Abstract
The emergence of novel mechanisms of β-lactam resistance and their gain by infectious micro-organisms are posing a huge clinical threat to human health. It creates a need to generate novel resistant antibiotic molecules to counter their attack. In the present study, a novel strategy is developed to synthesise β-lactamase-resistant penicillin G molecules by using the unique properties of iron nanoparticles. Spherical monodispersed iron nanoparticles were prepared by a simple chemical reduction method. The formation process of the iron nanoparticles was investigated by ultraviolet–visible spectroscopy and atomic force microscopy. Free amine groups introduced on the surface of native iron nanoparticles by coating a uniform layer of polyaniline were confirmed by Fourier transform infrared spectroscopy and scanning electron microscopy. Functionalised iron nanoparticles were then grafted to the C3 carboxyl group of the β-lactam ring of penicillin G in the presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride with a conjugation yield of 187.7 μg mg−1. These novel iron nanoparticle–penicillin G conjugates showed a very good growth inhibition against β-lactam-resistant Staphylococcus aureus. This work thus describes a novel strategy for synthesis of nanoconjugates which can be used to design novel bactericidal materials against β-lactam-resistant infectious micro-organisms.
1. Introduction
Infectious diseases have been a leading cause of mortality around the globe, especially in developing countries struggling with poor health services (World Health Report 2007) [Citation1]. With the increase in burden of bacterial infections, the infectious organisms are fuelled by antibiotic-resistant mechanisms. Because of these, clinical treatment of these infections is a big clinical challenge.[Citation2] Antibiotic-resistant bacteria are emerging as a threat to the favourable outcome of common infections in community and hospital settings.[Citation1,2] It is either due to horizontal gene transfer or unlinked point mutations in the pathogen genome. This acquired character in the microbes will be inherited to their offspring andwill result in a fully resistant colony.[Citation3] There are many mechanisms by which micro-organisms exhibit resistance to antimicrobials.[Citation2,3] Among all these antibiotic-resistance mechanisms, drug inactivation by β-lactamase is inducing maximum clinical threat to β-lactam antibiotic family.[Citation4] These enzymes specifically cleave the β-lactam ring at the position of amide bond, which is the reactive part in the β-lactam antibiotic.[Citation5]. As a result, the clinical usage of the entire range of β-lactam antibiotics is severely compromised. It is leading to limited therapeutic options, like development of new resistant antibiotic molecules.[Citation6] Over the last few decades, newer β-lactam antibiotics have been specifically designed by chemical modification of β-lactam ring at different positions as well various new formulations were developed.[Citation7–9] These compounds display potent antibacterial activity against gram-positive bacteria and show slightly higher stability towards hydrolytic cleavage by β-lactamase.[Citation7–9] However, clinical usage of these molecules/formulations is a big question because of their limited activity. This is attributed to their poor permeability through the microbial membranes.[Citation9] So this creates a need to develop new resistant antibiotic/bioactive molecules.[Citation6] Synthesis of nanosized particles for drug delivery with tailored physical and chemical properties is of great interest in development of new pharmaceutical products.[Citation10–12] Such nanosized materials are usually synthesised with various metal-based nanoparticles.[Citation10,Citation13] Among all the materials used so far for making nanosized drug conjugates, iron is considered as one of the best option. This is because of its unique properties like magnetism, safety and inorganic antibacterial activity.[Citation14–16] Iron ions are oligodynamic, as they cause bacteriostatic [Citation16] and bactericidal impacts.[Citation17–19] These properties make it a potential material for the synthesis of bioactive material, to inhibit microbial film formation,[Citation15] as a photo-killing agent of pathogenic bacteria [Citation17] and bactericidal agent.[Citation18,19]
In the present study, we have exploited the properties of iron (magnetite) nanoparticles and designed novel nanoconjugates with penicillin G to fight β-lactam-resistant micro-organisms. Magnetite nanoparticles were synthesised by chemical reduction method. These were further used to develop novel iron nanoparticle–penicillin G conjugates through covalent linkage of functionalised iron nanoparticles at C3 position in β-lactam ring of penicillin G molecules. Antibacterial activity of these novel iron nanoparticle–penicillin G conjugates was checked by using β- lactam-resistant Staphylococcus aureus USA 300 as model organism. These novel iron nanoparticle–penicillin G conjugates exhibited a significant antibacterial activity against β-lactam-resistant S. aureus. Our work thus describes a novel strategy for developing resistant drug molecules against infectious micro-organisms carrying extended-spectrum β-lactamase.
