Abstract
The precursors used to prepare high-quality nanoparticles by high-temperature decomposition are expensive and toxic organometallic compounds. Fe(OH)3, an inexpensive and environmentally friendly iron-containing compound, is for the first time introduced to act as an precursor to generate monodisperse iron oxide nanoparticles. The obtained nanoparticles are characterised by transmission electronic microscopy, IR, XRD and vibrating sample magnetometer. Organic-soluble ferromagnetic rod-like and superparamagnetic dot-like nanoparticles with the size ranging from 12 to 25 nm are obtained in a nonpolar solvent by adjusting reaction parameters, such as surfactant concentration and reaction time. Water-soluble nanoparticles can be synthesised when a bipolar solvent is used instead of nonpolar solvent. The results show that Fe(OH)3 is a promising precursor for the high-temperature decomposition method.
1. Introduction
Iron oxide nanoparticles are currently one of the most interesting materials due to their ultra-fine size, biocompatibility and unique magnetic properties. They are widely used, especially in biological fields, such as magnetic separation, drug delivery, magnetic resonance imaging and cancer hyperthermia Citation1–5. For all applications, the nanoaparticles with tunable size, narrow size distribution and high crystallinity are desirable for precisely controllable magnetic properties.
A variety of synthetic techniques have been developed to prepare iron oxide nanoparticles. The coprecipitation is a conventional approach to high-yield nanoparticles based on the coprecipitation of iron salts in alkaline aqueous solutions Citation6,Citation7; however, its inherent problems remain unsolved or difficult to address. The most prominent one is that the produced nanoparticles have broad size distribution, poor crystallinity and low-saturation magnetisation. Recently, the high-temperature decomposition method has been extensively developed to prepare high-quality monodisperse iron oxide nanoparticles. Although the precursors used are quite diverse including iron cupferron Citation8, ferrocene Citation9, iron acetylacetonate Citation10, iron pentacarbonyl Citation11, iron-oleate Citation12,Citation13 and so on, the mechanism of high-temperature decomposition is intrinsically the same. The precursor is firstly thermally decomposed for a burst of nucleation and then the nanoparticles are formed during slow growth. The key to generate monodisperse nanoparticles is to separate nucleation and growth, and the overlap of these two processes will result in polydispersity of the nanoparticles. Many factors, such as solvent and surfactant choice, concentration ratios, reaction times and temperature have dramatic effects on size, morphology and magnetic property of the nanoparticles Citation14–16. All of iron precursors presently used are toxic, expensive or pre-prepared organometallic compounds, and thus the use of an inexpensive, environmentally friendly and easy to find iron source as precursor is urgently expected. Many commercially available iron-containing compounds are investigated as iron sources for the high-temperature decomposition method. The challenging is the low solubility of iron-containing compounds in reaction solvent, which leads to the failure of subsequent high-temperature decomposition.
In this report, Fe(OH)3, a very common iron-containing compounds, is applied as the precursor to synthesise monodisperse organic-soluble iron oxide nanoparticles with tunable sizes. Furthermore, it is explored to generate water-soluble nanoparticles through one-pot high-temperature decomposition. FeO(OH) gives similar results to Fe(OH)3 Citation17; however, it is chemically unstable and thus not desired iron sources.
2. Experimental section
2.1. Materials
1-octadecene was purchased from Alfa Aesar. Iron (III) hydroxide oxide (Fe(OH)3), oleic acid and 1-methyl-2-pyrrolidinone were obtained from Aladdin Reagent. The other chemicals were analytical reagents and were purchased from Shanghai Chemical Reagent Corporation, China. All chemicals were used as received.
2.2. Synthesis of iron oxide nanoparticles
Monodisperse organic-soluble iron oxide nanoparticles were synthesised in a 50 mL three-neck round-bottom flask under an N2 flow. In brief, 0.53 g (5 mmol) of Fe(OH)3 was added to a mixture solution containing 15 mL of octadecene as solvent and oleic acid as surfactant. Oleic acid concentration was adjusted to control the size and morphology of the nanoparticles. The mixture was firstly heated to 110°C for 1 h for degassing, and then heated to 320°C at a heating rate of 3.3°C/min. After refluxing at this temperature for desired time, the mixture was cooled to room temperature. The precipitate containing iron oxide nanoparticles was collected by addition of excess ethanol and washed 4–5 times with a mixture of hexane and acetone. Finally, the nanoparticles were stored in hexane or chloroform.
