Abstract
An efficient single-step procedure for the synthesis of 2-substituted benzimidazole derivatives by nanocrystalline magnesium oxide as solid base catalyst under acetonitrile solvent at room temperature has been developed. The worldwide availability, larger scale synthesis, higher yields and shorter reaction times are the advantages of the present method.
1. Introduction
Benzimidazole moiety is a structural isostere of naturally occurring nucleotides; hence, it has been extensively utilised as a drug scaffold in the medicinal chemistry. Several promising anti-tumour active agents were found to contain the benzimidazole ring system. They were found to exert their anti-tumour activity by acting mainly as topoisomerases inhibitors,[Citation1–3] anti-angiogenic agents,[Citation4–6] and alkylating agents.[Citation7–9] In addition, the benzimidazole moiety is found in various synthetic pharmaceuticals displaying a broad spectrum of biological activity including anti-ulcer, anti-tumour and anti-viral effects.[Citation10] In the last years, it has been demonstrated that several bi- and ter-benzimidazole derivatives act as topoisomerase inhibitors.
Nanocrystalline oxides are of immense interest to scientist due to their enriched surface chemistry and high surface area. Ultrafine nanocrystallines of alkaline earth metal oxide, such as magnesium oxide, have many applications as catalyst,[Citation11–13] refractory materials,[Citation14] optically transparent ceramic windows,[Citation15,16] etc. In the field of catalyst, MgO has strong basic properties those are associated with catalysts by base in many organic reactions.[Citation17] Asymmetric Henry and Michael reaction,[Citation18] synthesis of organic carbonates,[Citation19] aldol condensation,[Citation20] etc., are some of the well-studied reactions those are catalysed by MgO nanoparticles. In recent years, I2 has also been used extensively as a synthetic reagent due to its inherent properties of low toxicity, electrophilicity and easy handling.[Citation21]
In continuation of our ongoing research on synthesis of benzimidazoles,[Citation22] herein we wish to report the use of nanocrystallines MgO and I2 as convenient, mild and efficient reagent for the preparation of benzimidazoles from o-phenylenediamine and aryl aldehydes in acetonitrile solvent at room temperature.
2. Results and discussion
Detailed studies on the reaction of o-phenylenediamine with aryl aldehydes to form 2-substituted benzimidazoles have shown that this nucleophilic addition and condensation reactions are influenced to a considerable extent by the reagent as shown in and . To find the effect of solvent on this reaction, several solvents including acetonitrile, dichloromethane, chloroform and methanol were investigated during the course of this study. After screening different solvents, it was found that the best solvent is acetonitrile in this reaction (, entry 1).
Table 1. Optimisation of solvent effect on the reactiona.
Scheme 1. Synthesis of 2-substituted benzimidazoles from o-phenylenediamine and aryl aldehydes in the presence of nanocrystalline MgO/I2.

A comparison of the catalytic effect of nano MgO with commercial MgO in the reaction was reported in . As can be seen in the table, nanocrystalline MgO displays a substantial activity in this reaction () than the commercial MgO under the same condition. Therefore, in this regard, the nanocrystalline MgO should be having more reactive sites than commercial MgO (irregular morphology with a surface area of 27 m2/g) and consequently higher agent activity. In addition to the above mentioned and with attention to the special structure of nanocrystalline MgO, it seems that the organic species should be able to diffuse faster on the surface of this solid agent rather than the commercial MgO.
Table 2. Survey of nanocatalyst effect in the reaction.
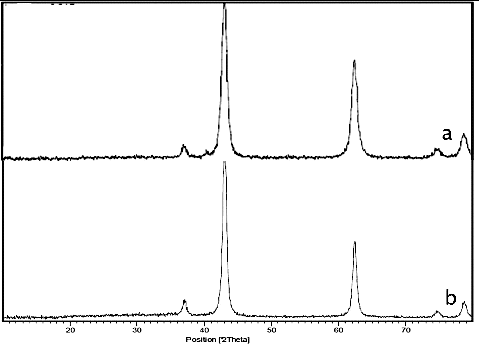
The crystallinity and sizes of nano MgO were evaluated by X-ray diffraction (XRD) technique before and after used as a catalyst (). The obtained crystallite sizes were between 12.8 and 17.5 nm that were determined by the use of the Scherrer equation, indicative of the nanocrystalline structure of the prepared MgO. Also, it was studied the XRD pattern of catalyst after six consecutive cycles for the synthesis of benzimidazoles. As can be seen in the figure, the crystallinity and sizes of catalyst were retained during the reaction process. This result can be confirmed by the proposed reaction mechanism.
