Abstract
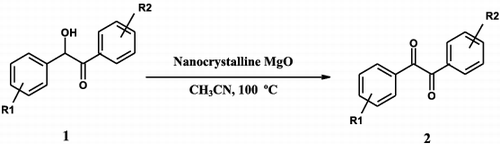
Nanocrystalline magnesium oxide (nano-MgO) has been used as an efficient catalyst for an improved synthesis of selective oxidation of benzoins to benzils inthe presence of acetonitrile as solvent in air atmosphere for the first time. Thepresent procedure has the following advantages: it is green, requires milder conditions and a shorter reaction time while providing higher yelds and selectiivity.
1. Introduction
In recent years, nanostructured materials have attracted great interest due to their particular physical and chemical properties.[Citation1] Among them, nanosized magnesium oxide (MgO) has a wide range of applications such as catalysts,[Citation2,3] refractory materials,[Citation4] optically transparent ceramic windows,[Citation5] etc. Magnesium oxide with large specific surface area is also a potential catalyst support for various reactions and a promising sorbent for chemisorptions and destructive adsorption of a variety of pollutants. In the field of catalysis, MgO has strong basic properties, which are associated with catalysis by bases in many organic reactions,[Citation6] Nanoscale supports create catalysts with more edges and corners, which can lead to higher performance of the catalyst.[Citation7]
1,2-Diketones are very important structural moieties in numerous biologically interesting compounds and are broadly utilised for construction of complex structures in organic synthesis. Among the numerous 1,2-diketones, benzil derivatives have received a special attention because of its practical applications in organic and pharmaceutical industry such as photosensitive and synthetic reagents.[Citation8] Benzil is extensively used as substrate in benzylic rearrangements and also acts as a starting material for the synthesis of many heterocyclic compounds [Citation9,10] exhibiting biological activity such as anticonvulsant derivative dilantin.[Citation11,12]
The oxidation of benzoins is one of the most efficient and practical methods for the synthesis of benzils. In general, the oxidation of benzoins to benzils has been used, such as hallium nitrate,[Citation13] ammonium nitrate–copper acetate,[Citation14] bismuth nitrate–copper acetate,[Citation15] and ferric nitrate.[Citation16] The use of homogeneous reagents for the oxidation of benzoin limits their practical utilisations over heterogeneous catalysts in terms of product recovery, product selectivity and environmental concerns.[Citation17,18] Many heterogeneous catalystssuch as ferric oxide–aluminum oxide,[Citation19] VOCl3,[Citation20] chromium trioxide on Kieselguhr,[Citation21] silica-supported manganese dioxide,[Citation22] ammonium chlorochromate adsorbed on alumina [Citation23] or silica,[Citation24] alumina or silica gel [Citation25] and commercial alumina [Citation26] were used to oxidise benzoins to benzils to overcome these difficulties but the desired product selectivity still remains a challenge for this interesting reaction. Recently, much attention has been focused on the development of green oxidation processes. Among various green oxidants, molecular oxygen has received more attention as the primary oxidant in the presence of heterogeneous catalysts for the oxidation of benzoin to benzyls.[Citation8,Citation27–29] However, there are few reports concerning the direct oxidation of benzoin to benzil with air catalysed by nanoparticles as nanocatalyst.
In the present study, we would like to report a simple and convenient method for the effective and selective oxidation of benzoin derivatives to corresponding benzils under air atmosphere using nanocrystalline magnesium oxide with high specific surface area of approximately 116 m2 g−1 and a crystallite size of approximately 12 nm as a novel and efficient catalyst ().
2. Results and discussion
In an initial study, for examination of catalytic activity of different catalysts such as Al2O3, MnO, MgO and nano-MgO in aerobic oxidation of benzoin to benzil, 1 mmol benzoin (1a), 2 mol% catalyst and 5 mL of dry acetonitrile [Citation30] was added in a round-bottom flask at 100 °C under stirring conditions. In all, only 2a was formed as a major product with 100% product selectivity. No product was obtained in the absence of the catalyst, indicating that the catalyst was necessary for this transformation. In the course of this study we found that MgO was the most effective catalyst (). Therefore, we decided to use nanocrystalline MgO with a high specific surface area as a catalyst with higher activity and better controlled selectivity.
