Abstract
Colloidal stability and coagulation kinetics of orthorhombic and monoclinic sulphur nanoparticles (SNPs) were determined in terms of their size distribution along a gradient of temperature, dilution and time-drive. Dynamic light scattering (DLS) was used to determine the size and shape of SNPs in the colloidal suspension. A simultaneous study with high-resolution scanning and transmission electron microscopy was undertaken to evaluate the shape and actual (core) size of SNPs. All the DLS-derived data were statistically analysed to determine the effect of temperature, dilution and time drive on the growth kinetics of SNPs in the colloidal medium. Polymer-stabilised orthorhombic (∼10 nm) and monoclinic (∼15 nm) SNPs were found to be highly stable in the variable range of physical conditions and validates their active use in antimicrobial drug formulations.
1. Introduction
Colloidal drug delivery systems present a range of therapeutic benefits, allowing researchers to cross barriers that have previously prevented efficient treatment while offering improved and more targeted absorption Citation1. Formulation of stable nanocolloids of such insoluble drugs capacitates their enhanced in vivo absorbance and availability Citation2,Citation3. Enhanced antimicrobial efficacy, translocation potential and biocompatibility of sulphur nanoparticles (SNPs) help the emergence of sulphur nanoparticles (SNPs) as future putative antimicrobial drug Citation4–6. Colloidal formulations of SNPs are thus expected to ease their administration into the biological system via drug or pesticide formulations. Hence, stability and rate of coagulation of these novel nanocolloids are absolutely essential to be determined. For this study, dynamic light scattering (DLS) was used to determine the size (in terms of hydrodynamic radius), shape (in terms of the Perrin factor) and coagulation kinetics of one non-capped colloidal sulphur (CS), one custom-made SNPs and two chemically synthesised allotropes (orthorhombic and monoclinic) of SNPs. Comparative statistical measures were applied to evaluate the viability of the aforementioned parameters along a range of temperature, time drive and dilution.
2. Materials and method
2.1. Characterisation of the tested sulphur particles
CS particles were prepared by acidifying sodium thiosulphate with sulphuric acid as described in the literature Citation7. Custom-made SNPs (in powdered form) were obtained from MK nano (MK Impex Corp., Canada). Orthorhombic SNPs were prepared via liquid phase precipitation method Citation5 and was stabilised with polyethylene glycol-400 (PEG-400). Monoclinic SNPs were prepared via water-in-oil micro-emulsion technique employing a polymer conjugate (Sorbitan monooleate and polysorbate-80) as the surfactant agent Citation8. Shape of the sulphur particles was observed with scanning electron microscopy (SEM) (FEI Quantum-200 MK-2, USA). Actual (core) size and allotropic nature of the SNPs were determined with transmission electron microscopy (TEM) (JEOL 2010 F) and X-ray diffraction (XRD) pattern (X’Pert PRO-MRD, PANalytical BV, the Netherlands). Microscopic and spectroscopic samples were prepared by adding a few drops of ultra sonically dispersed nanoparticles in ethyl alcohol on a glass slide (for SEM & XRD) or on a carbon-coated copper grid (for TEM).
2.2. Sample preparation for the stability study
Aqueous dispersions of colloidal and SNP allotropes were prepared through a novel filtration technique prior to studying their stability. Around 100 µL of the SNP colloids were suspended in 5 mL of ethyl alcohol to make a ethanol–sulphur slurry. For CS and custom-made SNPs, the ethanol–sulphur slurry was prepared by mixing 100 mg of each with 5 mL of ethanol. Ethanol–CS/SNPs slurry was then filtered (with Whatman filter paper-3, mesh size 125 mm) into five different dilutions of Millipore water. The resultant mixture was swirled gently to make a homogeneous colloidal suspension.
2.3. Determination of hydrodynamic radius, Perrin factor and coagulation kinetics
DLS (MALVERN Nano S (red badge), Ze-1600, UK) was used to determine the hydrodynamic radius (RH ), Perrin factor (F) and coagulation kinetics (Ck ) of both custom-made and synthesised SNPs at different temperature (from 25°C to 55°C with an interval of 10°C) and dilution (ethanol–sulphur slurry: water in the ratio of 1:5, 1:7, 1:9 and 1: 11). Orthorhombic and monoclinic SNPs were also analysed to determine the aforementioned features along a time drive of weeks (28 days with the interval of 7 days). As in the colloidal suspension, every collision is effective in forming an aggregate, the aggregation rate constant becomes equal to the collision rate constant. Hence, for each day, five independent rounds (with an interval of 10 min between two consecutive rounds) of experiment were performed to determine the diffusion-controlled (fast coagulation) mechanism for SNPs. The rate of change of the k fold aggregate concentration for both the synthesised SNPs were expressed using the Smoluchowski equation Citation9.
