Abstract
Aluminium oxide (Al2O3) and titanium dioxide (TiO2) nanoparticles (NPs) have been widely used in nanotechnology-based products. Recently, researchers and the public have raised concerns about the adverse effects of these NPs in biological systems, particularly in humans. The aim of this study was to investigate the possible adverse effects of these two common metal oxide NPs on human lung epithelium cells (A549) and to investigate NP size-dependent effects on these cells, considering both the primary and hydrodynamic particle size. NPs were found to inhibit cell viability and proliferation at the highest concentration level (10 mg/mL) included in this study, as measured by a clonogenic assay. Moreover, cell viability, proliferation and metabolism were impaired to a greater extent by the smaller NPs (5 nm TiO2 and 10 nm Al2O3) relative to the larger particles (200 nm TiO2 and 50 nm Al2O3) included in this study, as measured by cell proliferation and metabolism. Notably, the observed cytotoxic effects correlated to the primary size, rather than the hydrodynamic size. Similarly, NP cytotoxicity was found to be correlated with the NP surface area. These findings highlight the importance of including primary size and surface area information in NP characterisation in cytotoxicity studies.
1. Introduction
Nanotechnology is presently considered one of the greatest engineering innovations since the Industrial Revolution Citation1 and is estimated to become a $2.5 trillion market by 2015 Citation2. A major component of nanotechnology are engineered nanoparticles (NPs) defined as nanometre size particles (<100 nm), specifically synthesised to display a particular property Citation3. Aluminium oxide or alumina (Al2O3) is a commercially important nanomaterial widely used in abrasives, wear-resistant coatings on propeller shafts of ships, and drug delivery systems Citation4. Similarly, titanium dioxide or titania (TiO2) has been commonly used in white pigments, food colorants, and a variety of personal care products (e.g. sunscreens and cosmetic creams) Citation5. Such broad commercial applications have raised the concern about potential cytotoxicity of NPs in biological systems, particularly with regard to humans. Specifically, these common applications have highlighted the importance of investigating the cytotoxicity of those two NPs as they continue to be integrated into industry Citation6,Citation7.
Previous in vitro studies have shown that TiO2 and Al2O3 NPs can induce apoptosis and inflammatory responses in cells Citation8,Citation9. Recently, TiO2 NPs were shown to induce genotoxicity by up-regulating antioxidant genes to maximum transcription levels after exposure to one-tenth of the TiO2 concentration that caused noticeable growth inhibition Citation10. Similarly, Al2O3 NPs caused DNA damage and chromosomal aberrations in an in vivo rat model Citation4. Although many studies have investigated the potential toxic effects of NPs, the mechanisms of toxicity induced by NPs are still not clear. Particle size has been considered as an important parameter in determining the health effects of NPs because they fall in the transition zone between atoms and bulk materials; however, the effect of particle size on cytotoxicity is still not well understood. Oberdörster et al. Citation11 found that TiO2 NPs (20 nm) caused greater pulmonary effects in an in vivo rat model, compared to larger, but still submicronic TiO2 particles (250 nm). Sayes and Warheit Citation12 observed that 140 nm TiO2 NPs had higher toxicity relative to larger NPs (∼250 and 380 nm) studied. Moreover, Gurr et al. Citation5 reported that higher oxidative DNA damage was induced by 10 and 20 nm anatase TiO2 NPs compared to 200 nm TiO2 NPs. In contrast, Kim et al. Citation13 found no prominent size-dependent trend in cytotoxicity between TiO2 NPs (30 nm) and fine particles (1 µm). Similarly, Yamamoto et al. Citation14 concluded that the differences in size (90, 130 and 1600 nm) did not affect the mechanical toxicity of these particles. Mechanical toxicity relates to the physical stimulation of cells by insoluble particles rather than chemical toxicity due to metal ion release. For Al2O3, there are even fewer studies that have investigated size effects on cytotoxicity and they have not yielded conclusive results. Lin et al. Citation15, for example, found that exposure to smaller (13 nm) Al2O3 NPs resulted in a more significant depolarisation than 30 nm Al2O3 NPs; however, both sizes showed similar cytotoxicity in the 5–25 µg/mL dose ranges. Wagner et al. Citation16, on the other hand, found that Al NPs showed chemical-composition-dependent toxicity but neither size- nor surface-area-dependent cytotoxicity. Consequently, the size-dependency of cytotoxicity remains unclear for both types of NPs.
There is an additional debate about whether common optical-based methodologies used to assess cytotoxicity provide reliable data due to the interference of the dark nanomaterial. Common methods to assess the cytotoxicity of NPs include screening assays based on fluorescence or absorbance measurements following toxicant exposure and incubation with a fluorescent or colorimetric indicator dye of choice Citation17. The most common and accepted assays include 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Citation12,Citation18, lactate dehydrogenase (LDH) Citation19, reactive oxygen species (ROS) Citation20 and alamarBlue® Citation21. Casey et al. Citation22 found that there is a significant interaction between nanotubes and the assay dyes resulting in reduction of the associated absorption/fluorescent emission. Monteiro-Riviere et al. Citation23 reached similar conclusions reporting the interference of the carbon nanomaterials with the assay markers. Such results point to the importance of using multiple approaches in the evaluation of cytotoxicity and the potential interference of NPs in these common assays. In order to yield reliable results, the evaluation should not rely solely on the absorbance or fluorescence measurements.