2. Material and methods
2.1. Materials and apparatus
All the chemicals used were of analytical grade. Double-distilled deionised water was used throughout the work. Various instruments were used to conduct experiments like ultraviolet–visible (UV-Vis) double-beam spectrophotometer (Shimadzu UV-2550, Japan), Fourier transform infrared (FTIR) spectrophotometer (Thermo Scientific Instruments, USA), scanning electron microscope (SEM) (Model Joel JSM-6510, Japan), atomic force microscope (Agilent Technology, USA), ultra sonicator (Missonix, USA), orbital shaker incubator and biological oxygen demand test (BOD) incubator (Narang Scientific Works, New Delhi, India).
2.2. Synthesis and characterisation of native/functionalised iron nanoparticles
Chemical reduction method was used to prepare iron colloids.[Citation15] The size and morphology of iron nanoparticles were observed through atomic force microscope in air using magnetic AC (MAC) mode and analysed with PicoImage. Functionalisation of native iron nanoparticles was done by polymerisation of polyaniline at the surface of native iron nanoparticles in the presence of sodium chlorite as oxidant.[Citation20] To disperse a uniform layer of polyaniline over the surface of iron nanoparticles, the reaction mixture was sonicated for 2 min at room temperature with a constant power output of 3.0 watt with an on/off pulse of 30 seconds. The product was filtered under gravity and washed thrice with deionised water. These functionalised iron nanoparticles were suspended into deionised water (final concentration of 10 mg ml−1) and stored at 4 °C. The morphological studies of functionalised iron nanoparticles were carried out with SEM at Department of Chemistry, M.D. University, Rohtak. Functional groups on the nanoparticles were analysed by collecting FTIR spectrum as per manufacturer instructions.
2.3. Spectrophotometric assay for penicillin G
A novel spectrophotometric assay for estimation of penicillin G in aqueous solution was developed. Penicillin G stock solution was prepared in distilled water and scanned with UV-Vis double-beam spectrophotometer to generate continuous absorbance spectrum at various wavelengths between 190 and 1100 nm with a resolution of 1 nm using distilled water as a blank. With this continuous absorbance spectrum, wavelength corresponding to maximum absorbance (λmax) was analysed. It was further validated by taking absorbance of various penicillin G solutions at different concentrations. This method was validated for its accuracy, linearity, reproducibility and reliability.
2.4. Synthesis of iron nanoparticle–penicillin G conjugates
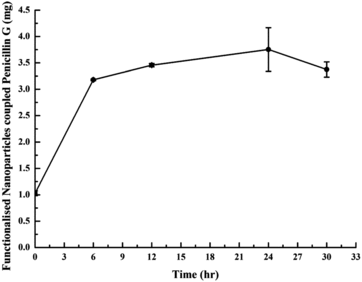
To synthesise iron nanoparticle–penicillin G conjugates, 0.5 ml of carbodiimide solution (25 μg ml−1 in 50 mM sodium phosphate buffer of pH 6.0) was mixed with 2.5 ml penicillin G solution (2 mg ml−1 in distilled water). The reaction mixture was sonicated for 10 min at room temperature with a constant power output of 0.5 watt with an on/off pulse of 30 seconds. Chemical coupling of penicillin G and iron nanoparticles was started by adding 2 ml of functionalised iron nanoparticles (20 mg) in reaction mixture followed by its incubation at 10 °C with continuous mixing at 50 rpm. The optimum time of incubation for iron nanoparticle–penicillin G conjugate formation was analysed by calculating the amount of free penicillin G in supernatant using UV spectrophotometric method after performing chemical coupling for