Table 1. The effect of variation of different catalyst for the oxidation of benzoin (1a) to benzil (2a) in liquid phase catalysis.a
The crystallite sizes determined by X-ray diffraction (XRD) were between 12.8 and 17.5 nm (determined by use of the Scherrer equation), indicative of the nanocrystalline structure of the prepared MgO. In addition the surface area was approximately 116 m2 g−1. The pore volume and pore size were also calculated from the N2 adsorption result; the pore size was approximately 21.1 nm and the pore volume approximately 0.69 cm3 g−1. The theoretical particle size was also calculated from surface area, assuming spherical particles, from the equation(1) where DBET is the equivalent particle diameter in nanometers, ρ is the density of the material in g cm−3 and S is the specific surface area in m2 g−1. The particle size calculated from Equation (1) was 14.4 nm, which confirmed the nanostructure of the MgO sample.[Citation31] The transmission electron microscopy (TEM) image of MgO nanostructure is shown in . As can be seen, the sample has a nanocrystalline structure with a plate-like shape.[Citation31]
Figure 1. TEM image of nanocrystalline MgO [Citation31].
![Figure 1. TEM image of nanocrystalline MgO [Citation31].](/cms/asset/89ff6f8e-22ee-4539-9d49-cc250f502646/tjen_a_869842_f0001_b.gif)
We initiated a solvent screen to explore the effect of different solvents on the oxidation of 1a and also summarised in . The results show the most yield of 2a was achieved at acetonitrile. When other solvents were used, no significant improvement in the yield was observed. It seems that in the oxidation procedure, the conversion of benzil depends directly on the polarity solvent and acetonitrile was chosen because of its high polarity. As shown in , at the catalyst to oxidant ratio of 1:2, the best yield of 2a was achieved (, Entry 9).
Table 2. The effect of molar ratio of catalyst and solvent on the yield of the oxidation of benzoin (1a) to benzil (2a) in liquid phase catalysis.a
Among the various oxidants (H2O2, m-perchlorobenzoic acid and air), it was concluded that the best activity and selectivity can be achieved by air itself under optimised reaction conditions (). We further observed that air present inside the reaction mixture itself is sufficient enough to derive this reaction and hence no additional supply of the air was pumped into the reaction mixture.
Table 3. The effect of variation of different oxidant for the oxidation of benzoin (1a) to benzil (2a) in liquid phase catalysis.a
As shown in , the oxidation of benzoins 1 by nanocrystalline MgO was carried out in good yield under mild reaction conditions. From the results in , it seems that the benzoins containing electron-donating group were found to be more reactive and couldbe oxidised more easily (1b and 1f). In contrast, the benzoins containing electron-withdrawing group have shown lower reactivity 1j. These results show the significant effect of substituents on the oxidation.
Table 4. Selective oxidation of benzoins 1 in the presence of nano-MgO under air atmosphere in liquidphase catalysis.a
This reaction is heterogeneous, taking place at the MgO nanocrystallines. On the other hand, the nanocatalyst MgO with high surface area and plate-like shape can lead to higher performance of the catalyst compared with the other catalysts. Moreover, the high efficiency of the nanoparticle oxides is caused not only by their high surface area but also by the high concentration of low coordinated sites and structural defects on their surface.[Citation31]
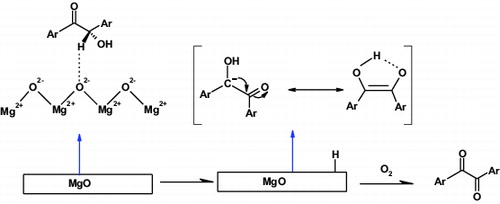
As shown in , a reaction mechanism for the oxidation of benzoin was proposed based on a similar reaction mechanism published by Azizian et al. [Citation33] and Skobridis et al. [Citation26]. The MgO materials exhibit a satisfactory adsorptive property, because of their higher specific surface area.[Citation34] Moreover, its strong basicity may be attributed to the easier adsorption of an α-proton from benzoin by a basic site of the catalyst. In this proposed mechanism, benzoin adsorbs reversibly onto active site of the catalyst, and then undergoes dehydrogenation from the α-C-atom, leading to a stabilised enediolate anion which reacts further. Although the exact mechanism of this novel MgO-catalysed aerobic oxidation reaction is not yet totally clear, we suggest that the reaction proceeds through an anionic intermediate, which reacts further with molecular oxygen to give benzil.
