Abstract
In this article, we present a surfactant-free method for the preparation of graphene/ZnS nanocomposites. The as-synthesised products were characterised by the X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectroscopy and ultraviolet-visible spectroscopy. It is shown that in the nanocomposites, individual ZnS nanoparticles are well-spread out on the graphene sheets. The good distribution of ZnS nanoparticles on graphene sheets would be promising for practical applications in future nanotechnology.
1. Introduction
Since reported by Rouff in 2006, graphene-based materials, which incorporate graphene sheets in a composite material, have initiated enormous scientific activities due to their excellent properties for various applications Citation1 such as nanoelectronics Citation2–4, sensors Citation5,Citation6, batteries Citation7–9, supercapacitors Citation10,Citation11 and hydrogen storage Citation2,Citation12,Citation13. On the other hand, the product cost of graphene is lower than that of carbon nanotubes (CNTs) Citation1. Therefore, studies on graphene-based materials used for substitutions for CNTs-based materials are worth carrying out.
To the best of our knowledge, the band gap of semiconductor nanoparticles can be tuned by controlling either the particle size Citation14 or the interactions of the particles with the surrounding matrix Citation15. Zinc sulphide (ZnS), an important member in the family of semiconductors, has been extensively investigated. By decorating ZnS with CNTs, a variety of excellent properties such as photocatalytic Citation16 and electronic have been observed Citation17. It is well-known that fruitful research works have been made into the preparation of CNTs/ZnS nanocomposites which are of many potential applications Citation16–19. However, to date, only a few literature works on the preparation and characterisation of graphene/ZnS nanocomposites have been reported.
Although Rajamathi et al. Citation20 synthesised graphene/sulphide nanocomposites by using H2S as a sulphide source, we always insist that the use of thio salts as a sulphide source can slow down the reaction kinetics and the growth of ZnS nanoparticles can be well-controlled. Hence, better distribution of ZnS nanoparticles on the graphene sheets can be expected Citation21. In recent research works, Wang and co-workers developed a solvothermal method to synthesise graphene/ZnS nanocomposites Citation22. Unfortunately, this method introduced a stabiliser called poly(acrylic acid) into the composites to ensure the better distribution of ZnS nanoparticles on the graphene sheets. In addition, previous works have demonstrated that the presence of foreign stabilisers is undesirable for most applications Citation23. Therefore, developing one optimum method for preparing graphene/ZnS nanocomposites is a worthwhile work for researchers to discover. In this article, we report a synthesis of graphene/ZnS nanocomposites by a surfactant-free method in relative mild reaction conditions.
2. Experimental
2.1. Materials and measurements
All chemicals used in our experiments were of reagent grade and used without further purification. The morphology and structure of the products were determined by transmission electron microscopy (TEM, JEM-2100) and X-ray diffraction (XRD, D/MAX2500, Rigaka) with Cu-Kα radiation. Samples for TEM were prepared by dropping the products on a carbon-coated copper grid after ultrasonic dispersion in absolute ethanol. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet FT-170SX spectrometer with KBr pellets in the 4000–400 cm−1 region. Ultraviolet-visible (UV-Vis) spectroscopy measurements were performed on a UV-2450 ultraviolet-visible spectrophotometer.
2.2. Synthesis of graphite oxide
The preparation of graphite oxide (GO) was carried out by a modified Hummers method Citation21,Citation24. In a typical synthesis, 1.0 g of graphite powder was put into cold (0°C) concentrated H2SO4 (50 mL). Then, KMnO4 (4.0 g) and NaNO3 (1.5 g) were added gradually under stirring and the temperature of the mixture was maintained below 10°C by cooling. The reaction mixture was continued for 3 h at the temperature below 10°C. Successively, the mixture was stirred at 35°C for 2 h, and then diluted with 80 mL of deionised (DI) water. Because the addition of water in concentrated sulphuric acid medium released a large amount of heat, the addition of water was performed in an ice bath to keep the temperature below 100°C. After adding all of the 80 mL of DI water, the mixture was stirred for 1 h, and was then further diluted to approximately 150 mL with DI water. After that, 12 mL of 30% H2O2 was added to the mixture to reduce the residual KMnO4. The mixture released a large number of bubbles and the colour of the mixture changed to brilliant yellow. Finally, the mixture was filtered and washed with 5% HCl aqueous solution (800 mL) to remove metal ions followed by 1.0 L of DI water to remove the acid. The resulting solid was dried at 60°C for 24 h. For further purification, the as-obtained GO was re-dispersed in DI water and then was dialysed for 1 week to remove residual salts and acids.