2.4. Statistical data analysis
Two way analysis of covariance (ANOCOVA) (R-2.13.2 software) was applied to understand the change in RH with respect to time interval between two consecutive rounds of experiment and between two consecutive weeks of experiment.
3. Results and discussion
3.1. Physical features of CS and SNPs
SEM micrographs revealed that CS particles (∼10 µm) were nearly spherical in shape with uneven surface topology (). Actual size of custom-made (∼60 nm), orthorhombic (∼10 nm) and monoclinic SNPs (∼15 nm) were determined with TEM (). XRD pattern for custom-made and one of the chemically synthesised (via liquid phase precipitation) SNPs were found to be identical to the orthorhombic sulphur Citation10. In comparison, SNPs prepared by water-in-oil microemulsion technique were formed in the monoclinic phase (data not given). The aggregation ratio characteristics of the adhesion between the SNPs were determined Citation11 by dividing RH value of SNPs with their actual size (obtained from TEM) ().
Figure 1. Scanning electron micrograph representing the shape and surface topology of the colloidal sulphur particles.
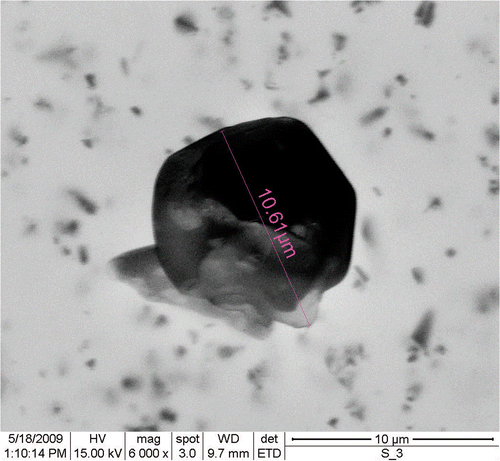
Figure 2. (A) Determination of actual (core) size of custom-made, (B) orthorhombic and (C) monoclinic SNPs.

Table 1. The aggregation ratio characteristics of the adhesion between the SNPs in use
3.2. Change in RH along time drive
CS and custom-made SNPs (both in powdered form) were not coated with any surfactants, and hence were not considered for the colloidal stability along time drive. The means of RH of monoclinic SNPs were found to increase from 87.4 nm (on first week) to 91.4 nm (on fourth week), while the means of RH of orthorhombic SNPs remained almost unaltered (from 87.1 to 88.38 nm) (). In contrast, RH for both the SNPs in ethanol–water suspension increased rapidly over consecutive rounds of experiment in a day. Time interval between sample preparation and each successive round of the experiment (T) have a more significant effect on the increase of RH than weeks (W), as the Pr (>F ratio) values of T were found to be much smaller than W (). Faster coagulation rate of SNPs in the diluted ethanol suspension can be logically correlated to the reduced density of the surfactants Citation12. The Perrin factor (F) for both orthorhombic and monoclinic SNPs indicates their oblate spheroid shape. It was observed that F for monoclinic SNPs was changed from 500.8 (first week) to 545.63 (fourth week) while the change in F for orthorhombic SNPs was also in a similar range (from 412.3 to 440.7). This phenomenon can be logically correlated to the change in RH over the weeks for respective SNPs.
Figure 3. (A) Effect of temperature, (B) time drive (C) and dilution on the hydrodynamic radius of custom-made and synthesised SNPs.

Table 2. Effect of weeks and time interval between sample preparation and each successive round of experiments on the hydrodynamic radius of SNPs
3.3. Change in RH along temperature and dilution
The RH of monoclinic SNPs was decreased (from 87.53 to 83.06) with the increase in temperature. In contrast, RH for orthorhombic and custom-made SNPs was found to be directly proportional to the change in temperature (). For all the SNPs in use, increase in dilution seems to impart statistically insignificant effect on the RH (). Changes in the F of SNPs were similar to their corresponding RH change in response to temperature and dilution.
3.4. Change in Ck kinetics along time drive
The concentrations for orthorhombic and monoclinic SNPs were estimated to be 1176 mg/L Citation10 and 18,000 mg/L (unpublished data), respectively, and were used to evaluate the coagulation half time (T 1/2). The T 1/2 for orthorhombic (1.387 × 1017 s) and monoclinic (9.065 × 1015 s) SNPs were evaluated from the Smoluchowski equation Citation9 using the second-order coagulation rate constant is equal to 1.226 × 10−17 m3/s in an aqueous solution at 25°C. The resulting half-times were high enough to ensure that the measurements were performed at the early stage of the coagulation process.