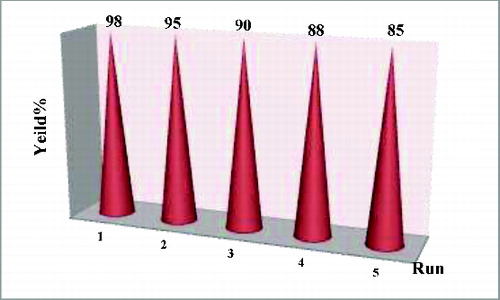
After completion of the reaction, the catalyst was reused for the next cycle without any appreciable loss of its activity. Similarly, reusability for the sequential reaction was also carried out and the catalyst was found to be reusable for five cycles ().
3. Experimental
Chemicals were purchased from Merck and Aldrich Chemical Company. Solvents were purified and dried according to standard procedures.[Citation30] NMR spectra were recorded with tetramethylsilane as the internal standard. Thin layer chromatography (TLC) was performed on glass-backed silica plates. Column chromatography was performed using silica gel (200–300 mesh) eluting with ethyl acetate and petroleum ether. 1H NMR spectra were recorded at 400 MHz, and 13C NMR spectra were recorded at 100 MHz (Bruker Avance). Chemical shifts (δ) are reported in parts per million. Coupling constants (J) are given in hertz. ESI-HRMS spectrometer was measured with a Finnigan LCQDECA ion trap mass spectrometer. IR spectra were recorded as KBr pellets on a PerkinElmer 781 spectrophotometer and on an Impact 400 Nicolet FTIR spectrophotometer. Melting points were determined in open capillaries using an Electrothermal Mk3 apparatus. The element analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyser carried out on PerkinElmer 240c analyser. XRD analysis was performed with an X-ray diffractometer (PANalytical X’Pert-Pro) using a Cu-Kα monochromatic radiation source and a Ni filter. TEM was performed with a Jeol JEM-2100UHR, operated at 200 kV.
3.1. Preparation of nanocrystalline MgO
Nanocrystalline MgO was prepared by means of a procedure reported elsewhere.[Citation31] In short, poly(vinyl alcohol) (PVA, MW 70,000) was dissolved in water at 90 °C under vigorous stirring to form a transparent solution. Mg(NO3)2·6H2O was dissolved in water containing PVA. The metal ion-to-PVA monomer unit molar ratio (M/PVA) was chosen as 1:3. Aqueous ammonia (25% w/w) was added dropwise at room temperature to the resulting viscous liquid mixture, with rapid stirring, to achieve careful pH adjustment to 10.5. After precipitation, the slurry was stirred for another 30 min and then heated under reflux at 80 °C for 20 h under continuous stirring. The mixture was cooled to room temperature, filtered and washed with hot deionised water for effective removal of the PVA. The final product was dried at 80 °C for 24 h and calcined at 700 °C.
3.2. General procedure for the oxidation of benzoins to benzils
To a mixture of benzoin (1 mmol), and nanocrystalline MgO (2 mol%) in a 100 mL round-bottom flask, acetonitrile (5 mL) was added. The reaction mixture was stirred at 100 °C in an atmospheric air for an appropriate time (monitored by TLC). On completion, the reaction mixture was filtered while hot and the residue was washed with hot acetonitrile (2× 5 mL). The product was obtained after removal of the solvent under reduced pressure followed by crystallisation or column chromatography. The catalyst was recovered from the residue after washing with CH2Cl2 (10 mL) followed by distilled water (200 mL) and then with acetone (15 mL). It can be re-used after drying at 80 °C for 2 h. The structures of the products were confirmed by spectral data and comparison with authentic samples prepared according to the literature methods.