The aim of this study was to investigate the possible adverse effects of two commonly used nanomaterials (TiO2 and Al2O3) on human lung epithelium cells (A549) to evaluate potential cytotoxicity and potential dependence on particle size. Both the primary (aerosol phase) and the hydrodynamic (colloid phase) particle sizes were investigated. Cell response to the NPs was assessed by both assays for cell viability/proliferation (clonogenic assay) and metabolism (alamarBlue®). The clonogenic (a.k.a. colony formation) assay was used to investigate the effect of NPs on cell viability (colony number) and proliferation (colony size) following nanomaterial exposure. This assay is independent of any absorbance or fluorescence measurements. In contrast, alamarBlue® was used to measure changes in cell metabolism due to nanomaterial exposure and is measured by absorbance. Based on separate but complementary measures of cell response, the goal is to determine whether NP size will provide some indication of cytotoxicity.
2. Materials and methods
2.1. NPs
This study investigated four different NP types, including 5 nm and 200 nm TiO2 and 10 nm and 50 nm Al2O3 (). The crystalline structure and purity for each NP type is included. Particles types were selected to investigate the size effect and were matched by the crystalline structure and purity as closely as possible and commercially available. While there may be subtle differences between the two materials, their surface chemistry are expected to be the same or very similar. All particle types were obtained from commercial sources and came in powder form except for the 10 nm Al2O3. The 10 nm Al2O3 NPs were dispersed in a water solution; there was no surfactant in those samples, as reported by the manufacturer.
Table 1. Commercial source and description of manufactured nanoparticles.
2.2. NP characterisation
Dynamic light scattering (DLS), differential mobility and electron microscopy methods were used to characterise the NP size. The hydrodynamic size of NP dispersions was measured with a 90 Plus Particle Size Analyzer (Brookhaven Instruments Corporation, Holtsville, NY), which is based on the principles of DLS. Particles were suspended in water to create 1 mg/mL solution of water, F-12K medium (Kaighn's Modification of Ham's F-12, American Type Culture Collection (ATCC, Manassas, VA) or cell culture medium (F-12K medium with 10% foetal bovine serum, ATCC, Manassas, VA). A 30 min bath sonication was performed to help the NPs disperse into solution.
The primary particle size distribution of each NP type was measured by a Scanning Mobility Particle Sizer (SMPS) spectrometer, as described in Citation24. The size distribution of 200 nm TiO2 NPs was measured using scanning electron microscopy (SEM, Zeiss supra 55, Carl Zeiss SMT Inc., Peabody, MA) because it was not possible to suspend them into a buffer that allowed their aerosolisation using an electrospray. 200 nm powders of TiO2 were dissolved in ethanol and dropped onto a silicon wafer. After the ethanol was evaporated, the samples were coated with platinum (Pt) and viewed using SEM. The particle diameter was determined by image analysis software Image J 1.41o (http://rsbweb.nih.gov/ij/).
The morphology and primary particle size of NPs were characterised by transmission electron microscopy (TEM). NPs were dispersed in ethanol with ultrasonication and subsequently dropped onto TEM copper grids. The images were obtained using a TEM (Philips CM12, Philips, Hillsboro, Oregon) using an accelerating voltage of 120 kV.
2.3. Cell cultures
The human carcinoma epithelial cell line A549 used in this study was purchased from ATCC (Manassas, VA, USA). This cell line has been widely used in particle cytotoxicity evaluation studies Citation12,Citation25,Citation26. Cells were cultured in growth medium, which consisted of F-12K medium (Kaighn's Modification of Ham's F-12, ATCC, Manassas, VA) supplemented with 10% foetal bovine serum (ATCC, Manassas, VA) and 2 mM L-glutamine and 100 U/mL penicillin/streptomycin. A549 cells collected between passages 3 and 10 (subcultures) were used in these experiments. Cells were maintained at 37°C in a 5% CO2 humidified incubator and the medium was changed every other day.
2.4. Clonogenic assay
Inhalation is the most significant exposure route for airborne NPs Citation27 and the lung is the major target of inhaled NPs Citation1. Consequently, the human carcinoma epithelial cell line A549 was chosen as a biological model to assess epithelial sensitivity to inhaled NPs. The clonogenic assay used in these studies was adopted from Citation28. A549 cells were seeded in a 12-well plate at a density of 150 cells per well in the growth medium (NP-free). A low density was required to obtain single cells and avoid colony overlap over time. The cells were allowed to grow for 12 h, which is shorter than the population doubling time (22 h) Citation17, and then the medium was removed. The A549 clones were cultured in growth medium containing NPs at concentrations of 0.1, 1 and 10 mg/mL in triplicate. A549 controls were cultured in the NP-free growth medium (0 mg/mL NPs). Following 2 days or 5 days treatment, the cells were washed with 1 × phosphate buffered saline (PBS), fixed in 4% paraformaldehyde (PFA) and the nuclei were stained using a 0.1% 4′,6-diamidino-2-phenylindole (DAPI) diluted in a 1 × PBS solution. Colony number per well and cells per colony were visualised using an inverted light microscope (IX81, Olympus, Center Valley, PA) and counts were recorded following 2- and 5-day treatments. This assay was also used to determine the effect of NP supernatants on cell proliferation. The supernatants were prepared by dispersing the NPs into the medium (10 mg/mL) and then the mixture was incubated for 24 h at 37°C. The NP-laden medium was then centrifuged at 16,000 × g for 20 min to remove the bulk NPs and filtered with 0.22 µm polyethersulfone syringe filters (Millipore Corporation).