Earlier literature suggests that elemental sulphur (ES) exerts its microbicidal action by impairing a number of components of cellular respiration. In contrast, microbes employ several means to reduce the load of sulphur toxicity from their cytosol and render ineffective ES as an antimicrobial agent Citation13. Efficacy of SNPs as potent antimicrobial agents has already been established Citation4–6. However, in order to promote these novel nanoformulations as future putative antimicrobial drug, their colloidal nature should have been studied. Colloidal stability of the prepared SNPs along variable range of temperature, dilution and time drive ensures their long-time storage essentially in their nanosized configuration. Moreover, SNPs are expected to surpass the resistive force of microbes owing to their diminutive size and surface modification Citation6. The cumulative result of the performed experiments suggest an excellent stability of the surfactant-coated SNP allotropes and persuade their use in colloidal drugs
Acknowledgements
This research was funded by NAIP-ICAR-World Bank (Comp-4/C3004/2008-09; and Department of Biotechnology (DBT), Govt. of India (BT/PR9050/NNT/28/21/2007 & BT/PR8931/NNT/28/07/2007) and ISI plan project for 2008-2011 for their generous financial support.
References
- Fanun , M . 2010 . Colloids in Drug Delivery , Boca Raton , FL : CRC Press/Taylor & Francis .
- Agarwal , A , Lvov , Y , Sawant , R and Torchilin , V . 2008 . Stable nanocolloids of poorly soluble drugs with high drug content prepared using the combination of sonication and layer-by-layer technology . J. Control Rel. , 128 ( 3 ) : 255 – 260 . doi: 10.1016/j.jconrel.2008.03.017
- Bybel , B , Neumann , D R , Kim , B Y , Amin , K and Rice , T . 2001 . Lymphoscintigraphy using 99mTc filtered sulfur colloid in chylothorax: A case report . J. Nucl. Med. Technol. , 29 ( 1 ) : 30 – 31 .
- Roy Choudhury , S , Nair , K K , Kumar , R , Gogoi , R , Srivastava , C , Gopal , M , Subhramanyam , B S , Devakumar , C and Goswami , A . 2010 . Nanosulfur: A potent fungicide against food pathogen Aspergillus niger . AIP Conf. Proc. , 1276 : 154 – 157 . doi: 10.1063/1.3504287
- Roy Choudhury , S , Ghosh , M , Mandal , A , Chakrovarty , D , Pal , M , Pradhan , S and Goswami , A . 2011 . Surface modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum . Appl. Microbiol. Biotechnol. , 90 : 733 – 43 . doi: 10.1007/s00253-011-3142-5
- Choudhury , SRoy , Roy , S , Goswami , A and Basu , S . Polyethylene glycol-stabilized sulphur nanoparticles: An effective antimicrobial agent against multidrug-resistant bacteria, J. Antimicrob. Chemother. doi:10.1093/jac/dkr591
- Fauque , G D . 1994 . Sulfur reductase from thiophilic sulfate reducing bacteria . Methods Enzymol. , 243 : 353 – 367 . doi: 10.1016/0076-6879(94)43026-8
- Guo , Y , Zhao , J , Yang , S , Yu , K , Wang , Z and Zhang , H . 2006 . Preparation and characterization of monoclinic sulfur nanoparticles by water-in-oil microemulsions technique . Powder Technol. , 162 ( 2 ) : 83 – 86 . doi: 10.1016/j.powtec.2005.12.012
- Kang , K and Redner , S . 1984 . Fluctuation effects in Smoluchowski reaction kinetics . Phys. Rev. A. , 30 : 2833 – 2836 . doi: 10.1103/PhysRevA.30.2833
- Kumar , R , Nair , K K , Alam , M I , Gogoi , R , Singh , P K , Srivastava , C , Roy Choudhury , S and Goswami , A . 2011 . A simple method for estimation of sulfur in nanoformulations by UV spectrometry . Curr. Sci. India. , 100 : 1542 – 1546 .
- Majzik , A , Patakfalvi , R , Hornok , V and Dékány , I . 2009 . Growing and stability of gold nanoparticles and their functionalization by cysteine . Gold Bull. , 42 ( 2 ) : 113 – 123 . doi: 10.1007/BF03214921
- Rao , A , Schoenenberger , M , Gnecco , E , Glatzel , T , Meyer , E , Brändlin , D and Scandella , L . 2007 . Characterization of nanoparticles using atomic force microscopy . J. Phys. Conf. Ser. , 61 : 971 – 976 . doi: 10.1088/1742-6596/61/1/192
- Sato , I , Shimatani , K , Fujita , K , Abe , T , Shimizu , M , Fujii , T , Hoshino , T and Takaya , N . 2011 . Glutathione reductase/glutathione is responsible for cytotoxic elemental sulfur tolerance via polysulfide shuttle in fungi . J. Biol. Chem. , 286 : 20283 – 20291 . doi: 10.1074/jbc.M111.225979