3.3. Spectroscopic data
3.3.1. Benzil (2a)
UV (CH3OH) λmax: 224 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.54 (t, 2H, CH, J = 7.6 Hz), 7.69 (t, 4H, CH, J = 7.6 Hz), 7.98 (d, 4H, CH, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 128.90 (4CH), 129.70 (4CH), 133 (2CH), 134.93 (2C), 194.30 (2CO); IR(KBr) cm−1 1650 (C=O, s), 1450, 1580 (C=C, m), 720 (CH, m); ESI-HRMS: calcd for C14H10O2 210.22800, found 210. 22752; Anal. Calcd. (%) C, 79.99; H, 4.79; O, 15.22; found C, 79.91; H, 4.73; O, 15.12.
3.3.2. 4,4′-Dimethoxybenzil (2b)
UV (CH3OH) λmax: 225 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.89 (s, 6H, OCH3), 6.97 (d, 4H, CH, J = 8.8 Hz), 7.95 (d, 4H, CH, J = 8.8 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 55.62 (2OCH3), 114.29 (4CH), 126.19 (4CH), 132.30 (2C), 64.86 (2C), 193.54 (2CO); IR (KBr) cm−1 1650 (C=O, s), 1586 (C=C, m), 769 (CH, m); ESI-HRMS: calcd for C16H14O4 270.27996, found 270. 27967; Anal. Calcd. (%): C, 71.10; H, 5.22; O, 23.68; found: C, 70.98; H, 5.19; O, 23.64.
3.3.3. 4-Dimethylaminobenzil (2c)
UV (CH3OH) λmax: 229 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.38 (s, 6H, CH3), 6.67 (d,2H, CH, J = 5.2 Hz), 7.48 (d, 2H, CH, J = 8.1 Hz), 7.61 (d, 2H, CH, J = 8.1 Hz), 7.8 (d,H, CH, J = 8.1 H), 8.28 (d, 2H, CH, J = 5.2 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 55.91 (2CH3), 111.20 (2CH), 128.90 (2CH), 129.01 (C), 129.70 (2CH), 135.37 (CH), 135.50 (C), 141.47 (C), 153.76 (C), 196.72 (CO); IR (KBr) cm−1 1650, 1700 (C=O, s), 1440, 1620 (C=C, m), 705 (CH, m); ESI-HRMS: calcd for C16H150NO2 253.29580, found 253.29563; Anal. Calcd. (%) C, 75.87; H, 5.97; N, 5.53; O, 12.63; found C, 75.82; H, 5.95; N, 5.50; O,12.61.
3.3.4. 4,4′-Dimethylbenzil (2d)
UV (CH3OH) λmax: 225 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.45 (s, 6H, CH3), 7.4 (d, 4H, CH, J = 7.2 Hz), 7.75 (d, 4H, CH, J = 7.6 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 21.95 (2CH3), 129.72 (4CH), 130.03 (4CH), 130.69 (2C), 146.11 (2C), 194.54 (2CO); IR (KBr) cm−1 1650, (C=O, s), 1586 (C=C, m), 769 (CH, m); ESI-HRMS: calcd for C16H14O2 238.28116, found 238.28088, Anal. Calcd. (%) C, 80.65; H, 5.92; O, 13.43; found C, 80.63; H, 5.90; O, 13.40.
3.3.5. 4-Methoxybenzil (2e)
UV (CH3OH) λmax: 228 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.76 (s, 3H, OCH3), 6.82 (d, 2H, CH), 7.34–7.61 (m, 5H, CH) 7.86 (d, 2H, CH); 13C NMR (CDCl3, 100 MHz) δ (ppm) 55.19 (CH3), 113.20 (2CH), 128.90 (2CH), 129.70 (2CH), 130.30 (C), 131.37 (CH), 133 (2CH), 133.26 (C), 164.69 (C),194.30 (CO); IR (KBr) cm−1 1650, (C=O, s), 1586 (C=C, m), 769 (CH, m); ESI-HRMS: calcd for C15H12O3 240.25398, found 240.2535; Anal. Calcd. (%) C, 74.99; H, 5.03; O, 19.98; found C, 74.91; H, 4.98; O, 19.96.