2.5. alamarBlue® assay
The alamarBlue® assays were performed in polystyrene, tissue-culture-treated, flat-bottom, 96-well plates containing 300 cells/well in F-12 K, with 10% FBS. After plates were incubated at 37°C for 12 h, the medium was removed. A total of 100 µL growth media containing NPs at concentrations of 0.1, 1 and 10 mg/mL was added and 0 mg/mL was served as the control to determine the baseline cell metabolism. A negative cell-free control was also included. The plates were cultured for 5 days at 37°C. After 5 days, the media were removed and the wells were rinsed thrice with 200 µL of fresh medium. Next, 100 µL of fresh medium and 10 µL of alamarBlue® reagent were added to each well. The plates were gently mixed and incubated for 4 h at 37°C. Post-incubation, the medium containing the alamarBlue® reagent was transferred to a new 96-well plate to avoid interference from NPs left on the well bottom. The absorbance was measured at 570 and 600 nm using a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Inc, Winooski, VT). The percent reduction of alamarBlue® was calculated using the manufacturer's formula:
2.6. Statistical analysis
Three independent experiments were conducted for each NP type and size. Control values were set as 100% and the results were expressed as percentage of the mean ± standard deviation. Student's t-test was used to compare differences between samples and the control. Multi-group comparisons of the means were carried out by two-way analysis of variance (ANOVA). Pearson correlation test was used to examine the correlation between the results of alamarBlue® and clonogenic assay. Statistically significant differences were set at p ≤ 0.05.
3. Results
3.1. NP characterisation
shows TEM micrographs of the four particle types used in this study. All particles showed similar spherical morphologies, except the 10 nm Al2O3, which formed needle-shaped aggregates. Since all samples were polydispersed, it was difficult to evaluate the mean size based on TEM images; therefore, the size distributions of the NPs were measured in solution and in the aerosol phase by DLS and Scanning Mobility Particle Sizer (SMPS), respectively (). Here, DLS was used to characterise particle size in solution (in water and cell culture medium with or without serum) while the SMPS was used to characterise the primary particle size (in aerosol phase). NPs agglomerate in solution; therefore, the particle size in solution (hydrodynamic size) is rarely similar to the primary particle size measured as aerosol. The hydrodynamic size is important because it may impact the cell's response to the nanomaterial exposure; however, the agglomeration state can also depend on several parameters, such as solution ionic strength, pH and surface charge Citation29,Citation30.
Figure 1. TEM images of particles used in this study. (a) 5 nm TiO2; (b) 200 nm TiO2; (c) 10 nm Al2O3 and (d) 50 nm Al2O3.
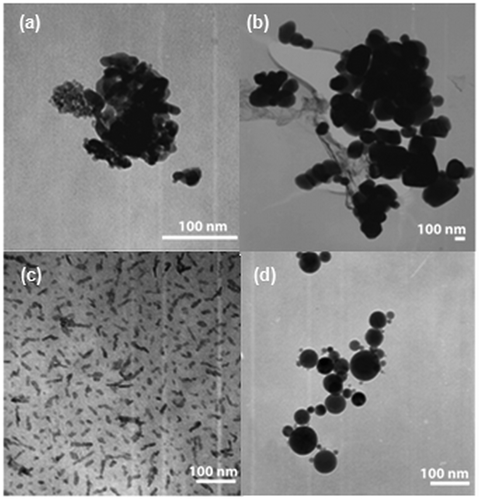
Table 2. Particle characterisation by DLS, SMPS.
All the tested particle types aggregated in solution form, as determined by DLS measurements. When the particles were dispersed in water, the smallest particles (5 nm TiO2 and 10 nm Al2O3) formed aggregates that were 74 and 9 times larger than the native particle sizes measured by the SMPS, respectively. The larger particles formed aggregates in water that were only about 1.5–2.5 times the size of the native (primary) particles, as measured by the SMPS. The size of the agglomerates of all types of NPs became larger (8–388 times the size of primary particles) when the NPs were suspended into the medium without serum due to a higher ionic strength. It is expected that the attractive forces (van der Waals forces) between particles became dominant over the electrostatic repulsive forces due to the increase of ionic strength as a result of the decreased thickness of the electrical double layer surrounding the particles Citation29. In the presence of serum, the hydrodynamic size decreased, as previously observed by Murdock et al. Citation30. The agglomerates of larger particles in serum-containing medium were roughly 1.6–10 times larger relative to the native particle sizes (measured by the SMPS). The proteins were likely adsorbed on the NP surface, and the layer of proteins then resulted in steric repulsion to deagglomerate the aggregates Citation31.
The SMPS results () show that 5 and 200 nm TiO2 as well as 10 nm Al2O3 samples were close to the expected size range reported by the manufacturers; however, the 50 nm Al2O3 particles were more than 50% larger than the manufacturer-reported sizes (50 nm). Different characterisation methods were used to determine the particle size, which may explain the discrepancy: The manufacturers used TEM while this study used SMPS measurements. The size measured by the SMPS are expected to be more representative of the native (primary) particle size because it provides the entire size distribution and better statistics.
3.2. Cell viability and proliferation in response to NP exposure
The clonogenic assay was used to assess cell viability and growth directly and to avoid the use of fluorescent or colorimetric indicators (e.g. MTT, neutral red) that could be impacted by nanomaterial interference Citation32. In this assay, cells were seeded at clonal density and the number of colonies that survived was a measurement of cell viability to a given treatment. Changes in the colony size indicated the relative change in proliferation following exposure to a given treatment Citation17.