The surface area of MgO nanocrystallines was approximately 116 m2·g−1. The pore volume and pore size were also calculated from the N2 adsorption result; the pore size was approximately 21.1 nm and the pore volume was approximately 0.69 cm3·g−1. The theoretical particle size was also calculated from the surface area, assuming spherical particles, from the equation,(1) where DBET is the equivalent particle diameter in nanometres, ρ is the density of the material in g·cm−3 and S is the specific surface area in m2·g−1. The particle size calculated from Equation (1) was 14.4 nm, which confirmed the nanostructure of the MgO sample.[Citation23] The transmission electron microscopic (TEM) image of MgO is shown in . As can be seen, the sample has a nanocrystalline structure with a plate-like shape. Also, dispersity and surface morphology of the catalyst were studied by scanning electron microscopy (SEM) technique (). The SEM analysis displayed that the MgO has a morphology of well-dispersed nanosheets.
Subsequent efforts were focused on optimising conditions to form the benzimidazole by using different amounts of nanocrystalline magnesium oxide at room temperature ().
Table 3. Optimising amount of nanocrystalline MgO.
As indicated in the table, the reaction showed an improvement in yield when using 0.18 g nanocrystalline MgO as an agent at room temperature (entry 4 in ). A ratio of 1:1.2:1:1 of nanocrystalline MgO/I2/aryl aldehyde/1,2-phenylendiamine was found to be the optimum amount for the reaction.
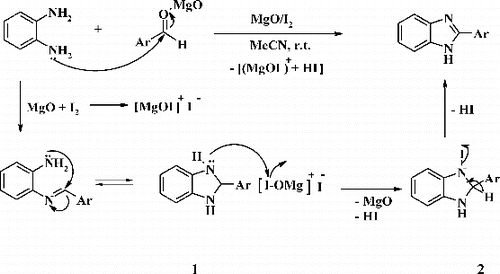
Regarding the mechanism of reaction, it probably involves the formation of [MgOI]+I− by the reaction of nano MgO with iodine molecule, which then reacts with the cyclic hydrobenzimidazoles 1 to afford intermediate 2 followed by the abstraction of hydrogen to yield the corresponding benzimidazoles ().
Scheme 2. Proposed mechanism for synthesis of 2-substituted benzimidazole derivatives using nanocrystalline MgO system.
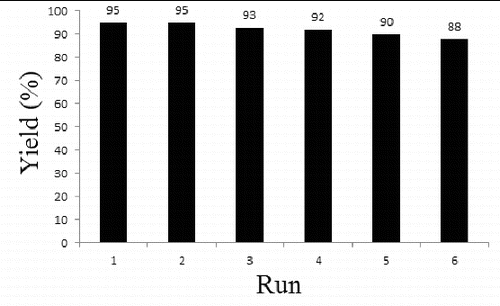
In ascertain and limitation of this methodology, the reaction of o-phenylenediamine with various aldehydes in the presence of nano MgO/I2 occurred. The results are summarised in . From the table, it was seen that the beneficial and useful benzimidazole derivatives were obtained from different aldehydes in optimised reaction conditions in excellent yields and short reaction times. The nanocrystalline MgO as a heterogeneous catalyst could be recycled through simple filtration after the reaction. Then, the recovered catalyst was used in the reaction by several runs (). However, the results indicate that the product yields were slightly decreased in the reaction processes. Moreover, reusability of the catalyst can be another interesting eco-friendly aspect of this reaction.
Table 4. Reaction of various aryl aldehydes with o-phenylenediamine.