different intervals like 0, 6, 12, 24 and 30 hrs. A final reaction was performed at these optimised parameters to achieve sufficient quantity of iron nanoparticle–penicillin G conjugates. Dialysis of the reaction mixture was performed in the presence of deionised water to remove unbound penicillin G and other by-products. Finally, the iron nanoparticle–penicillin G conjugates were recovered from the reaction by centrifugating the sample at 8000 rpm for 5 min at 4 °C. Pellet was suspended into 1 ml of deionised water and stored at 4 °C till further use. The amount of conjugated penicillin G was determined by measuring the unbound penicillin G content in the supernatant, and conjugation yield was calculated using the following formula:
2.5. Antimicrobial activity assay of iron nanoparticle–penicillin G conjugates against β-lactam-resistant S. aureus
The antimicrobial activity of iron nanoparticle–penicillin G conjugates, penicillin G and functionalised iron nanoparticles were evaluated against S. aureus USA 300, by standard disc diffusion assay. Microbial culture was grown on Luria–Bertani (LB) broth (prepared after adjusting pH 7.2 of a solution of tryptone (1.0%), yeast extract and sodium chloride (both 0.5%) in distilled water) at 37 °C with constant shaking at 200 rpm for 16 hrs at incubator shaker. A 100 μl of overnight grown culture (optical density = 1.0 at A600nm) was plated on LB agar medium. A sterile disc was placed in the centre of the culture-plated agar medium, and 20 μl of iron nanoparticle–penicillin G conjugates (∼75 μg conjugated penicillin with 400 μg iron nanoparticles), functionalised iron nanoparticles (400 μg) and penicillin G solution (75 μg) were added to the disc. The plates were incubated for 24 hrs at 37 °C. The microbial growth was evaluated in the plate and zone of growth inhibition was calculated. The results on the three plates corresponding to a particular sample were averaged.
3. Results and discussion
The discovery of antibiotics in the twentieth century was a breakthrough in the treatment of infections, but simultaneous emergence of resistance to these antibiotics among infectious organisms generated a threat to present and future medical advances. It generates a need to develop effective and resistant antimicrobial molecules.[Citation1–6] However, the steady discovery of novel antibiotics in the period 1940–1980 could not be sustained. Only few antibiotic classes joined the approved list after 1990 onwards. Along with this, crossover of the resistance for the related compounds is threatening the sharp depletion of the antibiotic resources for the future use. It creates a need to modify existing molecules for improved activity against emerging antibiotic-resistant pathogens.[Citation6–11] So, the present study was designed to develop β-lactamase-resistant penicillin G nanoconjugates which will help to overcome the clinical threats posed by broad spectrum β-lactamase-resistant micro-organisms.
3.1. Characterisation of native/functionalised iron nanoparticles
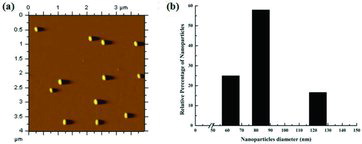
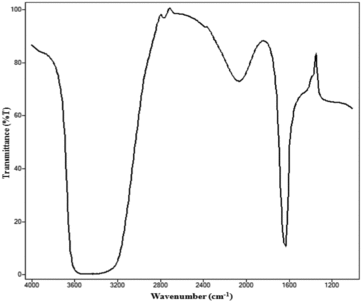
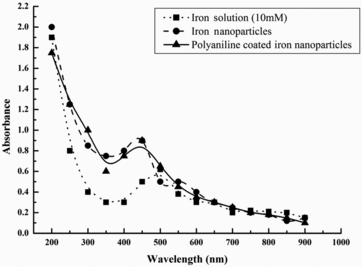