The effect of particle size and concentration on cell viability () and proliferation () was investigated through exposure to TiO2 and Al2O3 nanomaterials. Following a 5-day exposure at the highest concentration (10 mg/mL), a significant reduction in the colony number was observed for all particle types (), which indicated cell viability. The cell colony number relative to the control decreased to 88% and 87% for TiO2 (5 nm and 200 nm; p = 0.002 and 0.002), respectively. Similar trend was observed for Al2O3 where colony numbers decreased to 90% and 63% of controls (10 nm and 50 nm; p = 0.00 and 0.05), respectively. A significant decrease in the colony number was also measured after the 5-day exposure to 1 mg/mL 5 nm TiO2, but there were no significant decreases in viability (clone number) at NP lower dosages.
Figure 2. Effect of NP exposure on cell viability, as determined by the colony number after a 5-day exposure. Asterisk (*) represents significant difference from the control group (p ≤ 0.05).
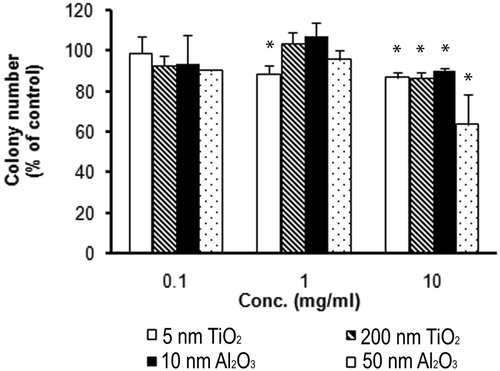
Figure 3. Effect of NP exposure on cell proliferation, as determined by average cell number per colony after: (a) 2-day exposure; (b) 5-day exposure. Asterisk (*) denotes significant difference from the control group or between two comparing groups (p ≤ 0.05).
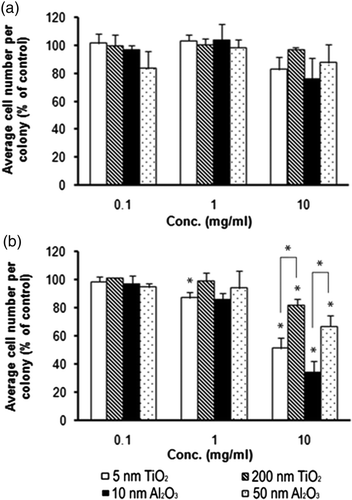
Cell proliferation was assessed by the average colony size following 2 and 5 days of exposure to the NP-laden medium (). As the cells divided and the colony size grew, a measure of proliferation was the increasing number of cells residing within a single colony over time. There was a slight, but not statistically significant, decrease in cell number within colonies after a 2-day exposure. By day 5 of exposure, a significant decrease in cell number was detected for all nanomaterials at the highest dosage (10 mg/mL) and for the smaller nanomaterials (5 nm TiO2 and 10 nm Al2O3) at the 1 mg/mL dose. Further, a dose-dependent decrease in cell number per colony was observed for all particle types after the 5-day exposure (). Results at the 10 mg/mL dose revealed an average colony size decrease by 50% for 5 nm TiO2 and 20% for 200 nm TiO2, respectively. Similarly, reductions of 65% and 33% were observed for 10 and 50 nm Al2O3, respectively. A size-dependent trend was also observed: the 200 nm TiO2 had 30% a higher proliferation rate relative to the smaller 5 nm TiO2 particles. Further, the 50 nm Al2O3 nanomaterials exhibited a 32% greater cell number relative to the smaller 10 nm particles. The two-way ANOVA with replication (n = 3) was used to analyse the data with particle dose and size as the variables. The results showed a dose-dependent effect (p < 0.001) and size-dependent effect (p < 0.001) on cell proliferation, which clearly demonstrated that NP size impacts on toxicity. This study also reported that the NP exposure was more detrimental on cell proliferation than cell viability. These results agree with the study by Herzog et al. Citation17, which found that for A549, colony size (cell proliferation) rather than colony number (cell viability) was dramatically affected by exposure to single-walled carbon nanotubes or carbon black NPs.
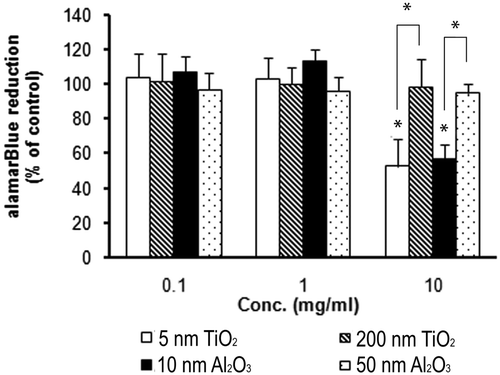
3.3. Cell metabolism in response to nanomaterial exposure
The alamarBlue® assay is a commonly used method for evaluating cellular metabolism, where metabolic activity results in the chemical reduction of the alamarBlue® reagent measured by absorbance Citation33,Citation34. Thus, a linear relationship can exist between the total cell metabolic activity and the absorbance measured. Due to the absorption capacity of some nanomaterials, this linear relationship may be altered at high NP concentrations (>0.025 mg/mL) Citation32. To prevent interference by the dark NPs during these measurements, in this study, most of the NPs were removed from the cell culture medium before measuring the absorbance.
Due to the variable reduction of the alamarBlue® reagent by different cell lines, the concentration reagent used can range from 5% to 25% and the incubation time can range from 2 to 72 h Citation33. The 4 h incubation time and 10% reagent used in this study were determined from initial experiments where the absorbance was measured over a range of time periods (0.5–24 h) to determine the optimal incubation period generating a linear relationship to cell density (data not shown).