The structure of products has been confirmed by physical and spectroscopic data such as infrared (IR), hydrogen nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR). In the IR spectra, the stretching frequency of aromatic C=C is formed in the region and ν = 1429–1600 cm−1. The stretching vibration of C–H in the alkyl groups was appeared at region and ν = 2903–3059 cm−1. In the 1H NMR spectra, one proton of N–H has chemical shift in δ = 7.1–15.2 ppm. The signals around δ = 6.84–9.1 ppm are assigned by protons of CH=CH of aromatic rings. In the 13C NMR spectra, one carbon of C=N has chemical shift in δ = 148.9–151 ppm.
3. Experimental section
3.1. Materials
All the materials were of commercial reagent grade. The aromatic aldehydes and o-phenylenediamine were purified by standard procedures and the purity was determined by thin layer chromatography (TLC).
3.2. Apparatus
The IR spectra were recorded as KBr pellets on a Perkin-Elmer 781 spectrophotometer and an Impact 400 Nicolet Fourier transform infrared spectrophotometer. The 1H NMR and 13C NMR were recorded in dimethylesulfoxide (DMSO) solvent on a Bruker DRX-400 spectrometer with tetramethylsilane as internal reference. The melting points obtained with a Yanagimoto micro melting-point apparatus are uncorrected. The XRD analysis was performed with an X-ray diffractometer (PAnalytical X’Pert-Pro) using a Cu–Ka monochromatic radiation source and a Ni filter. The TEM was performed with a Jeol JEM- 2100UHR, operated at 200 kV. The purity determination of the substrates and reaction monitoring were accomplished by TLC on silica-gel polygram SILG/UV 254 plates (from Merck Company).
3.3. Preparation of nanocrystalline MgO
Nanocrystalline MgO was prepared by means of a procedure reported elsewhere.[Citation23] In short, poly(vinyl alcohol) (PVA, MW 70,000) was dissolved in water at 90 °C under vigorous stirring to form a transparent solution. Mg(NO3)2 6H2O was dissolved in water containing PVA. The metal ion-to-PVA monomer unit molar ratio was chosen as 1:3. Aqueous ammonia (25% w/w) was added drop wise at room temperature to the resulting viscous liquid mixture, with rapid stirring, to achieve careful pH adjustment to 10.5. After precipitation, the slurry was stirred for another 30 min and then heated under reflux at 80 °C for 20 h under continuous stirring. The mixture was cooled to room temperature, filtered and washed with hot deionised water for effective removal of the PVA. The final product was dried at 80 °C for 24 h and calcined at 700 °C.
3.4. General experimental procedure for the tandem reaction
In a round-bottomed flask (50 mL) equipped with a magnetic stirrer, a solution of o-phenylenediamine (1.0 mmol), and aryl aldehyde (1.0 mmol) in MeCN (5 mL) was prepared. Nanocrystalline MgO (5.0 mmol) and solid I2 (3 mmol) were added and the mixture was stirred at room temperature for the time indicated in . The progress of the reaction was monitored by TLC (eluent: ether–EtOAc, 8:4). When the starting materials had completely disappeared, the mixture was quenched by adding H2O (10 mL), extracted with CHCl3 (4 × 12 mL) and the combined extracts were dried in CaCl2. The filtrate was evaporated and the corresponding benzimidazole was obtained as the only product (). The selected characterisation data for some of the prepared benzimidazoles are given below.
2-(3-Methoxyphenyl)-benzimidazole: yellow solid; mp = 200–202 °C (mp = 205–206 °C);[Citation24] IR (KBr)/ ν (cm−1) 3437 (NH), 1596 (C=N), 1541, 1464 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 3.9 (3H, s, Me), 7.05 (1H, m, Ar), 7.19 (2H, m, Ar), 7.45 (1H, s, Ar), 7.59 (2H, m Ar), 7.74 (2H, m, Ar), 12.9 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 56.0, 114.0, 115.2, 116.5, 117.8, 124.5, 130.5, 132.6, 139, 152.2, 159.1.
2-Phenyl-benzimidazole: pale yellow solid; mp. = 289–290 °C (mp = 288–290 °C);[Citation25] IR(KBr)/ν (cm−1) 3446 (NH), 1622 (C=N),1590, 1445 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.19 (2H, m, Ar), 7.48–7.64 (5H, m, Ar), 8.17 (2H, m, Ar), 12.9 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 111.1, 118.6, 121.9, 126.2, 128.6, 129.5, 130.0, 134.8, 143.5, 151.0; MS: m/z: 193 ([M-H]+, 100%).