Instead of octadecene, 1-methyl-2-pyrrolidinone was adopted to act as both solvent and surfactant to make water-soluble iron oxide nanoparticles. The synthetic procedure was the same as above-described except that refluxing temperature was 200°C, which is the boiling point of 2-pyrrolidinone. The obtained solution was washed with water and the nanoparticles were collected by a magnet.
3. Characterisation
The size and morphology of the nanoparticles were determined by transmission electronic microscopy (TEM, JEOL, JEM-200EX) operating at 120 kV. Samples were dropped from hexane or water onto a carbon-coated copper grid and dried at room temperature. IR spectra were recorded on a Nicolet Nexus 870 FT-IR spectrometer and powder samples were dried at 100°C under vacuum for 24 h prior to fabrication of the KBr pellet. Spectra were recorded with a resolution of 2 cm−1. Magnetic measurements were carried out with a vibrating sample magnetometer (VSM, Lakeshore 7407). XRD were recorded on a Rigaku Dmax-r C X-ray diffractometer using CuK-α radiation (λ = 1.540 Å) operated at 40 KV and 100 mA. Surface charge measurements were performed on a Zeta Potential Analyzer (Delsa 440SX, Beckman Coulter).
4. Results and discussion
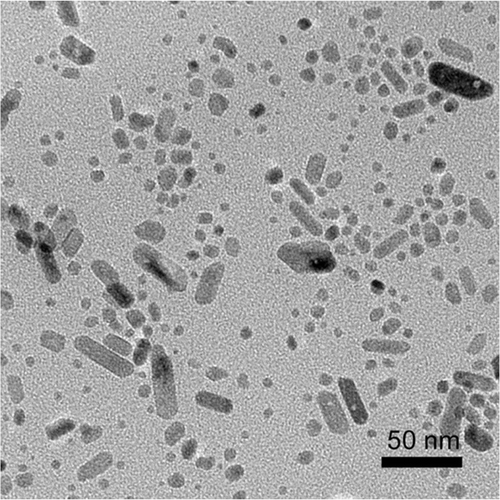
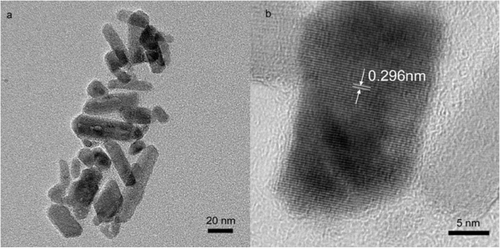
The synthesis of monodisperse organic-soluble iron oxide nanoparticles was carried out by dissolving Fe(OH)3 in octadecene in the presence of oleic acid as surfactant at elevated temperature. During reaction time, the solution turned from turbid brown to clear black, indicating the formation of the nanoparticles. When the molar ratio of oleic acid to Fe(OH)3 is zero, Fe(OH)3 is thermally decomposed to form rod-like iron oxide nanoparticles. TEM image shows that rod-like nanoparticles dispersed in hexane tend to aggregate because there is no surfactant to stabilise them (). The lattice spacing between two adjacent planes is 0.296 nm, corresponding to (200) plane of spinel-structured Fe3O4 (). When molar ratio is increased to less than 3, rod-like nanoparticles and monodisperse nanoparticles appear together (). It indicates that iron oleate formed by the reaction of Fe(OH)3 and oleic acid is the intermedium for monodisperse iron oxide nanoparticles, like iron acetylacetonate and iron pentacarbonyl as the precursors. This point is also evidenced by synthetic procedures for other nanoparticles. For an instance, a variety of cadmium sources are initially treated to form cadmium oleate intermedium before high-temperature decomposition to produce nanoparticles Citation18,Citation19.
Figure 1. TEM image of rod-like nanoparticles (a) dispersed in hexane and its corresponding HRTEM image (b).