Cell metabolism was measured using the alamarBlue® assay for a 5-day exposure to the NPs and was normalised by the nanomaterial-free control (0 mg/mL) (). Only the smaller NPs, 5 nm TiO2 and 10 nm Al2O3, at 10 mg/mL concentrations elicited significant decrease in cell metabolism, with 48% and 43% reduction rates, respectively. For the larger NPs, no significant difference was found. The two extra bars (with asterisks) in the 10 mg/mL concentration cases represent the comparison between different particle sizes. Differences observed in cell metabolism are in agreement with decreases in the clonal cell number ( and ) and confirm that smaller NPs exhibit greater cytotoxicity than larger particles.
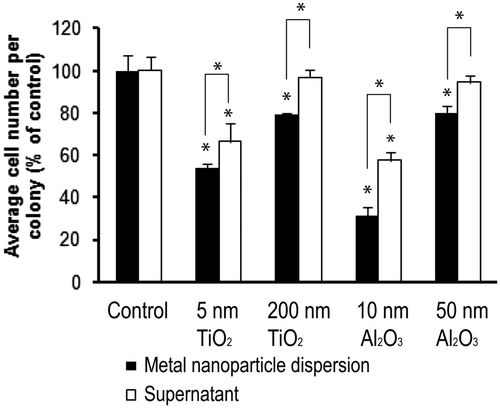
3.4. Impact of NP supernatants on cell proliferation
Horie et al. Citation35 concluded that the adsorption of medium components (e.g. serum proteins and Ca2+) must be taken into account when evaluating the cytotoxicity of low-toxicity materials like TiO2. It is not yet clear, however, how much cytotoxicity is caused by NPs (through physical interaction with the material or uptake of the nanomaterial) rather than medium component depletion or another indirect effect of nanomaterial exposure. To address this issue, in a separate experiment, the A549 cells were cultured in the presence of a medium laden with metal oxide NPs (dispersions) or nanomaterial conditioned medium (supernatants), where the nanomaterial is incubated and the bulk particles are removed. The effect of these exposures on cell proliferation was measured using the clonogenic assay and the results are reported in . The extra bars (with asterisks) represent the comparison between NP dispersions or NP supernatants data sets. Results showed that NP dispersions reduced cell proliferation as measured by colony size to a greater extent than the NP supernatants. The cell proliferation rate decreased significantly (to 66% for the 5 nm TiO2 and 57% for 10 nm Al2O3) following exposure to the supernatants. No significant reductions were observed for the supernatants from the larger particles.
4. Discussion
This study investigated the size-dependent effect of TiO2 or Al2O3 NPs on the viability, proliferation and metabolism of human epithelial A549 cells over a 5-day exposure. While the concentrations used in this study were relatively high, they are comparable to those used in previous nanotoxicity studies (0.5–5 mg/mL) utilising A549 cells Citation8,Citation9,Citation35,Citation36. In Citation35, for example, the viability of A549 cells was found to significantly decrease (<80% relative to the control group), following incubation with 7 nm TiO2 NPs at concentrations greater than 5 mg/mL. Similarly, Kim et al. Citation36 observed a 20% decrease in colony numbers (viability) after the A549 cells were exposed to 0.5 mg/mL of 20–40 nm TiO2 or 20 nm Al2O3 NPs for 10 days, as measured by the clonogenic assay. Sayes et al. Citation9 reported that a cytotoxic response in the A549 cells was observed at a dose of 1.5 mg/mL for 10 nm anatase TiO2 NPs by MTT and LDH assays. Finally, in the study by Chen et al. Citation8, a 34% reduction in A549 cell viability was observed at the highest concentration (2 mg/mL) of 75 nm TiO2 used. Further, A549 cells were reported to be less sensitive than other cells, such as human neuroblastoma SK-N-SH Citation37 and macrophage (THP-1) cell lines Citation38. This fact may partially explain why exposure to higher NP concentrations was required to detect changes in the cellular metrics used. Overall, those studies showed that TiO2 and Al2O3 NPs exhibited low cytotoxicity.
In this study, cell viability and proliferation were measured, independent of fluorescence or colorimetric measures that may be impacted by the nanomaterials Citation17,Citation32. The clonogenic assay is considered to be a reliable method to assess in vitro cytotoxicity Citation17. The cells were seeded at a low, clonal density to avoid the colonies overlapping during the 5-day experimental period. Before the NP-laden medium was added, cells were given 12 h to attach and spread; however, the cell density did not significantly change during this period since the population doubling time is 22 h.
To provide an additional and independent assessment of cytotoxicity, the alamarBlue® assay was used to measure the cell metabolism. The results from this assay showed a size-dependent trend in cell metabolism () at the highest concentrations studied. Further, at this dose level, both assays (clonogenic and metabolism) were in agreement with one another. The Pearson's correlation test performed between the clonogenic assay and the alamarBlue® for the 10 mg/mL group yielded a correlation coefficient of 0.94 (p = 0.05), indicating that a significant correlation exists between the two assays. It is appropriate to note, however, that the alamarBlue® assay may not exhibit a similar sensitivity to the clonogenic assay due to dying, abortive cells that can still be metabolically active and thus give misleading results Citation39.