2.3. Synthesis of graphene/ZnS nanocomposites
About 100 mg of GO was dispersed in 100 mL of ethylene glycol (EG) by ultrasonication for 30 min. Then, 0.41 mmol of thiourea was gradually added under vigorous magnetic stirring and the temperature of the mixture was heated up to 100°C. A separate 0.41 mmol of anhydrous ZnCl2 was dissolved into another 50 mL of EG and heated to 100°C. The solution of ZnCl2 is quickly added into the dispersion of GO and thiourea. The mixed solution was heated to 150°C. After about 4 h the solution became black. The resultant black power was isolated by centrifugation, washed with water and ethanol, respectively, and finally dried in a vacuum oven at 60°C for 24 h.
3. Results and discussion
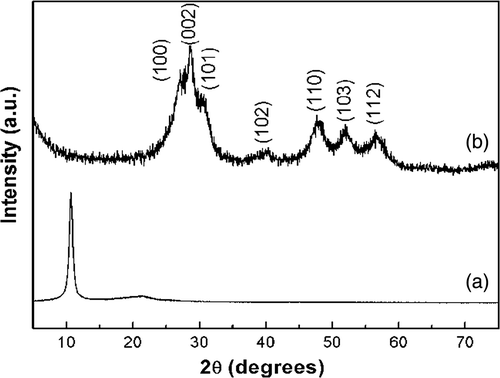
XRD measurements were employed to investigate the phase and structure of the synthesised samples. The XRD pattern of the as-prepared GO () shows a sharp peak at 2θ = 10.2°, corresponding to the (001) reflection of GO Citation25. shows that all the diffraction peaks of as-synthesised products can be indexed to hexagonal ZnS (JCPDS No. 80-0007). In the region of low angle, the XRD pattern does not show any diffraction peaks resulting from GO and/or graphite, suggesting that the graphene oxide was effectively reduced into graphene and the self-restacking of the as-reduced graphene sheets were well-prevented Citation21.
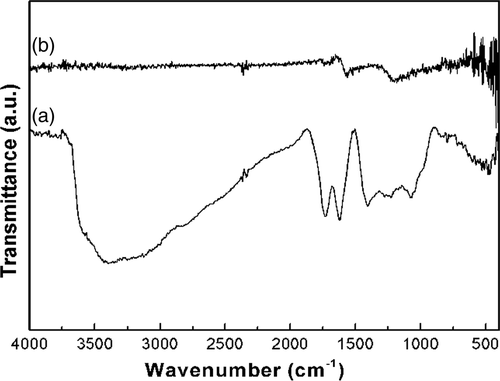
shows the FT-IR spectra of the GO and graphene/ZnS nanocomposites. The oxygen-containing functional groups of GO were revealed by the bands at 1057, 1230, 1400 and 1732 cm−1 (), which correspond to C–O stretching vibrations, C–OH stretching peak, C–O–H deformation peak and C=O stretching of COOH groups, respectively. The peak at 1618 cm−1 can be assigned to the vibrations of the adsorbed water molecules and also the skeletal vibrations of unoxidised graphitic domains Citation26. However, all these bands relating to the oxygen-containing functional groups almost vanished in the FT-IR spectra () of the graphene/ZnS nanocomposites, revealing that these oxygen-containing functional groups were almost removed, and thus the GO was transformed into graphene in the syntheses. shows that the peaks at about 1571 cm−1 can be attributed to the skeletal vibration of the graphene sheets.
EG has been widely used for synthesising graphene-based materials, in which it can act as the dispersing medium or the reducer Citation22,Citation27. In addition, Chen et al proposed that the use of sulphur-containing compounds as the reducing agent allows the de-epoxidation of GO more easily than would hydrazine Citation28. Therefore, thiourea in our method not only acts as the sulphide source but benefits the reduction of GO. In order to further prove reduction level of graphene oxide, we carried out elemental analyses for the as-synthesised composites. The C/H molar ratio is 43.60. These results are consistent with those reported in the literature Citation23, and further confirm that oxygen-containing functional groups were almost removed.