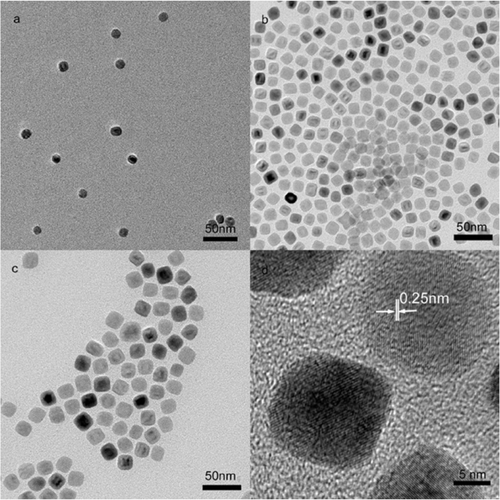
Monodisperse iron oxides nanoparticles with size deviation less than 10% are generated only in the case of molar ratio more than 3. At or above this ratio, Fe(OH)3 can completely react with oleic acid to form iron oleate intermedium. Oleic acid concentration has important effects on size and morphology of the nanoparticles. For example, the nanoparticles with 12, 15 and 18 nm in diameter are obtained when molar ratios are 3.2, 4.8 and 6.4, respectively (). With increasing oleic acid, the sizes of the nanoparticles increase and the shapes change from spherical to cubic. The observed lattice spacing of cubic 18 nm nanoparticles is 0.25 nm, corresponding to the (311) plane of Fe3O4. However, no nanoparticles can be obtained when molar ratio is greater than 10 because excess oleic acid will cover growth sites of the nanoparticles and consequently inhibit their growth. Similar phenomenon was observed in the reported work Citation14.
Figure 3. TEM images of 12 (a), 15 (b) and 18 nm (c) nanoparticles and HRTEM image (d) of 18 nm nanoparticles. The refluxing time is 30 min.
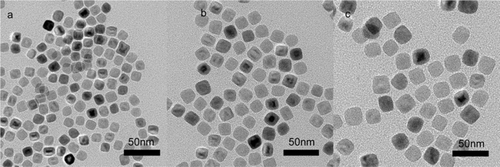
The size of the nanoparticles synthesised by simply increasing oleic acid concentration is less than 20 nm. To make relatively large nanoparticles one effective strategy is to apply ‘Ostwald Ripening’ principle in reaction system Citation20,Citation21. In essence, small nanoparticles dissolve during the prolonged refluxing time and redeposit on the large ones. shows TEM images of iron oxide nanoparticles obtained for prolonged refluxing time varied from 45 to 90 min at molar ratio of 6.4. The sizes of the nanoparticles increase from 20 to 22 and to 25 nm when the refluxing times are from 45 to 60 and to 90 min, respectively. It is noted that unlike the previous report Citation22, no further addition of Fe(OH)3 precursor is required using this method. When refluxing time is longer than 3 h, larger nanoparticles are obtained. The nanoparticles, however, become polydisperse in size. The excessive consumption of the precursor is most likely the major contributing factor for this time-dependent size growth. When there is no enough precursor, the formed monodisperse nanoparticles will be consumed for large nanoparticles, resulting in polydispersity of the nanoparticles. Thus, it appears that refluxing time of 3 h approaches the higher limit for making monodisperse nanoparticles under such synthetic conditions.
Figure 4. TEM images of 20 (a), 22 (b) and 25 nm (c) nanoparticles for prolonged reaction time. The molar ratio of oleic acid to Fe(OH)3 is fixed at 6.4.
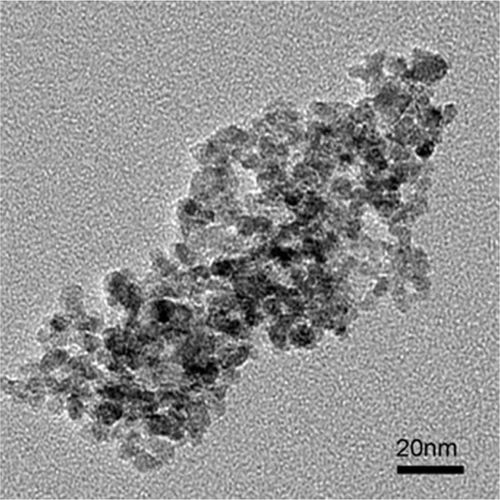
The above-synthesised nanoparticles are organic-soluble, and thus additional steps for surface modification are usually required to transfer them into water and make them active for biomedical applications Citation23,Citation24. In this report, Fe(OH)3 is also applied to generate water-soluble nanoparticles through one-pot reaction. 2-pyrrolidinone is chosen as solvent and stabiliser because it has strong polarity, a high-boiling point and coordination capacity with metal ions Citation25,Citation26. The synthetic procedure is based on above-mentioned method for producing organic-soluble nanoparticles, and refluxing temperature is fixed at 200°C, which is the boiling point of 2-pyrrolidinone. The obtained nanoparticles dispersed in water are shown in . It can be seen that the nanoparticles have a relatively broad size distribution and tend to aggregate. The low solubility of Fe(OH)3 in 2-pyrrolidinone may be the major factor for this phenomenon.