Using the NP dispersion and supernatants, the intrinsic cytotoxicity of the NPs versus the cytotoxicity caused by nutrient depletion was examined. shows the difference in proliferation (average cell number per colony) between NP dispersion groups and their supernatant groups. If medium component depletion contributed to the total decrease in the cell proliferation observed in the supernatant groups, then the difference between the two groups can be considered to be the intrinsic cytotoxicity of the NPs. The percent differences in proliferation were 13% and 17% for 5 nm and 200 nm of TiO2, respectively. Correspondingly, 26% and 15% decreases were measured for 10 and 50 nm Al2O3 particles. It is possible, however, that ROS present in the supernatants induced by NPs serves to inhibit the cell growth. In that case, the decrease in the average cell number per colony caused by the NPs would be larger than the intrinsic cytotoxicity calculated above; however, it is hard to clearly separate these two effects.
The main purpose of this investigation was to evaluate the possible size-dependent effects of two common NPs on A549 cells. While the overall TiO2 and Al2O3 NPs toxicity was low, results demonstrate that a low, a size-dependent cytotoxicity effect emerged. Based on the SMPS measurements (), the primary sizes of 5 nm TiO2 and 10 nm Al2O3 NPs were significantly smaller than those of 200 nm TiO2 and 50 nm Al2O3 particles. The primary particle sizes were the only difference within each group (TiO2 and Al2O3) and they lead to different cells responses. The reduction in cell proliferation for the smaller, 5 nm TiO2 NPs relative to the NP free control was around 50%, which was significantly higher than that of the larger, 200 nm TiO2 particle (20%). A similar trend was observed for Al2O3. It appears that changes in the primary particle size correlated to the detected NP cytotoxicity with the smaller particles exhibiting a higher cytotoxicity. Interestingly, both the 5 nm and 200 nm TiO2 formed similarly sized aggregates in the medium (in solution) and the same trend was observed with both Al2O3 NPs used in this study (). Consequently, the measured cytotoxicity did not correlate with the hydrodynamic particle size. Murdock et al. Citation30 also concluded that hydrodynamic size did not affect the toxicity after observing that the viability of A549 cells remained the same even though the hydrodynamic size of 39 nm TiO2 NPs changed.
Oh et al. Citation40 found that the shape of NPs affected their toxicity; however, the high (25) aspect ratio NPs showed less toxicity than lower (4.5 and 1.3) aspect ratio NPs. shows that 10 nm Al2O3 formed needle-shaped aggregates, but additional images verified the spherical shape of the native particles (supplemental data). Consequently, the higher cytotoxicity effect caused by 10 nm Al2O3 NPs compared to the 50 nm NPs may be due to size and not shape effects of the aggregates. It is also known that there are several chemical mechanisms of cytotoxicity, such as ROS generation Citation7,Citation41, metal ion release Citation26 and nutrient depletion Citation35. Since TiO2 and Al2O3 are insoluble and do not release metal ions Citation14,Citation35, cytotoxicity induced by chemical means are expected to be limited to ROS generation and nutrient depletion. Gurr et al. Citation5 reported that small TiO2 (anatase 10 and 20 nm) induced oxidative DNA damage, lipid peroxidation, micronuclei formation, and increased hydrogen peroxide and nitric oxide production with no significant differences observed for the larger 200 nm particles. The reason they proposed was that the smaller particles were easier to incorporate into cells. Similarly, Oh et al. Citation40 found that generated ROS per mass by small TiO2 NPs (anatase <50 nm, ∼40–120 µmol/g) was greater than that generated by the same crystal form of the larger particles (>80 nm, <40 µmol/g). This oxidative effect may be due to the increased surface area of the NP samples and may be partially responsible for the inhibition differences observed in this study.
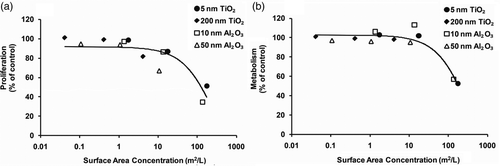
Horie et al. Citation35 explored the influence of nutrient depletion in the NP-treated medium. Their study demonstrated that TiO2 NPs exhibited protein and Ca2+ adsorption and both the Ca2+ and protein depletion that served to inhibit cell growth and metabolism. Since the smaller particles have higher surface area per volume, their adsorption ability is greater, resulting in greater nutrient depletion relative to larger particles. Thus, surface area differences may explain the size-dependent trend in the conditioned medium experiments (). An initial investigation was thus conducted to determine whether the observed reduction in cell response in this study was related to increase in the surface area. In this case, all mass concentration doses were converted to surface area doses assuming spherical morphology for all particle types for comparative purposes. Cellular metrics (viability, proliferation and metabolism) were then plotted against the calculated nanomaterial surface area. Results of that analysis showed no effect on viability (data not shown). show the data and exponential fit curves for proliferation and metabolism responses, respectively. Results showed that proliferation and metabolism decreased with increasing equivalent surface area. Van Hoecke et al. Citation42 observed a similar relationship between increasing NP surface area and a decrease in the reproduction rate of D. magna. Possible reasons for why NPs with larger equivalent surface area (small particles) may lead to more pronounced toxic effects may be a greater capacity to generate ROS and more nutrient depletion relative to smaller equivalent surface area particles (large particles). Further studies will be required to determine the extent and the specific mechanisms of this observed effect.
Figure 6. Cell response curves for 5 and 200 nm TiO2 and 10 and 50 nm Al2O3 NPs: (a) proliferation and (b) metabolism. Dose is expressed as surface area concentration (m2/L). The curve represents an exponential regression for the response.