2-(4-Chlorophenyl)-benzimidazole: yellow solid; mp = 290–291 °C, (mp = 291–293 °C);[Citation26] IR (KBr)/ ν (cm−1) 3442 (NH), 1598 (C=N), 1580, 1429 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.2 (2H, m, Ar), 7.50–7.89 (6H, m, Ar), 13 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 113.5, 123.7, 127.6, 128.3, 129.4, 133.4, 138.9, 151.8; MS: m/z: 229.0 (M+H)+.
2-(4-Bromophenyl)-benzimidazole: pale yellow solid; mp = 292–293 °C (mp = 250–252 °C);[Citation24] IR (KBr)/ ν (cm−1) 3449 (NH), 1628 (C=N), 1598, 1456 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.53 (2H, m, Ar), 7.83 (2H, m, Ar), 7.95 (2H, d, Ar, J = 4.8 Hz), 8.28 (2H, d, Ar, J = 4.8 Hz); 13C NMR (DMSO, 100 MHz)/ δ ppm: 102.5, 121.5, 141.6, 127, 128.1, 130.4, 138.2, 150.2.
2-(2-Chlorophenyl)-benzimidazole: yellow solid; mp = 231–233 °C, (mp = 232–234 °C);[Citation27] IR (KBr)/ ν (cm−1) 3444 (NH), 1591 (C=N), 1575, 1440 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.2 (2H, m, Ar), 7.50–7.89 (6H, m, Ar), 12.9 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 116.0, 122.7, 127.9, 130.4, 130.8, 131.6, 132.2, 132.5, 139.5, 149.6; MS: m/z: 228.5 (M+).
2-(2,3-Dichlorophenyl)-benzimidazole: yellow solid; mp = 224–226 °C, (mp = 224–226 °C);[Citation22] IR (KBr)/ ν (cm−1) 3095 (NH), 1624 (C=N), 1540, 1433 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.2 (2H, m, Ar), 7.53 (1H, m, Ar), 7.64 (2H, m, Ar), 7.82 (2H, m, Ar), 12.8 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 115.9, 122.9, 128.9, 130.5, 131.3, 132.1, 132.9, 133.2, 139.3, 149.1.
2-(3-Nitrophenyl)-benzimidazole: yellow solid; mp = 309–310 °C, (mp = 310–311 °C);[Citation25] IR (KBr)/ ν(cm−1) 3308 (NH). 1630 (C=N), 1531, 1482 (C=C, Ar); 1H NMR (DMSO, 400MHz)/ δ ppm: 7.47 (2H, m, Ar), 7.79 (2H, m, Ar), 7.92–7.95 (1H, m, Ar), 8.1–8.9 (2H, m, Ar), 9.1 (1H, s, Ar), 10.1 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 113.3, 121.2, 122.7, 125.2, 130.3, 132.1, 133.6, 139, 147.5, 152.7; MS: m/z: 238 ([M-H]+, 100%).
2-(4-Nitrophenyl)-benzimidazole: pale yellow solid; mp = 307–309 °C (mp = 312–314 °C);[Citation28] IR (KBr)/ ν (cm−1) 3421 (NH), 1606 (C = N), 1524, 1451 (C = C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.51–7.54 (2H, m, Ar), 7.83 (2H, m, Ar), 8.47 (2H, d, Ar, J = 8.4 Hz), 8.48–8.64 (2H, d, Ar, J = 8.4 Hz), 8.4 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 114.5, 121.7, 127.9, 128.7, 130.5, 137.2, 140.9, 152.8; MS: m/z: 239 (M+).
2-(2-Hydroxyphenyl)-benzimidazole: white solid; mp = 238–240 °C (mp = 240–242 °C);[Citation29] IR (KBr)/ ν (cm−1) 3325 (OH), 3055 (NH), 1593 (C=N), 1530, 1491 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 6.99–7.05 (2H, m, Ar), 7.27 (2H, m, Ar), 7.37 (1H, t, Ar), 7.66 (2H, m, Ar), 8.06 (1H, m, Ar), 13.19 (1H, s, OH), 13.19 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 112.1, 113.1, 117.7, 119.6, 123.3, 126.7, 132.2, 141.2, 152.2, 158.6; MS: m/z: 210 (M+).