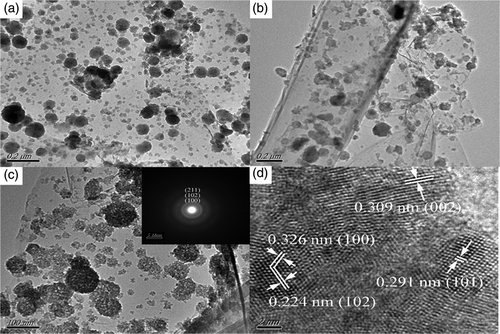
TEM and high-resolution TEM (HRTEM) analyses were performed on the as-prepared graphene/ZnS nanocomposites to determine their features in nanometer domain (). It can be clearly seen that the graphene sheet was well-decorated by ZnS nanoparticles (). As shown in , the ZnS nanoparticles have a size of about 40–70 nm and consist of a large number of tiny ZnS nanocrystals. Moreover, almost no nanoparticles were found outside of the graphene sheets. The HRTEM image is shown in and it can be seen that the ZnS nanoparticles are polycrystalline and the lattice fringes with interplanar distance of 0.224 nm, 0.291 nm, 0.309 nm and 0.326 nm can be assigned to the (102), (101), (002) and (100) planes of the hexagonal ZnS, respectively. The selected-area electron diffraction pattern (SAED) (inset in ) clearly shows the ring pattern arising from the hexagonal ZnS, further confirming the polycrystalline nature of the ZnS nanoparticles Citation29.
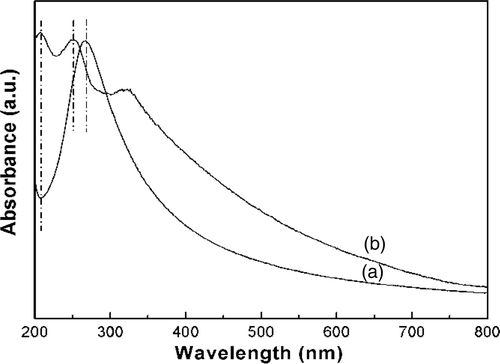
shows the UV-Vis absorption spectra of the as-synthesised graphene/ZnS nanocomposites, together with pure graphene Citation23 for comparison. For UV-Vis measurements, both pure graphene and the as-synthesised nanocomposites were dispersed in water by sonication. It can be seen that the graphene () shows a strong absorption peak at 269 nm, which is generally regarded as the excitation of π-plasmon of graphitic structure Citation30. However, this absorption peak was shifted to 252 nm (). From , we also see an absorption peak at 208 nm. We suppose that these phenomena probably arise from the coupling between graphene sheet and ZnS nanoparticles. Compared with that of bulk ZnS Citation31, the absorption peak of ZnS in nanocomposites was shifted to 322 nm. These phenomena can be explained by the smaller size of ZnS nanoparticles Citation14 and the interactions between ZnS and graphene sheets Citation15.
4. Conclusion
In conclusion, the graphene sheets decorated with a uniform and small diameter ZnS nanoparticles have been successfully prepared by the surfactant-free method in the relative mild reaction conditions. It is found that the size of ZnS particles is controlled in nanometer domain in the as-synthesised nanocomposites. The method in this article can be extended to the synthesis of other graphene/sulphide or CNTs/sulphide nanocomposites with various functions.
Acknowledgements
The authors thank the Research Foundation of Suqian College (No. 2011KY18) for the financial support.