In summary, this study evaluated the cytotoxicity induced by two commonly used NPs, TiO2 and Al2O3, using two independent assays. Cell metabolism (alamarBlue®) and cell proliferation (clonogenic) were found to be correlated. Both assays confirmed that all the studied NPs (5 nm and 200 nm TiO2 and 10 and 50 nm Al2O3) inhibited cell proliferation, but only effected subtle changes on cell viability. A dose-dependence decrease in the cell number per colony (proliferation) was observed for all particle types after a 5-day exposure. Moreover, the smaller NPs demonstrated greater inhibition on cell metrics than the larger particles, pointing to a size-dependent effect. Further, results exhibited a dose-dependent effect and size-dependent effect on cell proliferation. This dependence was correlated to the primary particle size and not the hydrodynamic particle size. By varying the primary particle size of a nanomaterial, smaller particles exhibit higher surface area relative to larger particles. This study also showed that the NP surface area was correlated with NP cytotoxicity, suggesting that small NPs are more toxic than larger particles. Al2O3 was also found to be more toxic than TiO2 in these studies. Thus, metrics other than mass should be considered in evaluating nanomaterial cytotoxicity and that further investigation of nanomaterials on human health is still needed.
Acknowledgements
This work was supported by the Nanoscale Science and Engineering Institute of the National Science Foundation under NSF Award Number DMR-0642573.
References
- Gwinn , MR and Vallyathan , V . 2006 . Nanoparticles: Health effects – Pros and cons . Environ. Health Persp. , 114 : 1818 – 1825 .
- Lux Research, The recession's impact on nanotechnology (2010). Available at www.luxresearchinc.com/blog/category/nanomaterials/
- Oberdörster , G , Oberdörster , E and Oberdörster , J . 2005 . Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles . Environ. Health Persp. , 113 : 823 – 839 .
- Balasubramanyam , A , Sailaja , N , Mahboob , M , Rahman , MF , Hussain , SM and Grover , P . 2009 . In vivo genotoxicity assessment of aluminium oxide nanomaterials in rat peripheral blood cells using the comet assay and micronucleus test . Mutagenesis , 24 : 245 – 251 .
- Gurr , JR , Wang , AS , Chen , CH and Jan , KY . 2005 . Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells . Toxicology , 213 : 66 – 73 .
- Depledge , MH , Pleasants , LJ and Lawton , JH . 2010 . Nanomaterials and the environment: The views of the royal commission on environmental pollution (UK) . Environ. Toxicol. Chem. , 29 : 1 – 4 .
- Nel , A , Xia , T , Madler , L and Li , N . 2006 . Toxic potential of materials at the nanolevel . Science , 311 : 622 – 627 .
- Chen , E , Ruvalcaba , M , Araujo , L , Chapman , R and Chin , WC . 2008 . Ultrafine titanium dioxide nanoparticles induce cell death in human bronchial epithelial cells . J. Exp. Nanosci. , 3 : 171 – 183 .
- Sayes , CM , Wahi , R , Kurian , PA , Liu , YP , West , JL , Ausman , KD , Warheit , DB and Colvin , VL . 2006 . Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells . Toxicol. Sci. , 92 : 174 – 185 .
- Wang , JX , Zhang , XZ , Chen , YS , Sommerfeld , M and Hu , Q . 2008 . Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii . Chemosphere , 73 : 1121 – 1128 .
- Oberdörster , G , Ferin , J and Lehnert , BE . 1994 . Correlation between particle-size, in-vivo particle persistence, and lung injury . Environ. Health Persp. , 102 : 173 – 179 .
- Sayes , CM and Warheit , DB . 2008 . An in vitro investigation of the differential cytotoxic responses of human and rat lung epithelial cell lines using TiO2 nanoparticles . Int. J. Nanotechnol. , 5 : 15 – 29 .
- Kim , HW , Ahn , EK , Jee , BK , Yoon , HK , Lee , KH and Lim , Y . 2009 . Nanoparticulate-induced toxicity and related mechanism in vitro and in vivo . J. Nanopart. Res. , 11 : 55 – 65 .
- Yamamoto , A , Honma , R , Sumita , M and Hanawa , T . 2004 . Cytotoxicity evaluation of ceramic particles of different sizes and shapes . J. Biomed. Mater. Res. , 68 : 244 – 256 .
- Lin , W , Stayton , I , Huang , Y , Zhou , X and Ma , Y . 2008 . Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549 . Toxicol. Environ. Chem. , 90 : 983 – 996 .
- Wagner , AJ , Bleckmann , CA , Murdock , RC , Schrand , AM , Schlager , JJ and Hussain , SM . 2007 . Cellular interaction of different forms of aluminum nanoparticles in rat alveolar macrophages . J. Phys. Chem. B. , 111 : 7353 – 7359 .
- Herzog , E , Casey , A , Lyng , FM , Chambers , G , Byrne , HJ and Davoren , M . 2007 . A new approach to the toxicity testing of carbon-based nanomaterials – The clonogenic assay . Toxicol. Lett. , 174 : 49 – 60 .
- Gojova , A , Guo , B , Kota , RS , Rutledge , JC , Kennedy , IM and Barakat , AI . 2007 . Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition . Environ. Health Persp. , 115 : 403 – 9 .
- Papageorgiou , I , Brown , C , Schins , R , Singh , S , Newson , R , Davis , S , Fisher , J , Ingham , E and Case , CP . 2007 . The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro . Biomaterials , 28 : 2946 – 2958 .