The formation of nanosized iron particles started almost immediately on the addition of alkali agent into acid solution of iron.[Citation15] It was followed by absorbance measurements. showed the absorption spectra of the iron nanoparticles generated after the reduction process. It had λmax at 450 nm and showed a slight shift from the λmax of iron solution, i.e. 500 nm, and might be due to surface modification in iron nanoparticles. Similar shift in absorbance spectra pattern was observed in earlier studies.[Citation17] AFM of iron nanoparticles showed agglomerates of small grains, with average diameter of 83 nm. However, few particles had diameters larger than 83 nm, probably formed because of aggregation. Atomic force micrographs showed that iron nanoparticles were approximately spherical ((a)). The histograms ((b)) of iron nanoparticles showed their size between 60 and 125 nm. The FTIR spectra of iron nanoparticles indicated that they were free from any absorbed molecule at their surface. Functionalisation of iron nanoparticles was performed by coating iron nanoparticles with a uniform layer of polymerised aniline. Sodium chlorite leads to polymerisation of aniline by generating reactive aniline radicals which will couple with each other to form a long chain polymer.[Citation20] After the polymerisation, the colour of nanoparticle solution was turned into light greyish green form. The formation of functionalised iron particles was followed by absorbance measurements (). It showed the unique absorption spectra with λmax at 450 nm. This unique absorption pattern might be due to the absorbance of light by the uniform layer of polyaniline over functionalised iron nanoparticles. Scanning electron micrographs showed that the size of functionalised iron nanoparticles was in a range from >100 to 200 nm. The size of native iron nanoparticles was increased after the functionalisation, which might be due to polyaniline coating over iron nanoparticles. Surface coating of polyaniline layer on iron nanoparticles was further confirmed by FTIR analysis (). Functionalised iron nanoparticles showed absorption peaks at 1635 cm−1 and at 3250–3600 cm−1 in FTIR spectra. Absorption peak at 1635 cm−1 is for the aromatic C=C stretching vibrations, while peaks at 3250–3600 cm−1 are because of amine group stretching vibrations. Among all other reagents, only polyaniline bears these functional groups, which clearly indicated the presence of polyaniline within these functionalised iron nanoparticles. Results of bothSEM and FTIR analyses confirmed the functionalisation of iron nanoparticles with free amine groups of polyaniline. These free amine group activated iron nanoparticles were used to develop novel penicillin G iron nanoconjugates in the presence of carbodiimde reagent.
Figure 1 UV–visible spectrum of iron solution (…▪…), native iron nanoparticles (-•-) and functionalised iron nanoparticles (-▴-).
3.2. Spectrophotometric assay of penicillin G
A λmax for penicillin G was observed at 217 nm after taking absorbance of penicillin G solution between 190 and 1100 nm with UV-Vis double-beam spectrophotometer. The λmax of penicillin G was found in UV region, this might be due to the presence of unique ring structure, as similar absorbance pattern was observed for another β-lactam antibiotic, i.e. flucloxacillin (having λmax at 219 nm).[Citation21] Penicillin G calibration curve showed linearity in the concentration range of 0–50 μg ml−1. Accuracy of the method was checked in terms of percentage recovery of 99.9% with a very small standard deviation of 0.02%. As per the guidelines of International Conference for Harmonisation, all these results showed reliability of the present method. This method was further used for determination of penicillin G in aqueous solution in the rest of the experiment.