References
- Stankovich , S , Dikin , DA , Dommett , GHB , Kohlhaas , KM , Zimney , EJ , Stach , EA , Piner , RD , Nguyen , ST and Ruoff , RS . 2006 . Graphene-based composite materials . Nature , 442 : 282 – 286 . doi: 10.1038/nature04969
- Du , AJ , Zhu , ZH and Smith , SC . 2010 . Multifunctional porous graphene for nanoelectronics and hydrogen storage: New properties revealed by first principle calculations . J. Am. Chem. Soc. , 132 : 2876 – 2877 . doi: 10.1021/ja100156d
- Westervelt , RM . 2008 . Applied physics – Graphene nanoelectronics . Science , 320 : 324 – 325 . doi: 10.1126/science.1156936
- Lee , BK , Park , SY , Kim , HC , Cho , K , Vogel , EM , Kim , MJ , Wallace , RM and Kim , JY . 2008 . Conformal Al2O3 dielectric layer deposited by atomic layer deposition for graphene-based nanoelectronics . Appl. Phys. Lett. , 92 : 203102 – 203104 . doi: 10.1063/1.2928228
- Du , HJ , Ye , JS , Zhang , JQ , Huang , XD and Yu , CZ . 2011 . A voltammetric sensor based on graphene-modified electrode for simultaneous determination of catechol and hydroquinone . J. Electroanal. Chem. , 650 : 209 – 213 . doi: 10.1016/j.jelechem.2010.10.002
- Keeley , GP , O'Neill , A , McEvoy , N , Peltekis , N , Coleman , JN and Duesberg , GS . 2010 . Electrochemical ascorbic acid sensor based on DMF-exfoliated grapheme . J. Mater. Chem. , 20 : 7864 – 7869 . doi: 10.1039/c0jm01527j
- Wang , XY , Zhou , XF , Yao , K , Zhang , JG and Liu , ZP . 2011 . A SnO2/graphene composite as a high stability electrode for lithium ion batteries . Carbon , 49 : 133 – 139 . doi: 10.1016/j.carbon.2010.08.052
- Wang , HL , Cui , LF , Yang , YA , Casalongue , HS , Robinson , JT , Liang , YY , Cui , Y and Dai , HJ . 2010 . Mn3O4-graphene hybrid as a high-capacity anode material for lithium ion batteries . J. Am. Chem. Soc. , 132 : 13978 – 13980 . doi: 10.1021/ja105296a
- Yao , J , Shen , XP , Wang , B , Liu , HK and Wang , GX . 2009 . In situ chemical synthesis of SnO2-graphene nanocomposite as anode materials for lithium-ion batteries . Electrochem. Commun. , 11 : 1849 – 1852 . doi: 10.1016/j.elecom.2009.07.035
- Chen , Y , Zhang , XO , Zhang , DC , Yu , P and Ma , YW . 2011 . High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes . Carbon , 49 : 573 – 580 . doi: 10.1016/j.carbon.2010.09.060
- Fan , ZJ , Yan , J , Zhi , LJ , Zhang , Q , Wei , T , Feng , J , Zhang , ML , Qian , WZ and Wei , F . 2010 . A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors . Adv. Mater. , 22 : 3723 – 3728 . doi: 10.1002/adma.201001029
- Lee , H , Ihm , J , Cohen , ML and Louie , SG . 2010 . Calcium-decorated graphene-based nanostructures for hydrogen storage . Nano Lett. , 10 : 793 – 798 . doi: 10.1021/n1902822s
- Wang , L , Lee , K , Sun , YY , Lucking , M , Chen , ZF , Zhao , JJ and Zhang , SBB . 2009 . Graphene oxide as an ideal substrate for hydrogen storage . ACS Nano , 3 : 2995 – 3000 . doi: 10.1021/nn900667s
- Murray , CB , Norris , DJ and Bawendi , MG . 1993 . Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites . J. Am. Chem. Soc. , 115 : 8706 – 8715 . doi: 10.1021/ja00072a025
- Akamatsu , K , Tsuruoka , T and Nawafune , H . 2005 . Band gap engineering of CdTe nanocrystals through chemical surface modification . J. Am. Chem. Soc. , 127 : 1634 – 1635 . doi: 10.1021/ja044150b