- Yang , H , Liu , C , Yang , DF , Zhang , HS and Xi , ZG . 2009 . Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition . J. Appl. Toxicol. , 29 : 69 – 78 .
- Kwon , YM , Xia , Z , Glyn-Jones , S , Beard , D , Gill , HS and Murray , DW . 2009 . Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro . Biomed. Mater. , 4 : 1 – 8 .
- Casey , A , Herzog , E , Lyng , FM , Byrne , HJ , Chambers , G and Davoren , M . 2008 . Single-walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cells . Toxicol. Lett. , 179 : 78 – 84 .
- Monteiro-Riviere , NA , Nemanich , RJ , Inman , AO , Wang , YY and Riviere , JE . 2005 . Multi-walled carbon nanotube interactions with human epidermal keratinocytes . Toxicol. Lett. , 155 : 377 – 384 .
- Wei , Z , Rosario , RC and Montoya , LD . 2010 . Collection efficiency of a midget impinger for nanoparticles in the range of 3–100 nm . Atmos. Environ. , 44 : 872 – 876 .
- Cha , KE and Myung , H . 2007 . Cytotoxic effects of nanoparticles assessed in vitro and in vivo . J. Microbiol. Biotech. , 17 : 1573 – 1578 .
- Limbach , LK , Wick , P , Manser , P , Grass , RN , Bruinink , A and Stark , WJ . 2007 . Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress . Environ. Sci. Tech. , 41 : 4158 – 4163 .
- Yang , W , Peters , JI and Williams , RO . 2008 . Inhaled nanoparticles – A current review . Int. J. Pharm. , 356 : 239 – 247 .
- Franken , NA , Rodermond , HM , Stap , J , Haveman , J and Van Bree , C . 2006 . Clonogenic assay of cells in vitro . Nat. Protoc. , 1 : 2315 – 2319 .
- Jiang , JK , Oberdörster , G and Biswas , P . 2008 . Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies . J. Nanopart. Res. , 11 : 78 – 89 .
- Murdock , RC , Braydich-Stolle , L , Schrand , AM , Schlager , JJ and Hussain , SM . 2008 . Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique . Toxicol. Sci. , 101 : 239 – 253 .
- Tantra , R , Tompkins , J and Quincey , P . 2010 . Characterisation of the de-agglomeration effects of bovine serum albumin on nanoparticles in aqueous suspension . Colloid. Surface B. , 75 : 275 – 281 .
- Monteiro-Riviere , NA , Inman , AO and Zhang , LW . 2009 . Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line . Toxicol. Appl. Pharm. , 234 : 222 – 235 .
- Gloeckner , H , Jonuleit , T and Lemke , HD . 2001 . Monitoring of cell viability and cell growth in a hollow-fiber bioreactor by use of the dye Alamar Blue (TM) . J. Immunol. Methods , 252 : 131 – 138 .
- Pettit , RK , Weber , CA , Kean , MJ , Hoffmann , H , Pettit , GR , Tan , R , Franks , KS and Horton , ML . 2005 . Microplate alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing . Antimicrob. Agents Ch. , 49 : 2612 – 2617 .
- Horie , M , Nishio , K , Fujita , K , Endoh , S , Miyauchi , A , Saito , Y , Iwahashi , H , Yamamoto , K , Murayama , H , Nakano , H , Nanashima , N , Niki , E and Yoshida , Y . 2009 . Protein adsorption of ultrafine metal oxide and its influence on cytotoxicity toward cultured cells . Chem. Res. Toxicol. , 22 : 543 – 553 .
- Kim , IS , Baek , M and Choi , SJ . 2010 . Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells . J. Nanosci. Nanotechnol. , 10 : 3453 – 3458 .
- Skandrani , D , Gaubin , Y , Beau , B , Murat , JC , Vincent , C and Croute , F . 2006 . Effect of selected insecticides on growth rate and stress protein expression in cultured human A549 and SH-SY5Y cells . Toxicol. In Vitro , 20 : 1378 – 1386 .
- Lanone , S , Rogerieux , F , Geys , J , Dupont , A , Maillot-Marechal , E , Boczkowski , J , Lacroix , G and Hoet , P . 2009 . Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines . Part. Fibre. Toxicol. , 6 : 1 – 12 .
- Casey , A , Herzog , E , Lyng , FM , Byrne , HJ , Chambers , G and Davoren , M . 2008 . Single walled carbon nanotubes induce indirect cytotoxicity by medium depletion in A549 lung cells . Toxicol. Lett. , 179 : 78 – 84 .
- Oh , W , Kim , S , Yoon , H and Jang , J . 2010 . Shape-dependent cytotoxicity and proinflammatory response of poly(3,4-ethylenedioxythiophene) nanomaterials . Small , 6 : 872 – 879 .
- Jiang , JK , Oberdörster , G , Elder , A , Gelein , R , Mercer , P and Biswas , P . 2008 . Does nanoparticle activity depend upon size and crystal phase? . Nanotoxicology , 2 : 33 – 42 .
- Van Hoecke , K , Quik , JTK , Mankiewicz-Boczek , J , De Schamphelaere , KAC , Elsaesser , A , Van Der Meeren , P , Barnes , C , McKerr , G , Howard , CV , Van De Meent. , D , Rydzynski , K , Dawson , KA , Salvati , A , Lesniak , A , Lynch , I , Silversmit , G , De Samber , B , Vincze , L and Janssen , CR . 2009 . Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests . Envion. Sci. Technol. , 43 : 4537 – 4546 .