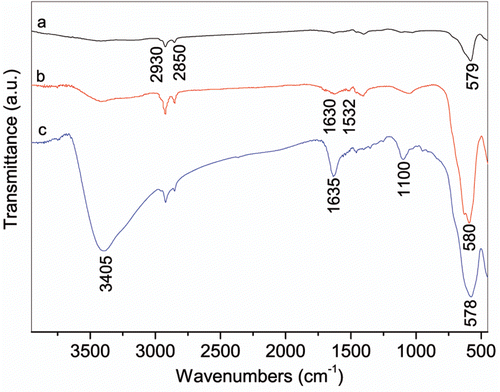
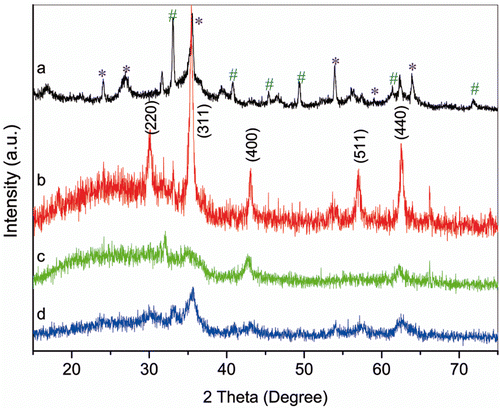
FTIR results of water-soluble nanoparticles as well as rod-like and organic nanoparticles are shown in . For water-soluble nanoparticles, the vibrational band of C=O shifts from 1686 cm−1 for pure 2-pyrrolidinone to 1635 cm−1, indicating that the O from C=O coordinates with Fe atom on the surface of the nanoparticles Citation25. The sharp peaks at 3405 and 1100 cm−1 are assigned to O–H from residual water and C–N vibrations, respectively. In contrast, the featured peaks at 2930 and 2850 cm–1, contributing to the asymmetric and symmetric –CH2 stretching, derives from octadecene for rod-like nanoparticles or oleic acid for monodisperse nanoparticles. The two peaks at 1630 and 1532 cm−1 due to the symmetric and asymmetric carboxylate (COO−) stretching, respectively, indicate that oleic acid is bound to the surface of the nanoparticles through covalent bond between carboxylate (COO−) and Fe atom Citation27,Citation28. The bands at around 580 cm−1 for Fe–O demonstrate that all of the obtained nanoparticles are iron oxide. Water-soluble nanoparticles are kept in water at pH = 5 for zeta potential measurement. The result shows that they are positively charged up to 50 mV, originating from the protonation of the tertiary amine group under acidic conditions. The crystallinity of Fe(OH)3 and the obtained nanoparticles is further confirmed by XRD patterns (). XRD pattern of Fe(OH)3 shows the peaks of both Fe(OH)3 (JCPDS file No. 38-0032, marked with *) and Fe2O3 · nH2O (JCPDS file No. 08-0097, marked with #), due to the transition of Fe(OH)3 to Fe2O3 · nH2O at room temperature Citation29. For the obtained nanoparticles, the respective (220), (311), (400), (511) and (440) peaks can be indexed to Fe3O4 planes (Magnetite, JCPDS file No. 85-1436). Moreover, the relatively high intensity of the (311) plane compared to other planes for rod-like nanoparticles indicates that rod-like nanoparticles are formed due to their preferential growth in the [311] direction Citation30.
Figure 7. XRD patterns of Fe(OH)3 (a), rod-like (b), organic- (c) and water-soluble (d) nanoparticles.
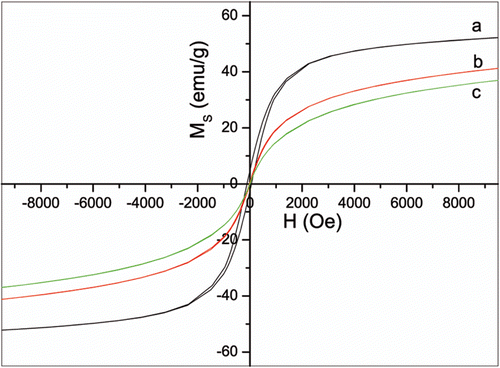
Magnetic property of the nanoparticles was measured at room temperature. Rod-like nanoparticles exhibit ferromagnetic behaviour with coercivity of 97 Oe. As expected, organic- and water-soluble nanoparticles show superparamagnetic feature with no hysteresis and perfect Langevin behaviour. The saturation magnetisations are 53, 44, and 41 emu/g for rod-like, organic-, and water-soluble nanoparticles, respectively ().