- Wu , HQ , Wang , QY , Yao , YZ , Qian , C , Zhang , XJ and Wen , XW . 2008 . Microwave-assisted synthesis and photocatalytic properties of carbon nanotube/zinc sulfide heterostructures . J. Phys. Chem. C , 112 : 16779 – 16783 . doi: 10.1021/jp8069018
- Du , JM , Fu , L , Liu , ZM , Han , BX , Li , ZH , Liu , YQ , Sun , ZY and Zhu , DB . 2005 . Facile route to synthesize multiwalled carbon nanotube/zinc sulfide heterostructures: Optical and electrical properties . J. Phys. Chem. B , 109 : 12772 – 12776 . doi: 10.1021/jp051284i
- Wei , DC , Liu , YQ , Cao , LC , Zhang , HL , Huang , LP and Yu , G . 2010 . Synthesis and photoelectric properties of coaxial Schottky junctions of ZnS and carbon nanotubes . Chem. Mater. , 22 : 288 – 293 . doi: 10.1021/cm900929h
- Gautam , UK , Bando , Y , Bourgeois , L , Fang , XS , Costa , PMFJ , Zhan , JH and Golberg , D . 2009 . Synthesis of metal-semiconductor heterojunctions inside carbon nanotubes . J. Mater. Chem. , 19 : 4414 – 4420 . doi: 10.1039/b903791h
- Nethravathi , C , Nisha , T , Ravishankar , N , Shivakumara , C and Rajamathi , M . 2009 . Graphene–nanocrystalline metal sulphide composites produced by a one-pot reaction starting from graphite oxide . Carbon , 47 : 2054 – 2059 . doi: 10.1016/j.carbon.2009.03.055
- Wu , JL , Shen , XP , Jiang , L , Wang , K and Chen , KM . 2010 . Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites . Appl. Surf. Sci. , 256 : 2826 – 2830 . doi: 10.1016/j.apsusc.2009.11.034
- Wang , P , Jiang , TF , Zhu , CZ , Zhai , YM , Wang , DJ and Dong , SJ . 2010 . One-step, solvothermal synthesis of graphene-CdS and graphene-ZnS quantum dot nanocomposites and their interesting photovoltaic properties . Nano Res. , 3 : 794 – 799 . doi: 10.1007/s12274-010-0046-0
- Li , D , Muller , MB , Gilje , S , Kaner , RB and Wallace , GG . 2008 . Processable aqueous dispersions of graphene nanosheets . Nat. Nanotechnol. , 3 : 101 – 105 . doi: 10.1038/nnano.2007.451
- Hummers , WS and Offeman , RE . 1958 . Preparation of graphitic oxide . J. Am. Chem. Soc. , 80 : 1339 – 1339 . doi: 10.1021/ja01539a017
- Nakajima , T , Mabuchi , A and Hagiwara , R . 1988 . A new structure model of graphite oxide . Carbon , 26 : 357 – 361 . doi: 10.1016/0008-6223(88)90227-8
- Xu , YX , Bai , H , Lu , G , Li , C and Shi , GQ . 2008 . Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets . J. Am. Chem. Soc. , 130 : 5856 – 5857 . doi: 10.1021/ja800745y
- Xu , C , Wang , X and Zhu , J . 2008 . Graphene-metal particle nanocomposites . J. Phys. Chem. C , 112 : 19841 – 19845 . doi: 10.1021/jp807989b
- Chen , WF , Yan , LF and Bangal , PR . 2010 . Chemical reduction of graphene oxide to graphene by sulfur-containing compounds . J. Phys. Chem. C , 114 : 19885 – 19890 . doi: 10.1021/jp107131v
- Yao , WT , Yu , SH and Wu , QS . 2007 . From mesostructured wurtzite ZnS nanowire/amine nanocomposites to ZnS nanowires exhibiting quantum size effects: A mild-solution chemistry approach . Adv. Funct. Mater. , 17 : 623 – 631 . doi: 10.1002/adfm.200600239
- Wang , X , Zhi , LJ , Tsao , N , Tomovic , Z , Li , JL and Mullen , K . 2008 . Transparent carbon films as electrodes in organic solar cells . Angew. Chem. Int. Ed. , 47 : 2990 – 2992 . doi: 10.1002/anie.200704909
- Li , JP , Xu , Y , Wu , D and Sun , YH . 2004 . Hydrothermal synthesis of novel sandwich-like structured ZnS/octylamine hybrid nanosheets . Solid State Commun. , 130 : 619 – 622 . doi: 10.1016/j.ssc.2004.03.016