3.3.6. 2-Pyridil (2f)
UV (CH3OH) λmax: 365 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 7.49 (t, 2H, CH, J = 8.1 Hz), 7.91 (t, 2H, CH, J = 8.1 Hz), 8.18 (d, 2H, CH, J = 8.1 Hz), 8.56 (d, 2H, CH, J = 8.1 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 122.27 (2CH), 127.99 (2CH), 137.26 (2CH), 149.46 (2CH), 151.65 (2C), 197.02 (2CO); IR (KBr) cm−1 1713 (C=O, s), 1274 (C=N, s), 1505 (C=C, m); ESI-HRMS: calcd for C12H18N2O2 212.20412, found 212.20361; Anal. Calcd. (%) C, 67.92; H, 3.80; N, 13.20; O, 15.08; found C, 67.90; H, 3.78; N, 13.10; O, 15.04.
3.3.6. 2,2′,4,4′-Tetramethoxybenzil (2g)
UV (CH3OH) λmax: 226 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.88 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 6.44 (s, 2H, CH), 6.53 (d, 2H, CH, J = 8.4 Hz), 7.81 (d, 2H, CH, J = 8.8 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 55.23 (OCH3), 55.80 (OCH3), 101.58 (2CH), 113.03 (2CH), 116.91 (2C), 131.22 (2CH), 161.83 (2C), 162.93 (2C), 186.91 (2CO); IR (KBr) cm−1 1630–1680 (C=O, s), 1470, 1618 (C=C, m), 738 (CH, m); ESI-HRMS: calcd for C18H18O6 330.33192, found 330.33164, Anal. Calcd. (%) C, 65.45; H, 5.49; O, 29.06; found C, 65.41; H, 5.43; O, 29.01.
3.3.7. 3-Methoxybenzil (2h)
UV (CH3OH) λmax: 234 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 3.72 (s, 3H, OCH3), 7.01 (t, 1H, CH), 7.1 (s, 1H, CH) 7.32 (d, 1H, CH), 751 (t, 2H, CH), 7.60 (t, 1H, CH), 7.72 (d, 2H, 2H);13C NMR (CDCl3, 100 MHz) δ (ppm) 54.00 (OCH3), 116.80 (CH), 120.13 (CH), 124.16 (CH), 128.90 (2CH), 129.70 (2CH), 131.05 (CH), 131.37 (CH), 132.88 (C), 135.27 (C), 163.09 (C), 193.41 (CO), 194.30 (CO); IR (KBr) cm−1 1660, (C=O, s), 1396, 1613 (C=C, m), 725 (CH, m); ESI-HRMS: calcd for C15H12O3 240.25396, found 240.25365; Anal. Calcd. (%): C, 74.99; H, 5.03; O, 19.98; found C, 74.95; H, 4.98; O, 19.94.
3.3.8. 4-Methylbenzil (2i)
UV (CH3OH) λmax: 223 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.34 (s, 3H, CH3), 7.3 (m, 2H, CH), 7.35 (m, 2H, CH), 7.42 (t, 1H, CH, J = 8 Hz), 7.55 (t, 2H, CH, J = 8, 7.2 Hz), 7.92 (d, 2H, CH, J = 7.92, 1.2 Hz); 13C NMR (CDCl3, 100 MHz) δ (ppm) 22.06 (CH3), 122.71 (2CH), 130.12 (2CH), 130.28 (2CH), 131.18 (2CH), 132.54 (2CH), 133.17 (C), 133.83 (C), 147.02 (C), 193.95 (CO), 194.08 (CO); IR (KBr) cm−1 1640, 1680, (C=O, s), 1420, 1630 (C=C, m), 724 (CH, m); ESI-HRMS: calcd for C15H12O2 224.25458, found 224.25433; Anal. Calcd. (%) C, 80.34; H, 5.39; O, 14.27; found C, 80.32; H, 5.35; O, 14.24.