2-(N,N-dimethylphenyl)-benzimidazole: yellow solid; mp = 293–295 °C (mp = 294.2–296.3 °C);[Citation30] IR (KBr)/ ν (cm−1) 3391 (NH), 1605 (C=N), 1518, 1459 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 6.84 (2H, d, Ar, J = 7.8 Hz), 7.43 (2H, m, Ar), 7.70 (2H, m, Ar), 8.21 (2H, d, Ar, J = 7.8 Hz), 15,2 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 39.5, 107.8, 111.8, 113.2, 125.1, 129.1, 131.5, 149.8, 153.2.
2-(4-Hydroxyphenyl)-benzimidazole; white solid; m.p = 254–255 °C (mp = 254.1–256.6 °C);[Citation30] IR (KBr)/ ν (cm−1) 3383 (OH), 3202 (NH), 1668 (C=N), 1600, 1457 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 6.91–7.50 (4H, m, Ar), 7.73–8.21 (4H, m, Ar), 9.7 (1H, s, OH), 15.2 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 113.0, 114.0, 116.9, 126.0, 131.6, 132, 149.9, 160.7.
2-(4-Methylphenyl)-benzimidazole: white solid; mp = 260–261 °C, (mp = 261–263 °C);[Citation27] IR (KBr)/ ν (cm−1) 3429 (NH), 1620 (C=N), 1587, 1433 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 2.36 (3, s, Me) 7.18 (2H, m, Ar), 7.34 (2H, d, Ar, J = 8.0 Hz), 7.57 (2H, m, Ar), 8.06 (2H, d, Ar, J = 8.0 Hz), 12.8 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 21.4, 115.5, 122.4, 125.9, 127.9, 130, 139, 140.0, 151.9.
2-(2-Nitrophenyl)-benzimidazole: pale yellow solid; mp = 212–214 °C (mp = 209–210 °C);[Citation28] IR (KBr)/ ν (cm−1) 3428 (NH), 1622 (C=N), 1530, 1458 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 7.54–7.57 (2H, m, Ar), 7.85–7.87 (2H, m, Ar), 7.99 (1H, t, Ar), 8.05 (1H, m, Ar), 8.12 (1H, d, Ar, J = 7.6 Hz), 8.37 (1H, d, Ar, J = 8.0 Hz), 13 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 114.5, 121.7, 127.9, 128.7, 130.5, 137.2, 140.9, 152.8. MS: m/z: 239 (M+).
2-(3-Hydroxyphenyl)-benzimidazole: yellow solid; mp = 245–247 °C, (mp = 245–247 °C);[Citation22] IR (KBr)/ ν (cm−1) 3434 (OH), 3243 (NH), 1588 (C=N), 1541, 1445 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 6.9 (1H, d, Ar, J = 5.6 Hz), 7.18 (2H, m, Ar), 7.33 (1H, t, Ar), 7.59 (4H, d, Ar, J = 6.6 Hz), 12.9 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 113.9, 115.6, 117.6, 117.8, 122.6, 130.5, 131.9, 139.9, 151.9, 158.3.
2-(2,5-Dimethoxyphenyl)-benzimidazole: yellow solid; mp = 197–198 °C, (mp = 197–198 °C);[Citation22] IR (KBr)/ ν (cm−1) 3408 (NH), 1622 (C=N), 1511, 1458 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 4.02 (3H, s, Me), 3.85 (3H, s, Me) 7.33 (2H, d, Ar, J = 6.0 Hz), 7.55 (2H, m, Ar), 7.89 (2H, m, Ar), 8.02 (1H, s, Ar), 15 (1H, s, NH); 13C NMR (DMSO, 100 MHz)/ δ ppm: 56.7, 57.0, 11.2, 114.4, 114.6, 114.6, 122.2, 126.3, 131.7, 146.1, 152.6, 153.8.