3.3. Synthesis of iron nanoparticle–penicillin G conjugates
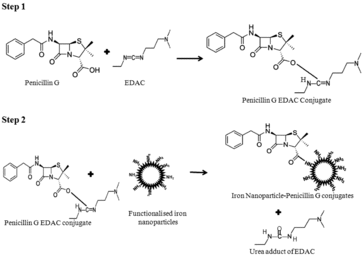
β-lactam antibiotics have dominated the clinical market and simultaneous development of natural products such as penicillin G into more potent forms through rational modification has increased the range of activity of these drugs. But widespread use of β-lactam has led to the emergence of resistant microbial strains having an enzyme beta lactamase. So β-lactam-type antibiotics need to be modified at various positions to resist the hydrolysis by β-lactamase.[Citation9] Recently, various research groups have identified the inorganic oxidising properties of iron nanoparticles which inhibit microbial growth in various environments.[Citation15–19] In the present study, iron nanoparticles were used as bulky side chain group to protect β-lactam ring against hydrolytic cleavage of β-lactamase, besides using their inorganic oxidising properties. A novel iron nanoparticle–penicillin G conjugate was developed after coupling iron nanoparticles at C3 position of β-lactam ring of penicillin G using carbodiimide reagent.[Citation22] outlines the construction of novel β-lactamase-resistant penicillin G iron nanoconjugate. This tactic will help to protect β-lactam ring from β-lactamase cleavage by developing steric hindrance to β-lactamase active site for substrate availability. Iron nanoparticle–penicillin G conjugates were developed after coupling iron nanoparticles at C3 position of β-lactam ring of penicillin G in the presence of carbodiimde reagent. In first step, the C3 carboxyl group of β-lactam ring was specifically activated by incubating penicillin G in presence of EDAC solution (N-(3-dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride). To activate −COOH group, carbodiimide reagent performed nucleophilic attack at COOH group at C3 position of β-lactam ring of penicillin G. It leads to the formation of a stable O-acylisourea adduct carrying activated EDAC as a leaving group. In the next step, free amine activated iron nanoparticles will be incubated with penicillin G EDAC, i.e. O-acylisourea adduct. The penicillin G EDAC, i.e. O-acylisourea adduct, reacted with free primary amine group introduced on the surface of functionalised iron nanoparticles resulted into desired amide linkage to prepare iron nanoparticle–penicillin G conjugates. After this amide bond formation, the activated carbodiimide group left as water-soluble urea derivative.
We expected that the presence of iron nanoparticles in C3 position of β-lactam ring of iron nanoparticle–penicillin G conjugates will generate steric hindrance for substrate availability for β-lactamase. It will induce stability to β-lactam ring against β-lactamase cleavage. This hypothesis was confirmed after checking the antimicrobial activity of iron nanoparticle–penicillin G conjugates against β-lactam S. aureus. The optimal time of incubation for iron nanoparticle–penicillin G conjugate formation was identified near 24 hrs () with a conjugation yield of 187.7 μg mg−1.
3.4. Antimicrobial activity of iron nanoparticle–penicillin G conjugates against β-lactam-resistant S. aureus
Antimicrobial activity was observed in triplicate against β-lactam-resistant S. aureus USA 300, in iron nanoparticle–penicillin G conjugates. An increased antimicrobial activity was observed in iron nanoparticle–penicillin G conjugates (11.0 ± 1.0 mm), in comparison to penicillin G (Nil) and functionalised iron nanoparticles (1.0 ± 0.5 mm). This increase is attributed to (1) active drug cleavage from the nanoparticle frame work and (2) covalent binding of the penicillin G to the iron nanoparticles.[Citation23] Along with this, sustained activity of iron nanoparticle–penicillin G conjugates indicated resistance of conjugated penicillin G nanoparticles against β-lactamase activity. This resistance towards β-lactamase cleavage proves successful implementation of our strategy for development of β-lactamase-resistant antimicrobial nanoconjugates. These nanoconjugates showed a stable antimicrobial activity against gram-positive (S. aureus) micro-organisms indicating their probable usage against β-lactam-resistant infectious micro-organisms. Thus, the present study describes a novel strategy for developing new β-lactamase-resistant, potent nanoconjugates which will help to combat the threat posed by the increased rate of evolution of antibiotic-resistant mechanisms in infectious Staphylococcus sp.
4. Conclusion
The present study describes a novel strategy to develop new resistant antibiotic molecules against the antibiotic-resistant mechanisms prevalent in the world of infectious microbes. This study has utilised the oxidising properties of iron nanoparticles to develop a β-lactamase-resistant, effective antibacterial conjugate which has shown a good activity against resistant micro-organisms. This strategy will be helpful to combat the threat posed by the increased rate of evolution of antibiotic-resistant mechanisms in infectious microbes by developing new resistant, highly effective antibiotic molecules.
Acknowledgements
Dr Munia Ganguli, Council of Scientific and Industrial Research-Institute of Genomics and Integrative Biology [CSIR-IGIB] is acknowledged for extending support for using AFM facility. Dr Manu Bhambi thanks the Department of Biotechnology (DBT) Bio-CARe scheme for the financial support.
References
- Nicolau DP. Current challenges in the management of the infected patient. Curr Opin Infect Dis. 2011;24:1–10.