3.3.9. 3-Bromo-4′-methylbenzil (2j)
UV (CH3OH) λmax: 227 nm; 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.42 (s, 3H, CH3), 7.34 (t, 1H, CH), 7.61 (d, 1H, CH), 7.74 (d, 3H, CH), 8.04 (s, H, CH), 8.59 (s, 1H, CH), 8.65 (s,1H, CH); 13C NMR (CDCl3, 100 MHz) δ (ppm) 55.65 (CH3), 144.44 (2CH), 123.21 (C), 129.12 (CH), 129.76 (C), 130.47 (2CH), 131.37 (CH), 132.44 (CH), 134.91 (CH), 137.46 (C), 165.18 (C), 192.04 (CO), 193.08 (CO); IR (KBr) cm−1 1640, 1700, (C=O, s), 1420, 1620 (C=C, m), 705, 730 (CH, m); ESI-HRMS: calcd for C15H11BrO3 319.15004, found 31914557; Anal. Calcd. (%) C, 56.45; H, 3.47; Br, 25.04; O, 15.04; found C, 56.43; H, 3.44; Br, 25.01; O, 15.01.
4. Conclusions
In conclusion, liquid phase aerial oxidation of benzoin was carried out using nanocrystalline MgO under milder reaction conditions. Very high activity and selectivity of the product was obtained. Moreover, the mild reaction conditions, high yield of products, ease of work-up and the ecologically clean selective procedure, will make the present method a useful and important addition to the present methodologies for the oxidation of benzoins to the corresponding benzils.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of the University of Kashan for supporting this work by Grant No. 256722/VIII.
References
- Zhou L, Xu J, Miao H, Wang F, Li X. Catalytic oxidation of cyclohexane to cyclohexanol and cyclohexanone over Co3O4 nanocrystals with molecular oxygen. Appl Catalysis A. 2005;292:223–228.
- Gulkova D, Solcova O, Zdrazil M. Preparation of MgO catalytic support in shaped mesoporous high surface area form. Microporous Mesoporous Mater. 2004;76:137–149.
- Climent MJ, Corma A, Iborra S, Mifsud M. MgO nanoparticle-based multifunctional catalysts in the cascade reaction allows the green synthesis of anti-inflammatory agents. J Catalysis. 2007;247:223–230.
- Faghihi-Sani MA, Yamaguchi A. Oxidation kinetics of MgO–C refractory bricks. Ceramics Int. 2002;28:835–839.
- Chen D, Jordan EH, Gell M. Pressureless sintering of translucent MgO ceramics. Scripta Materialia. 2008;59:757–759.
- Hattori H. Heterogeneous basic catalysis. Chem Rev. 1995;95:537–558.
- Safari J, Khalili SD, Rezaei M, Banitaba SH, Meshkani F. Nanocrystalline magnesium oxide: a novel and efficient catalyst for facile synthesis of 2,4,5-trisubstituted imidazole derivatives. Monotsh Chem. 2010;141:1339–1345.
- Kim KH, Park B, Lim JW, Kim JN. An efficient palladium-catalyzed synthesis of benzils from aryl bromides: vinylene carbonate as a synthetic equivalent of glyoxal. Tetrahedron Lett. 2011;52:3463–3466.
- Wynberg H, Kooreman HJ. The mechanism of the Hinsberg thiophene ring synthesis. J Am Chem Soc. 1965;87:1739–1742.
- Paudler WW, Barton JM. The synthesis of 1,2,4-triazine. J Org Chem. 1966;31:1720–1722.
- Dunnavant WR, James FL. Molecular rearrangements. I. The base-catalyzed condensation of benzil with urea. J Am Chem Soc 1956;78:2740–2743.
- Buck JS, Jcnkins SS. Catalytic reduction of alpha-diketones and their derivatives. J Am Chem Soc. 1929;51:2163–2167.
- Weiss M, Appel MJ. The catalytic oxidation of benzoin to benzil. J Am Chem Soc. 1948;70:3666–3667.
- Tymonko SA, Nattier BA, Mohan RS. Oxidation of benzoins to benzils using bismuth(III) nitrate-copper(II) acetate. Tetrahedron Lett. 1999;40:7657–7659.
- Paul AM, Khandekar AC, Shenoy MA. Silica supported ferric nitrate nonahydrate: selective oxidation of benzoins under mild conditions. Synthetic Commun. 2003;33:2581–2584.