5. Conclusion
In summary, Fe(OH)3 is introduced to act as an inexpensive and environmentally friendly precursor to generate organic- and water-soluble nanoparticles instead of organometallic compounds. By precisely adjusting reaction parameters, the nanoparticles with tailorable size and morphology are obtained. This method can be simply scaled up to produce gram-scale monodisperse nanoparticles. Thus, Fe(OH)3 may be a quite promising precursor for high-temperature decomposition to produce high-quality nanoparticles.
Acknowledgements
This work was supported by National Program on Key Basic Research Project (2009CB930300) and National Natural Science Foundation of China (No. 226007).
References
- Wang , X and Li , YD . 2007 . Monodisperse nanocrystals: General synthesis, assembly, and their applications . Chem. Commun. , 28 : 2901 – 2910 . doi: 10.1039/b700183e
- Jun , YW , Seo , JW and Cheon , A . 2008 . Nanoscaling laws of magnetic nanoparticles and their applicabilities in biomedical sciences . Accounts. Chem. Res. , 41 : 179 – 189 . doi: 10.1021/ar700121f
- Jeong , U , Teng , XW , Wang , Y , Yang , H and Xia , YN . 2007 . Superparamagnetic colloids: Controlled synthesis and niche applications . Adv. Mater. , 19 : 33 – 60 . doi: 10.1002/adma.200600674
- Laurent , S , Forge , D , Port , M , Roch , A , Robic , C , Elst , LV and Muller , RN . 2008 . Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications . Chem. Rev. , 108 : 2064 – 2110 . doi: 10.1021/cr068445e
- Wu , W , He , QG and Jiang , CZ . 2008 . Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies . Nanoscale Res. Lett. , 3 : 397 – 415 . doi: 10.1007/s11671-008-9174-9
- Cheng , FY , Su , CH , Yang , YS , Yeh , CS , Tsai , CY , Wu , CL , Wu , MT and Shieh , DB . 2005 . Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications . Biomaterials , 26 : 729 – 738 . doi: 10.1016/j.biomaterials.2004.03.016
- Liu , ZL , Liu , YJ , Yao , KL , Ding , ZH , Tao , J and Wang , X . 2002 . Synthesis and magnetic properties of Fe3O4 nanoparticles . J. Mater. Synth. Process , 10 : 83 – 87 . doi: 10.1023/A:1021231527095
- Rockenberger , J , Scher , EC and Alivisatos , AP . 1999 . A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides . J. Am. Chem. Soc. , 121 : 11595 – 11596 . doi: 10.1021/ja993280v
- Bhalerao , GM , Sinha , AK , Srivastava , H and Srivastava , AK . 2009 . Synthesis and studies of growth kinetics of monodispersed iron oxide nanoparticles using ferrocene as novel precursor . Appl. Phys. A – Mater. , 95 : 373 – 380 . doi: 10.1007/s00339-008-5068-z
- Sun , SH and Zeng , H . 2002 . Size-controlled synthesis of magnetite nanoparticles . J. Am. Chem. Soc. , 124 : 8204 – 8205 . doi: 10.1021/ja026501x
- Cheon , JW , Kang , NJ , Lee , SM , Lee , JH , Yoon , JH and Oh , SJ . 2004 . Shape evolution of single-crystalline iron oxide nanocrystals . J. Am. Chem. Soc. , 126 : 1950 – 1951 . doi: 10.1021/ja038722o
- Park , J , An , KJ , Hwang , YS , Park , JG , Noh , HJ , Kim , JY , Park , JH , Hwang , NM and Hyeon , T . 2004 . Ultra-large-scale syntheses of monodisperse nanocrystals . Nature Mater. , 3 : 891 – 895 . doi: 10.1038/nmat1251
- Jana , NR , Chen , YF and Peng , XG . 2004 . Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach . Chem. Mater. , 16 : 3931 – 3935 . doi: 10.1021/cm049221k
- Teng , XW and Yang , H . 2004 . Effects of surfactants and synthetic conditions on the sizes and self-assembly of monodisperse iron oxide nanoparticles . J. Mater. Chem. , 14 : 774 – 779 . doi: 10.1039/b311610g