- Wilke MH. Multiresistant bacteria and current therapy – the economical side of the story. Eur J Med Res. 2010;15:571–576.
- Gniadkowski M. Evolution of extended spectrum beta-lactamases by mutation. Clin Microbiol Infect. 2008;14:11–32.
- Bush K. Alarming beta-lactamase mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol. 2010;13:558–564.
- O’Callaghan CH. Structure activity relations and beta-lactamase resistance. Philos Trans R Soc Lond B. 1980;289:197–205.
- Fircanis S, McKay M. Recognition and management of extended spectrum beta lactamase producing organisms (ESBL). Med Health R I. 2010;93:161–162.
- Frezza M, Garay J, Chen D, Cui C, Turos E, Dou QP. Induction of tumor cell apoptosis by a novel class of N-thiolated beta-lactam antibiotics with structural modifications at N1 and C3 of the lactam ring. Int J Mol Med. 2008;21:689–695.
- Neu HC. Relation of structural properties of beta-lactam antibiotics to antibacterial activity. Am J Med. 1985;79:2–13.
- Karunaratne DN, Farmer S, Hancock RE. Synthesis of bulky beta lactams for inhibition of cell surface beta-lactamase activity. Bioconjug Chem. 1993;4:434–439.
- Abeylath SC, Turos E, Dickey S, Lim DV. Glyconanobiotics: novel carbohydrated nanoparticle antibiotics for MRSA and Bacillus anthracis. Bioorg Med Chem. 2008;16:2412–2418.
- Turos E, Shim JY, Wang Y, Greenhalgh K, Reddy GS, Dickey S. Antibiotic-conjugated polyacrylate nanoparticles: new opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17:53–56.
- Paszko E, Ehrhardt C, Senge MO, Kelleher DP, Reynolds JV. Nanodrug applications in photodynamic therapy. Photodiag Photodyn Ther. 2011;8:14–22.
- Raad II, Mohamed JA, Reitzel RA, Jiang Y, Dvorak TL, Ghannoum MA, Hachem RY, Chaftari AM. The prevention of biofilm colonization by multidrug resistant pathogens that cause ventilator associated pneumonia with antimicrobial coated endotracheal tubes. Biomaterials. 2011;32:2689–2694.
- Avasthi DK, Mishra YK, Singhal R, Kabiraj D, Mohapatra S, Mohanta B. Synthesis of plasmonic nanocomposites for diverse applications. J Nanosci Nanotechnol. 2010;10:2705–2712.
- Taylor EN, Webster TJ. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomedicine. 2009;4:145–152.
- Diao M, Yao M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009;43:5243–5251.
- Chen WJ, Tsai PJ, Chen YC. Functional Fe3O4/TiO2 core/shell magnetic nanoparticles as photokilling agents for pathogenic bacteria. Small. 2008;4:485–491.
- Gu H, Ho PL, Tsang KW, Wang L, Xu B. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram positive bacteria at ultralow concentration. J Am Chem Soc. 2003;125:15702–15703.
- Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effect of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomedicine. 2010;5:277–283.
- Li X, Li X. Oxidative polymerization of aniline using NaClO2 as an oxidant. Mater Lett. 2007;61:2011–2014.
- Dey S, Coudhary R, Vaithiyanathan S, Samal HB, Reddy YV, Krishna B. Spectrophotometric method developed for the estimation of flucloxacilin in bulk and dosage form using UV-Vis spectrophotometric method. Int J Pharma Bio Sci. 2010;1:35–43.
- Kachalova AV, Stetsenko DA, Gait MJ, Oretskaya TS. Synthesis of oligonucleotide 2′-conjugates via amide bond formation in solution. Bioorg Med Chem Lett. 2004;14:801–804.
- Turos E, Reddy GSK, Greenhalgh K, Ramaraju P, Abeylath SC, Jang S, Dickey S and Lim DV. Penicillin-bound polyacrylate nanoparticles: restoring the activity of b-lactam antibiotics against MRSA. Bioorg Med Chem Lett. 2007;17:3468–3472.