Lett. , 2 : 1003 – 1007 . doi: 10.1021/nl025674h
- Pastoriza-Santos , I and Liz-Marzan , LM . 2002 . Synthesis of silver nanoprisms in DMF . Nano Lett. , 2 : 903 – 905 . doi: 10.1021/nl025638i
- Wiley , B , Herricks , T , Sun , Y and Xia , Y . 2004 . Polyol synthesis of silver nanoparticles: Use of chloride and oxygen to promote the formation of single-crystal, truncated cubes and tetrahedrons . Nano Lett. , 4 : 1733 – 1739 . doi: 10.1021/nl048912c
- Sun , Y and Xia , Y . 2002 . Shape-controlled synthesis of gold and silver nanoparticles . Science , 298 : 2176 – 2179 . doi: 10.1126/science.1077229
- Jin , R , Cao , Y , Mirkin , CA , Kelly , KL , Schatz , GC and Zheng , JG . 2001 . Photoinduced conversion of silver nanospheres to nanoprisms . Science , 294 : 1901 – 1903 . doi: 10.1126/science.1066541
- Wiley , B , Xiong , Y , Li , Z , Yin , Y and Xia , Y . 2006 . Right bipyramids of silver: A new shape derived from single twinned seeds . Nano Lett. , 6 : 765 – 768 . doi: 10.1021/nl060069q
- Jensen , TR , Malinsky , MD , Haynes , CL and Van Duyne , RP . 2000 . Nanosphere lithography: Tunable localized surface plasmon resonance spectra of silver nanoparticles . J. Phys. Chem. B , 104 : 10549 – 10556 . doi: 10.1021/jp002435e
- Ershov , BG , Janata , E and Henglein , A . 1993 . Silver atoms and clusters in aqueous solution: Absorption spectra and the particle growth in the absence of stabilizing Ag+ ions . J. Phys. Chem. , 97 : 4589 – 4594 . doi: 10.1021/j100120a006
- Janata , E , Henglein , A and Ershov , BG . 1994 . First clusters of Ag+ ion reduction in aqueous solution . J. Phys. Chem. , 98 : 10888 – 10890 . doi: 10.1021/j100093a033
- Linnert , T , Mulavaney , P and Henglein , A . 1993 . Surface chemistry of colloidal silver: Surface plasmon damping by chemisorbed iodide, hydrosulfide (SH-), and phenylthiolate . J. Phys. Chem. , 97 : 679 – 682 . doi: 10.1021/j100105a024
- Rivas , L , Sanchez-Cortes , S , García-Ramos , JV and Morcillo , G . 2001 . Catalytic modification of gallic acid on a silver surface studied by surface-enhanced Raman spectroscopy . Langmuir , 17 : 574 – 577 . doi: 10.1021/la001038s
- Creighton , JA , Blatchford , CG and Albrecht , MG . 1979 . Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength . J. Chem. Soc. Faraday Trans. , 75 : 790 – 798 . doi: 10.1039/f29797500790
- Siekkinen , AR , McLellan , JM , Chen , JY and Xia , YN . 2006 . Rapid synthesis of small silver nanocubes by mediating polyol reduction with a trace amount of sodium sulfide or sodium hydrosulfide . Chem. Phys. Lett. , 432 : 491 – 496 . doi: 10.1016/j.cplett.2006.10.095
- Sun , YG and Xia , YN . 2002 . Shape-controlled synthesis of gold and silver nanoparticles . Science. , 298 : 2176 – 2179 . doi: 10.1126/science.1077229
- Dadosh , T , Sperling , J , Bryant , GW , Breslow , R , Shegai , T , Dyshel , M , Haran , G and Bar-Joseph , I . 2009 . Plasmonic control of the shape of the Raman spectrum of a single molecule in a silver nanoparticle dimer . ACS Nano , 3 : 1988 – 1994 . doi: 10.1021/nn900422w
- Mandal , M , Jana , NR , Kundu , S , Ghosh , SK , Panigrahi , M and Pal , T . 2004 . Synthesis of Aucore–Agshell type bimetallic nanoparticles for single molecule detection in solution by SERS method . J. Nanopart. Res. , 6 : 53 – 61 . doi: 10.1023/B:NANO.0000023227.17871.0f
- Leopold , N and Lendl , B . 2003 . A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride . J. Phys. Chem. B. , 107 : 5723 – 5727 . doi: 10.1021/jp027460u
- Nikoobakht , B and El-Sayed , MA . 2003 . Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method . Chem. Mater. , 15 : 1957 – 1962 . doi: 10.1021/cm020732l