2-(4-Methoxyphenyl)-benzimidazole: yellow solid; mp = 229–230 °C, (mp = 229.2–231.1 °C);[Citation30] IR (KBr)/ ν (cm−1) 3344 (NH), 1608 (C=N), 1506, 1461 (C=C, Ar); 1H NMR (DMSO, 400 MHz)/ δ ppm: 3.89 (3H, s, Me), 7.2 (2H, d, Ar, J = 8.0 Hz), 7.52 (2H, m, Ar), 7.79 (2H, m, Ar), 8.38 (2H, d, Ar, J = 8.0 Hz), 15.3 (1H, s, NH).; 13C NMR (DMSO, 100 MHz)/ δ ppm: 56.2, 114.1, 115.3, 115.5, 126.0, 130.6, 132, 148.9, 163.6.
4. Conclusion
We have reported an efficient and novel method for the synthesis of 2-substituted benzimidazoles from o-phenylenediamine and aryl aldehydes in the presence of nanocrystalline MgO/I2 in CH3CN. The corresponding products have been obtained in excellent yields, high purity and short reaction times. The obtained benzimidazoles have been characterised by physical and spectroscopic data such as UV, IR, 1H NMR and 13C NMR spectroscopy.
Acknowledgements
The authors are grateful to University of Kashan for supporting this work by Grant No. 159148/10.
References
- Alper S, Arpaci OT, Aki ES, Yalcin I. Some new bi- and ter-benzimidazole derivatives as topoisomerase I inhibitors. Farmaco. 2003;58:497–507.
- Sun Q, Gatto B, Yu C, Liu A, Liu LF, LaVoie EJ. Synthesis and evaluation of terbenzimidazoles as topoisomerase I inhibitors. J Med Chem. 1995;38:3638–3644.
- Sun Q, Gatto B, Yu C, Liu A, Liu LF, LaVoie EJ. Structure activity of topoisomerase i poisons related to hoechst 33342. Bioorg Med Chem Lett. 1994;4:2871–2876.
- Saulnier MG, Frennesson DB, Wittman MD, Zimmermann K, Velaparthi U, Langley DR, Struzynski C, Sang X, Carboni U, Li A, Greer A, Yang Z, Balimane P, Gottardis M, Attar R, Vyas D. 2-(1H-Imidazol-4-yl)ethanamine and 2-(1H-pyrazol-1-yl) ethanamine side chain variants of the IGF-1R inhibitor BMS-536924. Bioorg Med Chem Lett. 2008;18:1702–1707.
- Velaparthi U, Liu P, Balasubramanian B, Carboni J, Attar R, Gottardis M, Li A, Greer A, Zoeckler M, Wittman MD, Vyas D. Imidazole moiety replacements in the 3-(1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one inhibitors of insulin-like growth factor receptor-1 (IGF-1R) to improve cytochrome P450 profile. Bioorg Med Chem Lett. 2007;17:3072–3076.
- Wittman M, Carboni J, Attar R, Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C, Clarke W, Dell J, Eummer J, Frennesson D, Gottardis M, Greer A, Hansel S, Hurlburt W, Jacobson B, Krishnananthan S, Lee FY, Li A, Lin T-A, Liu P, Ouellet C, Sang X, Saulnier MG, Stoffan K, Sun Y, Velaparthi U, Wong H, Yang Z, Zimmermann K, Zoeckler M, Vyas D. Discovery of a 1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48:5639–5643.
- Schulz WG, Islam I, Skibo EB. Pyrrolo[1,2-a]benzimidazole-based quinones and iminoquinones. The role of the 3-substituent on cytotoxicity. J Med Chem. 1995;38:109–118.
- Skibo EB, Islam I, Heileman MJ, Schulz WG. Structure-activity studies of benzimidazole-based DNA-cleaving agents. Comparison of benzimidazole, pyrrolobenzimidazole, and tetrahydropyridobenzimidazole analogs. J Med Chem. 1994;37:78–92.
- Boruah RC, Skibo EB. A comparison of the cytotoxic and physical properties of aziridinyl quinone derivatives based on the pyrrolo[1,2-a]benzimidazole and pyrrolo[1,2-a]indole ring systems. J Med Chem. 1994;37:1625–1631.