- Sun Y, Ueyama N, Nakamura A. Air oxidation of p-substituted benzoin to the corresponding benzil catalyzed by Fe(II)-cysteine peptide complexes. Tetrahedron. 1992;48:1557–1566.
- Sachdev D, Naik MA, Dubey A, Mishra BG. Environmentally benign aerial oxidation of benzoin over copper containing hydrotalcite. Catalysis Commun. 2010;11:684–688.
- Muthupandi P, Sekar G. Zinc-catalyzed aerobic oxidation of benzoins and its extension to enantioselective oxidation. Tetrahedron Lett. 2011;52:692–695.
- Zhebin C, Zhengui S. Study on catalytic oxidation of benzoins by molecular oxygen in presence of ferric oxide supported on activated aluminum oxide. Chin J Org Chem. 2002;22:446–447.
- Kirihara M, Ochiai Y, Takizawa S, Takahata H, Nemoto H. Aerobic oxidation of α-hydroxycarbonyls catalysed by trichlorooxyvanadium: efficient synthesis of α-dicarbonyl compounds. Commun Chem. 1999:1387–1388.
- Lou JD, Zhang C, Wang GQ, Gao C. Oxidation of benzoins to benzils with chromium trioxide supported on kieselghur under viscous conditions. Synthetic React Inorg Metal Org Nanometal Chem. 2008;39:6–8.
- Khandekar AM, Paul, BM. Silica supported manganese dioxide: an efficient reagent for oxidation of benzoins. Synthetic Commun 2002;32:2931–2935.
- Zhang GS. Shi Z, Chen MF, Cai K. Ammonium chlorochromate adsorbed on alumina – a new reagent for the oxidation of alkohols and benzoins to the corresponding carbonyl-compounds. Synthetic Commun. 1997;27:953–956.
- Zhang GS, Shi QZ, Chen MF, Cai K. Ammonium chlorochromate adsorbed on silica-gel – a new reagent for the oxidation of alkohols and benzoins to the corresponding carbonyl-compounds. Synthetic Commun. 1997;27:3691–3696.
- Noroozi-Pesyan N, Dabbagh AH. Alumina and silica oxides as catalysts for the oxidation of benzoins to benzils under solvent-free conditions. Molecules. 2005;10:1364–1368.
- Skobridis K, Theodorou V, Weber E. A very simple and chemoselective air oxidation of benzoins to benzils by use of alumina. ARKIVOC. 2006;13:102–106. Available from www.arkat-usa.org
- Li B, Wang J, Fu J, Wang J, Zou C. Selective liquid phase oxidation of benzoin to benzil over transition metals doped MCM-41 with air. Catalysis Commun. 2008;9:2000–2002.
- Ebitani K, Ji HB, Mizugaki T, Kaneda K. Highly active trimetallic Ru/CeO2/CoO(OH) catalyst for oxidation of alcohols in the presence of molecular oxygen. J Mol Catalysis A. 2004;212:161–170.
- Venkatachalam G, Raja N, Pandiarajan D, Ramesh R. Binuclear ruthenium(III) Schiff base complexes bearing N4O4 donors and their catalytic oxidation of alcohols. Spectrochimica Acta Part A. 2008;71:884–891.
- Vogel AI. Text book of practical organic chemistry. 5th ed. London: Longman; 1989.
- Meshkani F, Rezaei M. Effect of process parameters on the synthesis of nanocrystalline magnesium oxide with high surface area and plate-like shape by surfactant assisted precipitation method. Powder Technol. 2010;199:144–148.
- Balalaie S, Golizeh M, Hashtroudi MS. Clean oxidation of benzoins on zeolite A using microwave irradiation under solvent-free conditions. Green Chem. 2000;2:277–278.
- Azizian S, Eftekhari-Bafrooei A, Bashiri H. Kinetics of catalytic oxidation of benzoin to benzil by alumina supported active MnO2. Kinet Catalysis. 2010;51:244–249.
- Li X, Xiao W, He G, Zheng W, Yu N, Tan M. Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surface A. 2012;408:79–86.