- Kwon , SG , Piao , Y , Park , J , Angappane , S , Jo , Y , Hwang , NM , Park , JG and Hyeon , T . 2007 . Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process . J. Am. Chem. Soc. , 129 : 12571 – 12584 . doi: 10.1021/ja074633q
- Bronstein , LM , Huang , XL , Retrum , J , Schmucker , A , Pink , M , Stein , BD and Dragnea , B . 2007 . Influence of iron oleate complex structure on iron oxide nanoparticle formation . Chem. Mater. , 19 : 3624 – 3632 . doi: 10.1021/cm062948j
- Yu , WW , Falkner , JC , Yavuz , CT and Colvin , VL . 2004 . Synthesis of monodisperse iron oxide nanocrystals by thermal decomposition of iron carboxylate salts . Chem. Commun. , : 2306 – 2307 . doi: 10.1039/b409601k
- Yu , WW and Peng , XG . 2002 . Formation of high-quality CdS and other II-VI semiconductor nanocrystals in noncoordinating solvents: Tunable reactivity of monomers . Angew. Chem. Int. Ed. , 41 : 2368 – 2371 . doi: 10.1002/1521-3773(20020703)41:13<2368::AID-ANIE2368>3.0.CO;2-G
- Yu , WW , Wang , YA and Peng , XG . 2003 . Formation and stability of size-, shape-, and structure-controlled CdTe nanocrystals: Ligand effects on monomers and nanocrystals . Chem. Mater. , 15 : 4300 – 4308 . doi: 10.1021/cm034729t
- Yang , HG and Zeng , HC . 2004 . Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening . J. Phys. Chem. B , 108 : 3492 – 3495 . doi: 10.1021/jp0377782
- Shevchenko , EV , Talapin , DV , Schnablegger , H , Kornowski , A , Festin , O , Svedlindh , P , Haase , M and Weller , H . 2003 . Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: The role of nucleation rate in size control of CoPt3 nanocrystals . J. Am. Chem. Soc. , 125 : 9090 – 9101 . doi: 10.1021/ja029937l
- Hyeon , T , Lee , SS , Park , J , Chung , Y and Bin Na , H . 2001 . Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process . J. Am. Chem. Soc. , 123 : 12798 – 12801 . doi: 10.1021/ja016812s
- Zhang , TR , Ge , JP , Hu , YP and Yin , YD . 2007 . A general approach for transferring hydrophobic nanocrystals into water . Nano Lett. , 7 : 3203 – 3207 . doi: 10.1021/nl071928t
- Wang , Y , Wong , JF , Teng , XW , Lin , XZ and Yang , H . 2003 . “Pulling” nanoparticles into water: Phase transfer of oleic acid stabilized monodisperse nanoparticles into aqueous solutions of alpha-cyclodextrin . Nano Lett. , 3 : 1555 – 1559 . doi: 10.1021/nl034731j
- Li , Z , Chen , H , Bao , HB and Gao , MY . 2004 . One-pot reaction to synthesize water-soluble magnetite nanocrystals . Chem. Mater. , 16 : 1391 – 1393 . doi: 10.1021/cm035346y
- Li , Z , Sun , Q and Gao , MY . 2005 . Preparation of water-soluble magnetite nanocrystals from hydrated ferric salts in 2-pyrrolidone: Mechanism leading to Fe3O4 . Angew. Chem. Int. Ed. , 44 : 123 – 126 . doi: 10.1002/anie.200460715
- Zhang , L , He , R and Gu , HC . 2006 . Oleic acid coating on the monodisperse magnetite nanoparticles . Appl. Surf. Sci. , 253 : 2611 – 2617 . doi: 10.1016/j.apsusc.2006.05.023
- Chen , ZP , Zhang , Y , Zhang , S , Xia , JG , Liu , JW , Xu , K and Gu , N . 2008 . Preparation and characterization of water-soluble monodisperse magnetic iron oxide nanoparticles via surface double-exchange with DMSA . Colloids Surf. A , 316 : 210 – 216 . doi: 10.1016/j.colsurfa.2007.09.017
- Au-Yeung , SCF , Denes , G , Greedan , JE , Eaton , DR and Birchall , T . 1984 . A novel synthetic route to “iron trihydroxide, Fe(OH)3”: Characterization and magnetic properties . Inorg. Chem. , 23 : 1513 – 1517 . doi: 10.1021/ic00179a009
- Wu , JJ , Lee , YL , Chiang , HH and Wong , DKP . 2006 . Growth and magnetic properties of oriented alpha-Fe2O3 nanorods . J. Phys. Chem. B , 110 : 18108 – 18111 . doi: 10.1021/jp0644661