- Navarrete-Vazquez G, Cedillo R, Hernandez-Campos A, Yepez L, Hernandez-Luis F, Valdez J, Morales R, Cortes R, Hernandez M, Castillo R. Synthesis and antiparasitic activity of 2-(Trifluoromethyl)benzimidazole derivatives. Bioorg Med Chem Lett. 2001;11:187–190.
- Gulkova D, Solcova O, Zdrazil M. Preparation of MgO catalytic support in shaped mesoporous high surface area form. Microporous Mesoporous Mater. 2004;76:137–149.
- Climent MJ, Corma A, Iborra S, Mifsud M. MgO nanoparticle-based multifunctional catalysts in the cascade reaction allows the green synthesis of anti-inflammatory agents. J Catal. 2007;247:223–230.
- Naeimi H, Rabiei Kh, Rezaei M, Meshkani F. Nanocrystalline magnesium oxide as a solid base catalyst promoted one pot synthesis of gem-dichloroaziridine derivatives under thermal conditions. J Iran Chem Soc. 2013;10:161–167.
- Faghihi-Sani MA, Yamaguchi A. Oxidation kinetics of MgO–C refractory bricks. Ceram Int. 2002;28:835–839.
- Chaim R, Shen ZJ, Nygren MJ. Transparent nanocrystalline MgO by rapid and low-temperature spark plasma sintering. Mater Res. 2004;19:2527–2531.
- Chen D, Jordan EH, Gell M. Pressureless sintering of translucent MgO ceramics. Scr Mater. 2008;59:757–759.
- Hattori H. Heterogeneous basic catalysis. Chem Rev. 1995;95:537–550.
- Choudary BM, Ranganath KVS, Pal U, Kantam ML, Sreedhar B. Nanocrystalline MgO for asymmetric henry and michael reactions. J Am Chem Soc. 2005;127:13167–13171.
- Kantam ML, Pal U, Sreedhar B, Choudary BM. An efficient synthesis of organic carbonates using nanocrystalline magnesium oxide. Adv Synth Catal. 2007;349:1671–1675.
- Choudary BM, Chakrapani LT, Kumar KVR, Kantum ML. Direct asymmetric aldol reaction catalyzed by nanocrystalline magnesium oxide. Tetrahedron 2006;62:9571–9576.
- Paquette LA. Encyclopedia of reagents for organic synthesis. New York (NY): Wiley, 1995. Vol. 4, p. 2796.
- Naeimi H, Alishahi N. A simple, mild and efficient one-pot synthesis of 2-substituted benzimidazoles in the presence of H2O2/HCl under microwave irradiation. J Chin Chem Soc. 2012;59:1001–1005.
- Meshkani F, Rezaei M. Facile synthesis of nanocrystalline magnesium oxide with high surface area. Powder Technol. 2009;196:85–88.
- Montanari F, Passerini R. Richerche sugli eterociclici: spetri di assorbimento UV e proprietá cromoforiche - Nota 1: imidazoli, benzimidazoli e fenil-benzimidazoli. Bull Sci Chim Ind Bologna 1953;11:42–45.
- Nagawade RR, Shinde DB. BF3.OEt2 Promoted solvent-free synthesis of benzimidazole derivatives. Chin Chem Lett. 2006;17:453–456.
- Nagawade R, Shinde DB. TiCl4 Promoted synthesis of benzimidazole derivatives. Indian J Chem. 2007;46:349–353.
- Xiangming H, Huiqiang M, Yulu W. p-TsOH Catalyzed synthesis of 2-arylsubstituted benzimidazoles. Arkivoc 2007;xiii:150–154.
- Ramana DV, Kantharaj E. Synthesis of 2-substituted benzoxazoles and benzimidazoles based on mass spectral ortho interactions. J Chem Soc Perkin Trans. 1995;2:1497–1501.
- Addison AW, Burke PJ. Synthesis of some imidazole- and pyrazole- derived chelating agents. J Heterocycl Chem. 1981;18:803–805.
- Navarrete-Vázquez G, Moreno-Diaz H, Aguirre-Crespo F, León-Rivera I, Villalobos-Molina R, Muñoz-Muñizd O, Estrada-Sotoa S. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg Med Chem Lett. 2006;16:4169–4173.